Abstract
The objective of this study to evaluate heme oxygenase (COHb), leptin and coenzyme Q10 (CoQ10) in pre-eclamptic women. Also Zinc, copper, Iron, total iron binding capacity, Ferritin and uric acid were assessed. 120 female subjects were included in this study. They were divided into, 60 female with normal pregnancy attending the outpatient clinic, 60 pre-eclamptic patients were recruited from obstetrics and gynaecology department El-kasr El-Aini hospital. The results showed that in pre-eclampatic group, leptin level was significantly increased while COHb and CoQ10 was significantly decreased. It is concluded that hemeoxygenase, leptin and coenzyme CoQ10 can be considered as new markers for prediction of pre-eclampsia.
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-012-0226-7) contains supplementary material, which is available to authorized users.
Keywords: Pre-eclampsia, Hemeoxygenase, Carbon monoxide, Leptin, Coenzyme Q10
Introduction
Pre-eclampsia, pregnancy-specific disorder developing after 20 weeks of gestation, affects both the mother and the unborn baby [1]. This condition affects 8–10 % of all pregnancies worldwide and defined by gestational hypertension and proteinuria, contributes substantially to the prenatal morbidity and mortality of both mother and newborn. It is thought to be an early placental dysfunction, characterized by insufficient invasion of the spinal arteries by the trophoblast, placental ischemia, and impaired perfusion of the uteroplacental unit which can lead to fetal growth restriction [2].
Heme oxygenase (HO) is the rate-limiting enzyme in the degradation of heme to form carbon monoxide (CO), bilirubin and free iron [3]. It is involved in the control of vascular tone, regulating anti-inflammatory and antiapoptotic responses as well as reducing oxidative stress [4].
Leptin is described as a major placental protein which exhibits metabolic and physiological functions in a normal pregnancy. Leptin is one of the most important adipose derived hormones that not only secreted by the white adipose tissue but also synthesized by placenta. In pre-eclampsia, patients placental production of leptin is increased under hypoxic conditions so it is used as a marker of placental ischemia [5].
Coenzyme Q10 (CoQ10) is an essential component of oxidative phosphorylation at mitochondrial level, and also functions to stabilize cell membranes as well as acting as a potent antioxidant antihypertensive, anti-atherogenic, neuroprotective and bioenergetic [6]. CoQ10 affects the function of all cells in the body, making it essential for the health of all tissues and organs [7].
Trace elements, including zinc (Zn), copper (Cu) and iron (Fe) are involved in the regulation of blood pressure by participating in the structures of several enzymes. It has been hypothesized that an imbalance of Zn and Cu status might be involved in the etiology of human hypertension [8].
Subjects and Methods
Subjects
We investigated a 60 female with normal pregnancy, attending the outpatient clinic and 60 pre-eclamptic patients were recruited from obstetrics and gynaecology department, El-kasr El-Aini hospital. The study was approved by the Human Ethics Committee of the Faculty of Pharmacy, Suez Canal University.
The inclusion criteria for pre-eclamptic subjects include pregnancy with the duration more than 20 weeks gestation and child bearing age and exclusion of other medical complications (Liver and Renal disease), chronic hypertension and twin pregnancy. Written informed consents were taken from all subjects included in the study.
Laboratory Methods
Sample Collection
Blood sample was divided into two parts: first part fresh blood was drawn from an artery in heparenized syringe and aspirated into the Blood Gas Analyzer, the second part was drawn from a vein in vials under complete aseptic condition and serum was separated.
Biochemical Analysis
Determination of Liver Enzymes and Bilirubin
Commercial kit Purchased from BioMed Diagnostics (Oregon, USA) based on the method described by Reitman and Frankel, [9] was used for determination of ALT and AST activity. Additionally, the determination of serum Bilirubin was described by Gordon [10], method, Commercial kit Purchased from High Performance Diagnostic Reagents (USA).
Determination of Serum Uric Acid and Creatinine
The kit Purchased from Biocon Diagnostik (Germany), based on the method described by Weir et al. [11] was used for uric acid detection.
