Abstract
This study was undertaken to determine the association of inflammatory biomarker, oxidative stress and antioxidant capacity marker with fetal haemoglobin (HbF) level among sickle cell trait and sickle cell disease (SCD) patients in Chattisgarh. The study group consisted of 51 SCD (SS) patients with painful episode, 49 SCD (SS) patients with steady state, 50 sickle cell trait (AS) and 50 controls. Malondialdehyde (MDA), CRP, total antioxidant power (FARP), total thiol and HbF levels were quantified. We found a significant positive (p < 0.0001) association between CRP and MDA levels and its inverse association with HbF level in SS patients. We also observed that antioxidant capacity had significantly positively (p < 0.0001) associated with HbF level. The protective effect of HbF was found, because the increase in HbF levels resulted in decrease in lipid peroxidation and inflammation in SCD patients. A decrease in the HbF level and its antioxidant capacity has been associated with the pathogenesis of SCD. These finding may explain the high level of HbF is ameliorating oxidative stress and inflammation in SCD patients.
Keywords: C-reactive protein, Oxidative stress, Antioxidant capacity, HbF level, Sickle cell disease
Introduction
Fetal haemoglobin (HbF) is a major ameliorating factor in sickle cell disease (SCD). HbF inhibits the polymerization of sickle haemoglobin (HbS) and its levels are likely to be regulated as a multigenic trait [1, 2]. The occurrence of some complication of sickle cell anemia (SCA) in Chattisgarh like enlarged spleen, infection, painful episode and vaso-occlusive crisis, inflammatory mediator activation, oxidative stress and endothelial dysfunction are reduced by increased concentration of HbF.
SCD has long been recognized as an inflammatory condition and oxidative stress play important role in pathophysiology of SCA [3, 4]. Imbalance between production and elimination of reactive oxygen species (ROS) can damage cell structures, including lipids, membranes, proteins and nucleic acids resulting in cell death or altered cell function. It is now well established that ROS mediate inflammatory process and may be involved in oxidative reactions such as lipid peroxidation and protein oxidation [5–7]. Although results are sometimes contradictory, patients with SCA are shown to have high oxidative stress. Normal RBCs are usually, subjected to oxidative stress as a result of continuous ROS production that accompanies Hb autoxidation [8], a condition that increases two times more in SCA [9], leading to a continuous inflammatory response, oxidative stress and associated with endothelial dysfunction, inflammation and multiple organ damage. To counter the destructive effects of these oxidants, there are endogenous antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase [10], which help to detoxify ROS [11, 12]. These antioxidants are better known as oxygen radical scavengers. There is gross depletion of hydrophilic and hydrophobic antioxidants, and there is depressed antioxidant enzyme activity that is functionally linked to the increased need for antioxidant defence under chronic inflammatory condition.
Some recent studies have shown that the HbF protective effect is primarily mediated by decreased intravascular sickling, resulting in reduced oxidative stress and also in increased nitric oxide bioavailability [13]. Hydroxyurea (HU) treatment may therefore be correlated to lower oxidative status and better antioxidative defense. The true relationship between oxidative status and CRP with HU treatment remains unclear. To the best of our knowledge, no previous study has evaluated the relationship of CRP with oxidative stress and antioxidant markers among sickle cell patients in Chattisgarh.
The present study was to investigate the level of the inflammatory biomarker C-reactive protein, oxidative stress marker and antioxidant marker in sickle cell trait and SCD patients in Chattisgarh and its association with HbF level.
Materials and Methods
Subject
Random 51 sickle cell patients with painful episode (VOC) and 49 sickle cell patients in steady state (without having pain, defined as the absence of a manifest painful crisis, acute cerebrovascular disease, or acute chest syndrome) were taken in the study. The period between the painful crises, during which the patient is symptom free, is considered the steady state. None of the patients was on any kind of treatment (other than folic acid supplementation), nor had any patient received blood transfusions during the 4 months prior to sample collection. Fifty sickle cell trait patients (asymptomatic state from patients) and 50 normal control individuals were included in the study. Mean age of sickle cell patients was 20 years, sickle cell trait was 26 years and normal individuals had mean age 24 years.
Estimation of HbF Level
The 5 ml blood was collected after obtaining proper consent from all individuals. EDTA blood was used for the determination of the HbF % by high-performance liquid chromatography. Serum or plasma were prepared from of whole blood and stored at −80 °C until further analysis.
Estimation of Malondialdehyde
The concentrations of malondialdehyde (MDA) were evaluated from the reaction resulting in the formation of thiobarbituric acid reactive substances according to Satoh method [14]. Samples were precipitated using 20 % trichloroacetic acid and after centrifugation, precipitate was wash with 0.05 M H2SO4. The 3 ml TBA (0.22 %) and H2SO4 were added in the precipitate and incubate for 30 min in boiling water bath and cool under tap water. Then n-butanol (4 ml) was added by vigorous shaking. After centrifugation, upper butanol layer were taken for absorbance at 532 nm using spectrophotometer.
Estimation of C-Reactive Protein, Ferric Reducing Ability of Plasma and Total Thiol
Inflammatory biomarker C-reactive protein was measured by nephelometry [15]. Antioxidant capacity of serum was determined by measuring ability of serum to reduce ferric tripyridyltriazine (Fe3+-TPTZ) complex to ferrous (Fe2+-TPTZ) form. The complex between Fe2+ and TPTZ was gives a blue color and absorbance was taken at 593 nm by using spectrophotometer. The colour formation was linearly related to the amount of reductants [16].
