Abstract
Cinnamon has been used as an anti-diabetic agent for centuries but only in recent few years its mechanism of action has been under investigation. Previous studies showed that cinnamon might exert its anti-diabetic effect via increasing glucose transporter isotype-4 (GLUT4) gene and glycoprotein contents in fat cells. To study if hydro-alcoholic cinnamon extract (HACE) enhances GLUT4 translocation from intracellular compartments of nuclear or endoplasmic reticulum membranes (N/ER) into the cytoplasmic membrane (CM). C2C12 myoblastic cell line were seeded in DMEM plus 20 % FBS and differentiated to myotubes using 2 % horse serum. After myotubes formation, 100 or 1,000 μg/ml HACE, as intervention, and as control 1 % DMSO were added for 3 h. Cells were washed and homogenized followed by ultracentrifuge fractionation, protein separation by SDS-PAGE and GLUT4 detection using semi-quantitative Western blotting. Data analysis was done by two-independent samples t test for comparison of mean ± SD of GLUT4 percent in categories. GLUT4 contents were higher in CM of groups 100 and 1,000 μg/ml HACE and lower in 1 % DMSO treated myotubes (CI = 0.95, P < 0.05). For N/ER reverse results were obtained (CI = 0.95, P < 0.05). As our results have shown HACE induces GLUT4 translocation from intra-cell into cell surface. We conclude that cinnamon maybe a choice of type-2 diabetes mellitus treatment because its extract enhances GLUT4 contents in CM where it facilitates glucose entrance into the cell. However it is necessary to trace the signaling pathways which are activated by HACE in muscular tissue.
Keywords: Anti-diabetic, Cinnamon, Diabetes type-2, GLUT4, Muscle
Introduction
Diabetes is the most prevalent metabolic disease worldwide and the most cases are insulin resistant or type-2 diabetic patients [1]. Insulin resistance results to pathologic complications such as hyperinsulinemia, hyperglycemia and glucose toxicity, glucose intolerance, hypertriglyceridemia, high blood pressure, neuropathy, retinopathy, nephropathy, ketoacidosis and death [2–4]. The most important rout to reduce blood glucose concentrations are glucose exposal as a resource of cell energy and glycogen synthesis following its uptake into the cell via glucose transporters or GLUTs which are trans-membrane glycoproteins; this process essentially is mediated by insulin [5–7]. Adipocytes and myocytes are two major glucose disposing tissues [7, 8] that their most important and abundant glucose transporter is GLUT4 which as same as other glucose transporters facilitates glucose transport from extracellular environment into the cytosol [9]; Transcription and intracellular movement of GLUT4 gene product into the cytoplasmic membrane mediated by insulin signal transduction pathway; So GLUT4 dependent glucose transport is considered insulin dependent [7, 8].
Treatment strategies for diabetes are based on chemical agents that maybe unfavorable for most peoples both ethnically and mentally. Most peoples favored to treat their disease by/via a natural agent/way [10, 11]; for example for centuries Asian peoples used herbs to treat or prevent diabetes. In this case cinnamon, green tea, turmeric and bitter melon are good examples [12–15]. Previous studies have shown that 1–6 g/day cinnamon powder intake reduces blood glucose, total cholesterol and LDL-Cholesterol in human [16]. Also it is shown that methyl hydroxychalecon, a cinnamon derived component, mimics insulin effects on 3T3-L1 cell line and increases glycogen synthase kinase, glucose uptake and insulin receptor (IR) contents [1]. Other studies have shown GLUT4 enhancement under different concentrations of cinnamon extract or dihydrocinnamic acid (DHH105), a derivative of cinnamon, on GLUT4 content in animal adipose tissue [17] or 3T3-L1 cell line, a good model for studding fat tissue metabolism [14]. But muscular tissue is the most important site for glucose metabolism which uses more than 75 % of body glucose expenditure under insulin impact [18–21]. As the rate limiting step of blood glucose level expenditure is regulated by GLUT4 glycoprotein presence in the cytoplasmic membrane [22, 23], the objective of current study was to investigate the effect of cinnamon water extract on GLUT4 contents of cytoplasmic membrane (CM) and nuclear/endoplasmic (N/ER) reticulum fractions of differentiated C2C12 myotubes. Here we test the hypothesis if the hydro-alcoholic cinnamon extracts (HACE) increase GLUT4 quantities in cytoplasmic membrane. For this purpose we measured GLUT4 quantities in CM and N/ER of HACE exposed or non-exposed C2C12 myotubes.