Additionally, the method described by Bartel [12] was used for determination of creatinine kinetic, the kit purchased from High Performance Diagnostic Reagents (POINTE, SCIETIFIC, INC, USA).
Determination of HO
COHb percentage of the arterial blood was performed gasometrically by Blood Gas Analyzer (Bayer Rapidlab 865, USA) according to the method described by Hampson et al. [13].
Determination of Serum Leptin
This method was carried out according to Considine and Sinha [14] for quantitative determination of circulating leptin concentration in human serum by ELISA method, commercial kit purchased from (DRG, USA).
Determination of Serum CoQ10
CoQ10 measurement was performed using high-performance liquid chromatography (HPLC) assay described by Mosca et al. [15]. Briefly, 400 μl of serum was supplemented with 50 μl of a 1,4-benzoquinone solution then Mixture was vortexed for 10 s. After 10 min 1 ml of n-propanol was added. Mixture was vortexed for 10 s and centrifuged at 10,000 rpm for 2 min at 4 °C finally 100 μl of supernatant was injected into the HPLC.
Determination of Serum Zn and Cu
The determination of Zn and Cu by atomic absorption Spectrophotometer according to the method of Sinaha and Cabrielli [16]. Using Perken–Elmer 2380 atomic absorption spectrophotometer.
Determination of Serum Fe and TIBC
Fe and TIBC in human serum was determined using the colorimetric method of [17], commercial kit purchased from Stanbio, USA.
Determination of Serum Ferritin
The quantitative determination of circulating ferritin concentration in human serum by ELISA method was performed according to Corti et al. [18]. The kit was supplied from Monobind Inc, USA.
Statistical Analysis
Data will be expressed as mean ± SD. Significance was assessed by student t test. All statistical tests were done employing SPSS program version 16 (SPSS Software, SPSS Inc., Chicago, USA) and the differences were considered significant at p ≤ 0.05 [19].
Results
Liver Enzymes and Bilirubin
Serum ALT and AST levels showed significant increase in pre-eclampsia group compared to normal pregnant group. However, serum bilirubin level was significantly decreased (p < 0.05) in pre-eclampsia group compared to normal pregnant group (Table 1).
Table 1.
Liver enzymes and Bilirubin in different studied groups
| Parameters | Normal pregnant | Pre-eclampsia |
|---|---|---|
| ALT (U/l) | 6.6 ± 0.34 | 9.7 ± 0.65a |
| AST (U/l) | 9.3 ± 0.28 | 13.0 ± 0.56a |
| Bilirubin (mg/dl) | 0.33 ± 0.02 | 0.26 ± 0.01a |
Number of patients in each group = 60
aSignificant difference when comparing pre-eclampsia group with normal pregnant group at p ≤ 0.05
Serum Uric Acid and Creatinine
There was significant increase (p < 0.05) in serum uric acid level in pre-eclampsia group compared to normal pregnant group. However, serum creatinine level was significantly increased (p < 0.05) in pre-eclampsia group compared to normal pregnant groups at p < 0.05 (Tables 2, 3).
Table 2.
Serum uric acid and creatinine in different studied groups
| Parameters | Normal pregnant | Pre-eclampsia |
|---|---|---|
| Uric acid (mg/dl) | 5.0 ± 0.15 | 7.1 ± 0.32a |
| Creatinine (mg/dl) | 0.75 ± 0.02 | 1.07 ± 0.04a |
Number of patients in each group = 60
aSignificant difference when comparing pre-eclampsia group with normal pregnant group at p ≤ 0.05
Table 3.
Serum Fe, TIBC and ferritin in different studied groups
| Parameters | Normal pregnant | Pre-eclampsia |
|---|---|---|
| Fe (μg/dl) | 67.3 ± 4.92 | 85.3 ± 4.64a |
| TIBC (μg/dl) | 428.7 ± 3.85 | 386.0 ± 4.73a |
| Ferritin (ng/dl) | 38.2 ± 1.09 | 79.6 ± 1.02a |
Fe, iron, TIBC, total iron binding capacity, Number of patients in each group = 60
aSignificant difference when comparing pre-eclampsia group with normal pregnant group at p ≤ 0.05
Serum Iron, TIBC and Ferritin
There was significant increase (p < 0.05) in serum Fe and TIBC levels in pre-eclampsia group compared to normal pregnant group. Moreover, serum TIBC level was significantly decreased in normal pregnant group compared to pre-eclampsia group.