Materials and Methods
Hydro-Alcoholic Cinnamon Extract
HACE was prepared according to the method described by R.A. Anderson et al. [24] with modifications; 200 g cinnamon powder which was prepared from cinnamon barks routinely imported from china and is selling in Iran, dissolved in 500 ml 0.1 N acetic acid (Merck) and boiled for 20 min and centrifuged. One part of the 2,000 rpm supernatant mixed with 4 part absolute ethanol (Merck) and kept in 4 °C overnight; then the mixture was filtered using Whatman No. 1 filter paper and dried in a 60 °C oven; resulted powder dissolved in dimethylsulfoxide (DMSO) (Sigma) with a concentration of 100 mg/ml as stock solution.
C2C12 Primary Culture and Differentiation
C2C12 cell line purchased from Pasture Institute of Iran. About 8 × 104 C2C12 myoblast cells were seeded in DMEM (Sigma) containing 4 mmol/l glutamine, 0.025 mol/l glucose, 1 mmol/l sodium pyrovate, 0.018 mol/l NaHCO3 (Sigma-Aldrich), 100 U/ml Penicillin G (BIOCHROMA AG), 100 μg/ml streptomycin (BIOCHROMA AG) and 10 % foetal bovine serum (FBS) (Sigma) in 250 ml tissue culture flasks. Cell culture medium was exchanged each day until reaching to near confluent conditions. In all steps of cell culture flasks were kept in a humidified incubator with 5 % CO2 and 37 °C.
Differentiation of Myoblasts to Myotubes
Myoblasts differentiation to myotubes induced by the addition of DMEM supplemented with 2 % heat inactivated horse serum (HS) (Gibco™) and polynucleated myotubes formation monitored microscopically until day 5 of differentiation induction (Fig. 1). Differentiation medium was replenished each 48 h.
Fig. 1.
Microscopic images of C2C12 culture and differentiation steps. Fibroblast like C2C12 myoblasts (1) were grown in DMEM + 20 % FBS and kept in 37 °C with 5 % CO2 until reaching about 80 % confluence (2). Then culture medium replenished with DMEM + 2 % horse serum for inducing myoblast differentiation to myocytes (3&4) and polynuclear myotubes (5&6). Cells on day 6 were prepare for adding Hydro-Alcoholic Cinnamon Extract (HACE) or DMSO as intervention or vehicle categories respectively
Affecting Hydro-Alcoholic Cinnamon Extract (HACE) on Myotubes
Two different concentrations of HACE were prepared to assess if HACE has any effect on GLUT4 content of CM and N/ER. Concentrated 100 mg/ml HACE was diluted with DMEM + 2 % HS and two concentrations 100 and 1,000 μg/ml were prepared separately. For comparison of the effect of different concentrations of HACE with a control group, prepared two different concentrations were added to related culture flasks, as vehicle categories, containing differentiated myotubes 6 day after differentiation initiation. Study design was as follow: three flasks for DMEM + 2 % HS containing 1,000 μg/ml HACE, three flasks for DMEM + 2 % HS containing 100 μg/ml HACE (two categories with different concentrations) and three else flasks containing 1 % DMSO as vehicle group. As Cao et al. [14] have shown the best results for GLUT4 enhancement in 3T3-L1 under HACE treatment on time exposure of 3 h, we also kept the myotubes in such exposure time status.
Subcellular Fractionation
After 3 h exposure to HACEs or DMSO myotubes were washed 3 times with ice cold phosphate buffer (pH 7.4) then detached from flasks with scraper and homogenized with tissue homogenizer for 3 min in HES buffer, pH 7.4; 225 mM sucrose (Sigma); 4 mM Na2EDTA (Merck), 20 mM HEPES (Applichem); 1 mM phenylmethylsulfonyl fluoride and one tablet/dl anti-protease cocktail (Sigma). Cell homogenate was centrifuged according to the instruction presented by Tortorella et al. [25] for dexamethasone effect on C2C12 myotubes GLUT4 contents; the first spin performed at 19,000 g for 20 min; To crude plasma membrane or Nuclear/Endoplasmic reticulum (N/ER) of myotubes resulted pellet was homogenized in HES buffer and layered on a buffer composed of 1.12 M sucrose in 20 mM HEPES and 1 mM Na2EDTA buffer and centrifuged further at 1,00,000 g for 1 h. Interphase portion of this step was contained plasma membrane particles that were floated on HCS and resulted pellet included of N/ER particles. Interphase particles were aspirated and centrifuged at 40,000 g for 20 min to be pelleted; the pellet was re-suspended in PBS, pH 7.4, containing protease inhibitors. N/ER particles were re-suspended in a buffer containing 50 mM NaCl (Merck), 2 % Nonidet P-40 (Applichem), 0.5 % sodium deoxycholate (Applichem), 0.2 % SDS (Merck), 20 mM Tris (pH 7.4) (Merck) and protease inhibitor cocktail. All centrifugation steps were done at 4 °C with Beckman coulter ultracentrifuge and Type 90 Ti rotor. Also g force and round per minute (RPM) values for each step of centrifugation were calculated by the Beckman coulter ultracentrifuge calculating software [26].
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (PAGE) and Western blotting: Total protein concentrations were analyzed using Bradford reagent (Sigma-Aldrich) and proteins were separated by a 10 % SDS-PAGE according to the Laemmli instruction [27]. Briefly 100 μg protein was loaded to SDS-PAGE wells and separated with 30 mAmp for 3 h. Separated proteins were transferred to the nitrocellulose membrane at 4 °C for mAmp overnight. Blotted membranes were blocked using 2 % blocking agent included in immunoblotting kit (Amersham ECL Advance Western blotting) and was prepared in Tweenated phosphate buffer saline containing 1 % Polysorbate-20 (TBS), pH 7.5, for 3 h with gently shaking. After 3 × 15 min washing in TBS, blotted paper immersed in 2 % blocking agent containing mouse anti-GLUT4 antibody 1F8 (Santa Cruz Biotech) with a dilution of 1:200 for 4 h. After repeating wash steps goat anti-mouse IgG-HRP (SantaCruz Biotech) was added with a concentration of 1:1,000 in 2 % blocking agent for 5 h with gently shaking. After rewashing the membrane, chemiluminescent detection reagents were added according to the kit instruction and in a dark room. GLUT4 bands were visualized using mammography films (Kodak) and its related reagents.
Quantifications of GLUT4 Bands and Data Analysis
Resulted bands from Western blotting were quantified by densitometry and comparing with together for percent determination. Data were a percent that divided between intervention group and its equal vehicle group. Comparative histograms plotted in Excel and data were analyzed by spss ver 11.5 software with two independent samples t test.
Results and Discussions
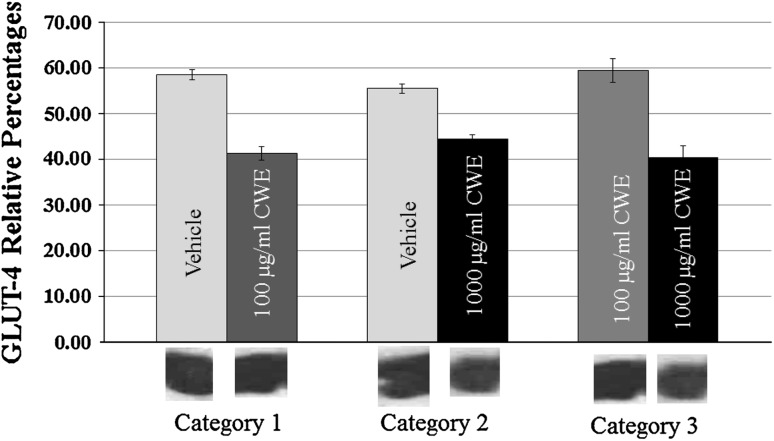
Comparison of GLUT4 percentages in N/ER fractions of C2C12 myotubes that exposed to 100 or 1,000 μg/ml HACE (intervention group) or 1 % DMSO (vehicle) for 3 h showed that GLUT4 quantities are lesser in groups exposed to HACEs. Statistically Glut4 percentages were significantly different between intervention and vehicle groups (with CI = 0.95, P = 0.001 both for 100 and 1,000 μg/ml HACE). Also comparison of myotubes exposed to 100 μg/ml HACE for 3 h have significantly more Glut4 glycoproteins than 1,000 μg/ml HACE group (Fig. 2) (CI = 0.95, P = 0.006). This effect was dose dependent meaning that Glut4 quantities of N/ER were lesser in that group exposed to 1,000 μg/ml in comparison to the 100 μg/ml HACE. However, Glut4 quantities were increased in CM fraction of that groups exposed 3 h to HACE concentrations of 100 or 1,000 μg/ml than DMSO exposed category (CI = 0.95, P = 0.000 both for 100 or 1,000 μg/ml); As it is revealed from Fig. 3 this effect was dose-dependent as the group exposed to 1,000 μg/ml cinnamon significantly has higher percentages of GLUT4 quantities in comparison to the 100 μg/ml HACE or DMSO exposed myotubes; myotubes that were exposed to 100 μg/ml HACE had more Glut4 glycoproteins than vehicle (Fig. 3).