COHb, Leptin and CoQ10 Levels
There was significant decrease (p < 0.05) in mean value of blood COHb level in pre-eclampsia group compared to normal pregnant group. The mean value of serum leptin level was significantly increased (p < 0.05) in pre-eclampsia group compared to normal pregnant group. Serum CoQ10 level was significantly increased (p < 0.05) in normal pregnant group compared to control group. While, the value of CoQ10 in pre-eclampsia group was significantly decreased (p < 0.05) compared to control group. Also serum CoQ10 level was significant decrease (p < 0.05) in pre-eclampsia group compared to normal pregnant group (Table 4).
Table 4.
COHb, leptin and CoQ10 in different studied groups
| Parameters | Normal pregnant | Pre-eclampsia |
|---|---|---|
| COHb (%) | 0.8 ± 0.02 | 0.2 ± 0.02a |
| Leptin (ng/ml) | 24.7 ± 0.96 | 71.8 ± 1.33a |
| CoQ10 (μmol/l) | 1.03 ± 0.02 | 0.44 ± 0.01a |
Number of patients in each group = 60
COHb carboxyhemoglobin, CoQ10 coenzyme Q10
aSignificant difference when comparing pre-eclampsia group with normal pregnant group at p ≤ 0.05
Serum Cu and Zn Levels
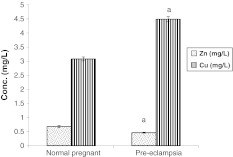
Serum Zn level was significantly decreased in pre-eclampsia group compared to normal pregnant group. However, there was significant increase (p < 0.05) in Cu level in pre-eclampsia group compared to normal pregnant group (Fig. 1).
Fig. 1.
Serum zinc and copper levels (mg/l) in different studied groups. Number of patients in each group = 60. a Significant difference when comparing pre-eclampsia group with normal pregnant group at p ≤ 0.05
Discussion
In the present study, it was found that the level of COHb was significantly decreased in pre-eclampsia group when compared with normal pregnant, indicating the decrease in HO activity. These results were in agreement with Bainbridge et al. [20], who stated that women who have pre-eclampsia have significantly decreased CO concentration when compared with healthy pregnant women. Increasing blood pressure during pre-eclampsia may lead to vasoconstriction and decrease blood flow to the placenta which results in decreased HO activity, so it will lead to a decrease in its catalytic products (CO and bilirubin).
Hemeoxygenase catalyses the oxidation of heme in the presence of NADPH and cytochrome P-450 to produce CO and bilirubin which promote vasodilatation and protection of endothelium and other cells from oxidative injury [21]. CO, which is produced naturally at low levels, may prevent the placental cell death and possess vessel-relaxing, cytoprotective activities that may prevent syncytiotrophoblast cell death and injury to foetus and mother [20].
Decreased CO production inhibits cGMP, increases the resistance of feto-placental and utero-placental circulation and decreases blood perfusion. The syncytiotrophoblast and endothelium are in close contact with the hemoglobin of maternal and fetal blood. When HO reduced in pre-eclampsia., hemoglobin and heme undergoes autoxidation to produce superoxide and hydrogen peroxide resulting in inhibition of prostacyclin synthase enzyme and inactivation of endothelium derived relaxing factors. All this could lead to ischemia, hypoxia and dysfunction of the placenta [21].