Fig. 2.
Paired comparisons of Glut4 relative percentages obtained from Nuclear/Endoplasmic Reticulum (N/ER) fractions of myotubes between categories 100 and 1,000 μg/ml Hydro-Alcoholic Cinnamon Extract (HACE) treated and their concurrent vehicle (1 % DMSO) myotubes and with together. Data were gathered from Western-blot band scans. In all cases mean ± SD were compared using independent samples t test for P < 0.05 (CI = 0.95)
Fig. 3.
Paired comparisons of Glut4 relative percentages obtained from Cytoplasmic membrane (CM) fractions of myotubes between categories 100 and 1,000 μg/ml Hydro-Alcoholic Cinnamon Extract (HACE) treated and their concurrent vehicles (1 % DMSO) myotubes and with together. Data were gathered from Western-blot band scans. In all cases mean ± SD were compared using independent samples t test for P < 0.05 (CI = 0.95)
The anti-diabetic effect of Cinnamomi cassiae extract (Cinnamon bark: Lauraceae) in a type II diabetic animal model (C57BIKsj db/db) was reported by Kim and coworkers [23]. The potential Insulino-mimetic activity, cellular glucose metabolism enhancement and hypoglycemic properties of cinnamon were reviewed by Gruenwald et al. and Kirkham et al. [28, 29]. Cinnamon ingredients have been shown to enhance glucose metabolism via insulin signal transduction pathway in adipocyte cells and tissues [14, 23, 30]. Liao et al. [31] have shown that chromatographic extract of Cirsium Japonicom (CJ) decrease levels of glucose, triglycerides, cholesterol, insulin and adiponectin but increase plasma leptin levels in diabetic rat model. Although they have shown some beneficial effect for CJ but GLUT4 contents of adipose tissue was not changed significantly. Although CJ and cinnamon both have antidiabetic effect on diabetic rats and their main components are polyphenols but our results, and also other studies on cinnamon phenolics, are in discordant with obtained results for CJ. These discrepancies may be due to a variety of factors such different study models, unknown plants ingredients and so forth. In the other hand such results confirm that structural similarities between polyphenols are not good evidences for their antidiabetic effects to be addressed and each new investigated component needs to be assessed by both in vivo and in vitro models. Another study is investigated GLUT4 transcription, phosphate inositid-3 kinase activity and glucose uptake, to find out the effects of cholorogenic acid, frolic acid and berbein in L6 myotubes; the effect of mentioned components was compared with the effect of metformin and thiazolidindiones; cholorogenic and frolic acid did enhance GLUT4 transcription without and with a PI3-K pathway independent and dependent mechanisms respectively [32]. Another research team has reported Epigallocatechin-3-O-gallate (EGCG), a phenolic component of green tea, increases GLUT4 translocation from cytosolic compartments to the CM in L6 myotubes; this effect was an adenosine monophosphate protein kinase (AMPK) and PI3-K dependent event [33]. Also we have shown that HACE increases GLUT4 contents of CM in C2C12 myotubes. Then we hypothesize that EGCG and cinnamon polyphenols have same effects on GLUT4 intracellular compartments movements/translocation and this movement may be PI3-K dependent which the direction is from cytosol to the cytoplasmic membrane. Cao et al. [13] have investigated the effect of green tea ingestion on different genes in insulin signal transduction and GLUT1, GLUT2, GLUT3 and GLUT4 in muscular and hepatic tissues of diabetic rats. They have reported that 1 g/kg green tea per day increases hepatic GLUT1/4 significantly but not in muscular tissue. In the other hand 2 mg/kg green tea had increased GLUT2 and GLUT4 in the muscle significantly; this is an evidence of dose-dependency. The later result is in accordance with our study result; in our study also HACE had increased GLUT4 contents of muscular tissue culture model, C2C12 myotubes that this effect was dose-dependent (see results). Also Deng et al. [34] have shown that resveratrol, an ingredient of Polygonum cuspidatum and red wine did enhance glucose uptake and GLUT4 movement to the CM in C2C12 myotubes. However, other in vivo, ex vivo studies and human trials which have been explored antidiabetic effect or mechanism by which cinnamon exert antidiabetic effect all are in accordance with our study results [14, 16, 35–38]. But studies for cinnamon effect on the GLUT4 transcription and/or its intracellular movement in conjunction to signaling pathways are rare and the mechanism of its components is under investigation. Kim et al. [17] have studied 3,4-dihydroxyhydrocinnamic acid (DHH105), a derivative of cinnamon ingredients, which shows the highest activity of glucose uptake in vitro. They have shown that glucose uptake by epididymal adipocytes of diabetic rats were enhanced under different concentrations of DHH105. They have been proposed that DHH105 exert its effect via GLUT4 and Akt pathway and mentioned DHH105 as a good candidate for diabetes treatment. Cao et al. [14] have investigated the effect of cinnamon extract on GLUT4 transcription and its content in intracellular and cytoplasmic membrane of adipose tissue cell culture model, 3T3-L1 cell line. They have studied cinnamon water extract and its polyphenols, fractionated by HPLC, effects on GLUT4 transcription and its glycoprotein contents in resulted fat cells of 3T3-L1. They have shown that HACE and its polyphenols enhance the GLUT4 gene expression and CM contents. Also this study is in accordance with our results for a muscular tissue cell culture model. Then it is concluded that cinnamon could be a good choice for diabetes type 2 treatment because its ingredients up-regulate GLUT4 gene expression and glycoprotein movement from intracellular compartments to the CM where GLUT4 facilitate glucose entrance from extra cell to the intra cell space.
Acknowledgments
This work is supported by research grants from Shahid Sadoughi Yazd University of medical sciences and Yazd research center for In Vitro Flocculation (IVF); so we thank all University authors, professors and staffs who help us especially Prof. Abbas Aflatoniyan, Dr. Mohammad Ali Abdoli, Mr. Mehrdad Soleymani, Mrs. Farzaneh Fesahat and Mrs. Fatemeh Sadeghiyan all for their kindly co-working and guidelines.
Contributor Information
Abdorrahim Absalan, Email: a.r.absalan@gmail.com, Email: abdorrahim.absalan@modares.ac.ir.
Javad Mohiti-Ardakani, Email: mohiti_99@yahoo.com.
Hossein Hadinedoushan, Email: hhadi_2000@yahoo.com.
Mohammad Ali Khalili, Email: Khalili59@hotmail.com.
References
- 1.Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J Am Coll Nutr. 2001;20:327–336. doi: 10.1080/07315724.2001.10719053. [DOI] [PubMed] [Google Scholar]
- 2.Larijani B, Zahedi F, Aghakhani Sh. Epidemiology of diabetes mellitus in Iran. Iran J Diabet Lipid Disord. 2002;1(1):1–8.