In this study, it was found that serum leptin level increased significantly in pre-eclampsia group compared to normal pregnant groups. These results were in agreement with Mumtaz et al. [22]. Over-expression of placental leptin and leptin receptors triggers nor-adrenaline turnover within the brown adipose tissue so that sympathetic activity is increased in the fetal maternal unit, stimulating fetal wastage and sudden intrauterine demise. A study by Mise et al. [23] supported that augmented leptin levels reflect placental hypoperfusion and hypoxia in severe pre-eclampsia. Mumtaz et al. [22], explained that the high leptin level may be increased in women with increased gestational age because not only adipose tissue is the source of leptin, but also in pregnancy foetus, placenta, amniotic fluid, the increase in plasma volume and extra vascular fluid accumulation leads to increase in the maternal weight and also BMI which is responsible for the increase in serum leptin level. Masuyama et al. [24], added that, the elevated leptin levels may represent the adaptation mechanism of the foeto-placental system, which attempts to compensate for the impaired placental perfusion and to provide the metabolic needs of the foetus. Pre-eclampsia induces inflammatory mediators, such as tumor necrosis factor-α and interleukin-6, which may in turn trigger leptin release.
During pre-eclampsia, it was found that a significant decrease in plasma level of CoQ10 compared to normal pregnant women. These data closely resembled the few reports about this antioxidant during human gestation Teran et al. [25] who stated that CoQ10 level decrease during pre-eclampsia.
Increase of CoQ10 during normal pregnancy most likely depends on the increase in LDL, which is the main carrier of CoQ10 in plasma [26] and the increase in cholesterol level which is the biosynthetic pathway for CoQ10 [27].Decrease of CoQ10 during pre-eclampsia is due to direct reaction with free NO in reversible manner resulting in inhibition of mitochondrial complex I activity [26]. Gürkan and Bozdağ–Dündar [28] stated that CoQ10 reduces blood viscosity and improves blood flow thus reducing the systolic and diastolic pressure.
Our study found that serum iron and ferritin were significantly increased in pre-eclampsia while TIBC level was significantly decreased when compared to normal pregnancy. Similar results were obtained by Adam et al. [29], Taheripanah and Farkush [30] and Zafar and Iqbal [31].
Normal pregnant women have a decreased serum iron and ferritin because of feto-placental demand and the required expansion of red cell mass [31]. While in pre-eclampsia increased serum iron, ferritin and decreased TIBC may occur consequent to oxidative stress, enhanced lipid peroxide formation and decreased serum antioxidant buffering against redox-active iron which lead to endothelial dysfunction in pre-eclampsia [32].
Walsh [32] demonstrated that increased serum Fe is due to destruction of red blood cells which promotes lipid peroxidase activity and induces endothelial cell damage. While increased serum ferritin is due to elevated Fe storage in reticulo-endothelial cells.
On the other hand, Adam et al. [29] found that serum Fe measurement has not any valuable role in the prediction of pre-eclampsia. Additionally, Taheripanah et al. [30] demonstrated that there is no relation between serum ferritin level changes and anaemia in pregnancy. While Dreyfuss et al. [33] stated that Fe deficiency and severe anaemia during pregnancy is associated with a woman’s increased risk of death and pre-eclampsia.
In this study, it was found that serum Cu level was significantly increased in pre-eclamptic group when compared with normal pregnant women, while Zn level was significantly reduced in pre-eclamptic group when compared with and normal pregnant women. These results were in agreement with Kumar et al. [34] and Lou et al. [35].
Estrogen also rises during pregnancy and becomes higher as pregnancy continues, which may be associated with a rise in ceruloplasmin, an enzyme to which copper is tightly bound [36].
Nutritional deficiencies of both macro- and micro-nutrients are a common health problem, particularly among the women of reproductive age. The risk is further increased with pregnancy because of increased requirements of various nutrients like Zn and Cu [37]. The changes in levels of these nutrients during pregnancy could affect pregnancy, delivery, and outcome of pregnancy through alterations in maternal and concept’s metabolism Jain et al. [38] and Ziaei et al. [39] contradict our study and found that maternal serum copper level is lower in pre-eclamptic women as compared to healthy pregnant while [40], show that there is no significant differences in the serum levels of Cu between pre-eclampsia group and healthy pregnant individuals.
Zinc acts as an essential element for many metabolic pathways, regulates immune system, and acts as cofactor for activation of many enzyme systems including antioxidant enzymes. Zinc supplementation during pregnancy has been shown to improve the immune system of the developing foetus and reduce the incidence of pregnancy-induced hypertension, preterm delivery, and low birth weight [41]. Zn level was significantly reduced in pre-eclamptic group when compared with healthy control group [38].
Zinc in maternal serum decreased due to hemodilution, increased urinary excretion and increased transfer from the mother to the growing foetus [42]. In addition, low dietary intake and accelerated metabolism might be other contributing factors [40] Zinc is required for the proper functioning of enzymes like superoxide dismutase, which is required for scavenging free radicals. Deficient concentrations of these elements during pregnancy may cause impairment of antioxidant potential of cells by decreasing superoxide dismutase activity, as well as increased lipid peroxidation, leading to increase in blood pressure [38].
On the other hand, the results of Golmohammed et al. [40] showed no significant differences in the serum level of Zn between pre-eclampsia group and healthy pregnant group [40]. Kumru et al. [42] who stated that pre-eclampsia was associated with increased Cu/Zn ratio which arises from a placental insufficiency condition. While Golmohammed et al. [40], study showed that these elements did not play a prominent role in the pathogenesis of pre-eclampsia.
Conclusion
It is clear that the HO system through direct actions as well as actions of the main metabolites, CO and biliverdin/bilirubin, may afford the opportunity for the development of novel antihypertensive therapies.
Hemeoxygenase, leptin and CoQ10 can be consider as parameters for early prediction of pre-eclampsia, and hence the prevention of its complications.
Recommendation The supplementation with CoQ10 during pregnancy may inhibit the development of pre-eclampsia and prevent the increase in blood pressure.
Electronic Supplementary Material
Fig 1. HPLC Chromatogram showing serum coenzyme Q10 standard. Supplementary material 1 (DOCX 71 kb)
Fig. 2. HPLC Chromatogram showing serum coenzyme Q10 in control group. Supplementary material 2 (DOCX 77 kb)
Fig. 3. HPLC Chromatogram showing serum coenzyme Q10 in normal pregnant group. Supplementary material 3 (DOCX 73 kb)
Fig. 4. HPLC Chromatogram showing serum coenzyme Q10 in preeclampsia group. Supplementary material 4 (DOCX 74 kb)
References
- 1.Makris A, Xu B, Yu B, Thornton S, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta. 2006;27:445–451. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res. 2010;36(2):239–247. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 3.Cao JK, Inoue X, Drummond LG, Abraham NG. Physiological significance of heme oxygenase in hypertension. Int J Biochem Cell Biol. 2009;41:1025–1033. doi: 10.1016/j.biocel.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 5.Sucak A, Kanat-Pektas M, Gungor T, Mollamahmutoglu L. Leptin levels and antihypertensive treatment in pre-eclampsia. Singap Med J. 2010;51(1):39–43. [PubMed] [Google Scholar]
- 6.Littarru GP. Biomedical and clinical aspects of coenzyme Q. Clin Investig. 1993;71(8):587–588. doi: 10.1007/BF00184478. [DOI] [PubMed] [Google Scholar]
- 7.Cheng B, Yuan Q, Sun X, Li W. Enhanced production of coenzyme Q10 by overexpressing HMG-CoA reductase and induction with arachidonic acid in Schizosaccharomyces pombe. Appl Biochem Biotechnol. 2010;160:523–531. doi: 10.1007/s12010-008-8386-x. [DOI] [PubMed] [Google Scholar]