- 3.Kannappan S, Jayaraman T, Rajasekar P, Ravichandran MK, Anuradha CV. Cinnamon bark extract improves glucose metabolism and lipid profile in the fructose-fed rat. Singap Med J. 2006;47:858–863. [PubMed] [Google Scholar]
- 4.Martin S, Slot JW, James DE. GLUT4 trafficking in insulin-sensitive cells. A morphological review. Cell Biochem Biophys. 1999;30:89–113. doi: 10.1007/BF02737886. [DOI] [PubMed] [Google Scholar]
- 5.Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9–26. doi: 10.4067/S0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- 6.Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W, et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007;39:222–229. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- 7.Sparling DP, Griesel BA, Olson AL. Hyperphosphorylation of MEF2A in primary adipocytes correlates with downregulation of human GLUT4 gene promoter activity. Am J Physiol Endocrinol Metab. 2007;292:E1149–E1156. doi: 10.1152/ajpendo.00521.2006. [DOI] [PubMed] [Google Scholar]
- 8.Kotliar N, Pilch PF. Expression of the glucose transporter isoform Glut-4 is insufficient to confer insulin-regulatable hexose uptake to cultured muscle-cells. Mol Endocrinol. 1992;6:337–345. doi: 10.1210/me.6.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Han J, Park S, Lee W. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Diabetes. 2003;52:A532. doi: 10.1016/s0003-9861(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 10.Al-Achi A. Herbs that affect blood glucose levels. Women’s Health Primary Care. 2005;8:325–330. [Google Scholar]
- 11.Drew AK, Myers SP. Safety issues in herbal medicine: implications for the health professions. Med J Aust. 1997;166:538–541. doi: 10.5694/j.1326-5377.1997.tb123246.x. [DOI] [PubMed] [Google Scholar]
- 12.Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem. 2000;48:849–852. doi: 10.1021/jf9904517. [DOI] [PubMed] [Google Scholar]
- 13.Cao H, Hininger-Favier I, Kelly MA, Benaraba R, Dawson HD, Coves S, et al. Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem. 2007;55:6372–6378. doi: 10.1021/jf070695o. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. 2007;459:214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Shih CC, Lin CH, Lin WL, Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009;123:82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 17.Kim W, Khil LY, Clark R, Bok SH, Kim EE, Lee S, et al. Naphthalenemethyl ester derivative of dihydroxyhydrocinnamic acid, a component of cinnamon, increases glucose disposal by enhancing translocation of glucose transporter 4. Diabetologia. 2006;49:2437–2448. doi: 10.1007/s00125-006-0373-6. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Nedachi T, Kanzaki M. Regulation of glucose transporters by insulin and extracellular glucose in C2C12 myotubes. Am J Physiol Endocrinol Metab. 2006;291:E817–E828. doi: 10.1152/ajpendo.00194.2006. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 21.Tulipano G, Spano P, Cocchi D. Effects of olanzapine on glucose transport, proliferation and survival in C2C12 myoblasts. Mol Cell Endocrinol. 2008;292:42–49. doi: 10.1016/j.mce.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 25.Tortorella LL, Pilch PF. C2C12 myocytes lack an insulin-responsive vesicular compartment despite dexamethasone-induced GLUT4 expression. Am J Physiol Endocrinol Metab. 2002;283:E514–E524. doi: 10.1152/ajpendo.00092.2002. [DOI] [PubMed] [Google Scholar]
- 26.Beckman Coulter I. 1998–2010. Available from: https://www.beckmancoulter.com/wsrportal/wsrportal.portal?_nfpb=true&_windowLabel=UCM_RENDERER&_urlType=render&wlpUCM_RENDERER_path=%2Fwsr%2Fresearch-and-discovery%2Fproducts-and-services%2Fcentrifugation%2Frotors%2Findex.htm&wlpUCM_RENDERER_t=3. Accessed 28 Dec 2009.
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 29.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11:1100–1113. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Choung SY. Antihyperglycemic and antihyperlipidemic action of cinnamomi cassiae (cinnamon bark) extract in C57BL/Ks db/db mice. Arch Pharm Res. 2010;33:325–333. doi: 10.1007/s12272-010-0219-0. [DOI] [PubMed] [Google Scholar]
- 31.Liao Z, Chen X, Wu M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch Pharm Res. 2010;33:353–362. doi: 10.1007/s12272-010-0302-6. [DOI] [PubMed] [Google Scholar]
- 32.Prabhakar PK, Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine. 2009;16:1119–1126. doi: 10.1016/j.phymed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Li Q, Liang J, Dai X, Ding Y, Wang J, et al. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine. 2010;17:14–18. doi: 10.1016/j.phymed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes. 2008;57:1814–1823. doi: 10.2337/db07-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson RA. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc Nutr Soc. 2008;67:48–53. doi: 10.1017/S0029665108006010. [DOI] [PubMed] [Google Scholar]
- 36.Baker W, Gutierrez-Williams G, White CM, Kluger J, Coleman C. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. 2008;31:41–43. doi: 10.2337/dc07-1711. [DOI] [PubMed] [Google Scholar]
- 37.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009;22:507–512. doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed] [Google Scholar]
- 38.Nahas R, Moher M. Complementary and alternative medicine for the treatment of type 2 diabetes. Can Fam Physician. 2009;55:591–596. [PMC free article] [PubMed] [Google Scholar]