- 8.Canatan H, Bakan I, Akbulut M, Halifeoglu I, Cikim G, Baydas G, Kilic N. Relationship among levels of leptin and zinc, copper, and zinc/copper ratio in plasma of patients with essential hypertension and healthy normotensive subjects. Biol Trace Elem Res. 2004;100:117–123. doi: 10.1385/BTER:100:2:117. [DOI] [PubMed] [Google Scholar]
- 9.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 10.Gordon ER. The conjugates of bilirubin. Hepatology. 1975;2:19. [Google Scholar]
- 11.Weir MR, Flack JM, Applegate WB. Tolerability, safety, and quality of life and hypertensive therapy: the case for low-dose diuretics. Am J Med. 1996;30(101(3A)):83S–92S. doi: 10.1016/S0002-9343(96)00271-9. [DOI] [PubMed] [Google Scholar]
- 12.Bartels H. Serum creatinine and creatinine clearance. Clin Chim Acta. 1972;8(23):961–963. [PubMed] [Google Scholar]
- 13.Hampson NB, Scott KL, Zmaeff JL. Carboxyhemoglobin measurement by hospitals implications for the diagnosis of carbon monoxide poisoning. J Emerg Med. 2006;31(1):13–16. doi: 10.1016/j.jemermed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Considine RV, Sinha MK. Serum immunoreactive—leptin concentrations in normal weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 15.Mosca F, Fattorini D, Bompadre S, Littarru GP. Assay of coenzyme Q10 in plasma by a single dilution step. Anal Biochem. 2002;305:49–54. doi: 10.1006/abio.2002.5653. [DOI] [PubMed] [Google Scholar]
- 16.Sinaha SN, Cabrielli ER. Serum copper and zinc levels in various pathological conditions. Am J Clin Pathol. 1970;54:570–577. doi: 10.1093/ajcp/54.4.570. [DOI] [PubMed] [Google Scholar]
- 17.Burtis CA, Ashford ER. Tietz textbook of clinical chemistry. 2. Philadelphia: W.B. Saunders Company; 1994. [Google Scholar]
- 18.Corti MC, Gaziano M, Hennekens CH. Iron status and risk of cardio-vascular disease. J Ann Epidemiol. 1997;7:62–68. doi: 10.1016/S1047-2797(96)00112-3. [DOI] [PubMed] [Google Scholar]
- 19.Saeys Y, Inza I, Larraaga P. A review of future selection techniques in bioinformatics. Bioinformatics. 2007;23:2507–2517. doi: 10.1093/bioinformatics/btm344. [DOI] [PubMed] [Google Scholar]
- 20.Bainbridge SA, Belkacemi L, Dickinson M, Graham CH, Smith GN. Carbon monoxide inhibits hypoxia/reoxygenation-induced apoptosis and secondary necrosis in syncytiotrophoblast. Am J Pathol. 2006;169:774–783. doi: 10.2353/ajpath.2006.060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangol DS, Chen HP. Role of hemeoxygenase-2 in pregnancy-induced hypertension. Int J Gynecol Obstet. 2004;85:44–46. doi: 10.1016/S0020-7292(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 22.Mumtaz F, Memon A, Yousfani S, Tahir S, Khushk I, Memon M, Memon A. Role of serum leptin level as a marker of severity of pre eclampsia. J Ayub Med Coll Abbottabad. 2008;20(1):13–15. [PubMed] [Google Scholar]
- 23.Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, Itoh H, Mori T, Masuzaki H, Hosoda K, Ogawa Y, Nakao K. Augmented placental production of leptin in pre-eclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab. 2009;83(9):3225–3229. doi: 10.1210/jc.83.9.3225. [DOI] [PubMed] [Google Scholar]
- 24.Masuyama H, Nakatsukasa H, Takamoto N, Hiramastsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in pre-eclampsia. J Clin Endocrinol Metab. 2007;92:2672–2679. doi: 10.1210/jc.2006-2349. [DOI] [PubMed] [Google Scholar]
- 25.Teran E, Hernandez I, Nieto B. Coenzyme Q10 supplementation during pregnancy reduces the risk of pre-eclampsia. Int J Gynaecol Obstet. 2009;105(1):43–45. doi: 10.1016/j.ijgo.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Teran E, Racines-Orbe M, Vivero S, Escudero C, Molina G, Calle A. Pre-eclampsia is associated with a decrease in plasma coenzyme Q10 levels. Free Radic Biol Med. 2003;35(11):1453–1456. doi: 10.1016/j.freeradbiomed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Noia G, Littarru GP, Santis M, Oradei A, Mactromarino C, Trivellini C, Caruso A. Coenzyme Q10 in pregnancy. Fetal Diagn Ther. 1996;11:264–270. doi: 10.1159/000264313. [DOI] [PubMed] [Google Scholar]
- 28.Gürkan AS, Bozdağ-Dündar O. Coenzyme Q10. J Fac Pharm Ankara. 2005;34(2):129–154. [Google Scholar]
- 29.Adam B, Malatyalioglu E, Alvur M, Talu C. Magnesium, zinc and iron levels in pre-eclampsia. J Matern Fetal Med. 2001;1(4):246–250. doi: 10.1080/714904340. [DOI] [PubMed] [Google Scholar]
- 30.Taheripanah R, Farkush B. Relation between serum ferritin and iron parameters with preeclampsia. J Fam Plan Reprod Health Care. 2007;2(1):87–91. [Google Scholar]
- 31.Zafar T, Iqbal Z. Iron status in preeclampsia. Prof Med J. 2008;15(1):74–80. [Google Scholar]
- 32.Walsh SC. Lipid peroxidation in pregnancy. Hypertension. 1994;13:1–8. [Google Scholar]
- 33.Dreyfuss ML, Stoltzfus RJ, Shrestha JB, Pradhan EK, LeClerq SC, Khatry SKSRS, Katz J, Albonico M, West KP. Hookworms malaria and vitamin a deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr. 2000;130(10):2527–2536. doi: 10.1093/jn/130.10.2527. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124(3):259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Lou SG, Amirabi A, Yazdian M, Pashapour N. Evaluation of serum calcium, magnesium, copper, and zinc levels in women with pre-eclampsia. Iran J Basic Med Sci. 2008;4(33):231–234. [Google Scholar]
- 36.Atamer Y, Koc¸yigit Y, Yokus B, Aytac A, Atamer A, Ceylan A. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;119:60–66. doi: 10.1016/j.ejogrb.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstorm KD. Hypertensive disorders in pregnancy in Williams obstetrics. 22. New York: McGraw-Hill; 2005. [Google Scholar]
- 38.Jain S, Sharma P, Kulshreshtha S, Mohan G, Singh S. The role of calcium, magnesium and zinc in pre-eclampsia. Biol Trace Elem Res. 2010;133:162–170. doi: 10.1007/s12011-009-8423-9. [DOI] [PubMed] [Google Scholar]
- 39.Ziaei S, Ranjkesh F, Faghihzadeh S. Evaluation of 24-hour urine copper in preeclamptic vs. normotensive pregnant and non-pregnant women. IJFS. 2008;2(1):9–12. [Google Scholar]
- 40.Golmohammed S, Amirabi A, Yazdian M, Pashapour N. Evaluation of serum calcium, magnesium, copper, and zinc levels in women with pre-eclampsia. Iran J Basic Med Sci. 2008;33(4):231–234. [Google Scholar]
- 41.Osendrep SJM, West SE, Black RE. The need for maternal zinc supplementation in developing countries. J Nutr. 2003;2003(133):817–827. doi: 10.1093/jn/133.3.817S. [DOI] [PubMed] [Google Scholar]
- 42.Kumru S, Aydin S, Simsek M, Sahin K, Yaman M, Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in pre-eclamptic and healthy pregnant women. Biol Trace Elem Res. 2003;2003(94):105–112. doi: 10.1385/BTER:94:2:105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig 1. HPLC Chromatogram showing serum coenzyme Q10 standard. Supplementary material 1 (DOCX 71 kb)
Fig. 2. HPLC Chromatogram showing serum coenzyme Q10 in control group. Supplementary material 2 (DOCX 77 kb)
Fig. 3. HPLC Chromatogram showing serum coenzyme Q10 in normal pregnant group. Supplementary material 3 (DOCX 73 kb)
Fig. 4. HPLC Chromatogram showing serum coenzyme Q10 in preeclampsia group. Supplementary material 4 (DOCX 74 kb)