Total thiol level of serum was estimated using the spectrophotometric method [17]. The 200 μl of serum was mixed with 0.6 ml of Tris–EDTA buffer, 3.16 ml of absolute methanol and 10 mm DTNB reagent. After 15 min incubation, sample was centrifuge at 3,000 rpm for 10 min and absorbance was taken at 412 nm.
Statistical Analysis
Results were presented as mean ± SD. Student’s t were used to compare normally distributed variables between groups. Karl Pearson correlation test was used to identify associations between parameter. A p value of ≤0.05 was considered statistically significant.
Results
The mean values of CRP, MDA, FARP, total thiol (T-Sh) and HbF levels are shown in Table 1. Forty-nine sickle cell patients with steady state had significant (p < 0.0001) higher level of HbF in comparison with those SS patients who had painful episode (VOC).
Table 1.
Level of MDA, C-reactive protein, total antioxidant power (FARP) and total thiol and HbF level among SCD, sickle cell trait and control individuals
| Parameter | Controls (AA) | AS patients | SS patients in steady state | SS patients in painful episode (VOC) |
|---|---|---|---|---|
| (n = 50) | (n = 50) | (n = 49) | (n = 51) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| HbF | 0.12 ± 0.13 | 1.06 ± 0.74 | 24.43 ± 4.93 | 14.74 ± 5.18 |
| CRP | 0.03 ± 0.08 | 1.07 ± 0.73 | 2.12 ± 1.06 | 11.62 ± 6.28 |
| MDA | 0.174 ± 0.129 | 1.612 ± 0.957 | 2.826 ± 0.667 | 4.698 ± 1.378 |
| FRAP | 746.94 ± 173.462 | 512 ± 103.177 | 454.877 ± 83.677 | 340.568 ± 66.510 |
| Total thiol (T-Sh) | 368.44 ± 31.461 | 332.5 ± 67.056 | 246.979 ± 25.208 | 171.294 ± 39.762 |
Values are expressed in mean with standard deviation (mean ± SD)
n Number of subjects
Correlation of CRP, MDA, FARP, T-Sh with HbF level
Table 2 indicates the correlations between inflammatory biomarker CRP, oxidative stress marker (MDA), total antioxidant power (ferric reducing ability of plasma) and total thiol (T-Sh) with HbF levels. In the SS with painful episode (CRP r2 = 0.567; MDA r2 = 0.664; FARP r2 = 0.848; T-Sh r2 = 0.641) and SS with steady state (CRP r2 = 0.597; MDA r2 = 0.565; FARP r2 = 0.643; T-Sh r2 = 0.606), the CRP and MDA levels were shown significant negative (p < 0.0001) correlation with HbF level and FARP and total thiol concentration were shown significant (p < 0.0001) positive correlation with HbF level. Correlation of HbF level with CRP, MDA, FARP, total thiol (T-Sh) levels were not significant among sickle cell trait and control individuals and all parameters were in normal range.
Table 2.
Correlation of CRP, MDA, FARP, T-Sh with HbF level among SCD, sickle cell trait and control individuals
| Condition | Correlation (r) CRP and HbF |
Correlation (r) MDA and HbF |
Correlation (r) FARP and HbF |
Correlation (r) Total thiol and HbF |
|---|---|---|---|---|
| Control (AA) | 0.012 | −0.108 | 0.232 | 0.081 |
| Sickle cell trait AS | 0.015 | 0.075 | −0.097 | 0.208 |
| SS patients with steady state | −0.773* | −0.752* | 0.802* | 0.779* |
| SS patients with VOC (painful episode) | −0.753* | −0.815* | 0.921* | 0.801* |
r Pearson correlation coefficient
* p < 0.0001
Association Between Inflammation, Oxidative Stress, Total Thiol and FARP
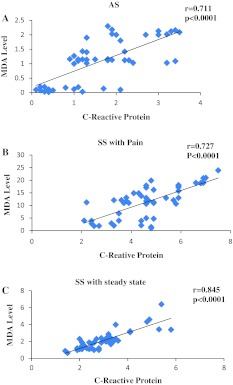
The mean plasma MDA concentration in sickle cell trait and two group of SCD with steady state and painful episode was significantly higher than that of the control group (Table 1). The values of CRP, MDA, antioxidant and total thiol concentration were in normal range in the control group. MDA concentration was significantly (p < 0.0001) positively correlated with inflammatory C-reactive protein among sickle cell traits (AS) (n = 50; r2 = 0.505), SS patients with painful episode (VOC) (n = 51; r2 = 0.528) and SS patients in steady state (n = 49; r2 = 0.714) (Fig. 1a–c).
Fig. 1.
The graphs show the correlations between C-reactive protein and MDA level among SCD in steady state and painful episode and sickle cell trait. a AS, n = 50, b SS in painful episode, n = 51, c SS in steady state, n = 49
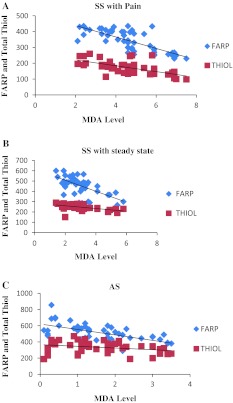
Further evidence of total antioxidant and total thiol concentration in serum were significantly negatively (p < 0.0001) associated with inflammatory marker CRP and MDA levels (Fig. 2a–c) in SS patients with painful episode (VOC) (FARP + CRP r2 = 0.419; FARP + MDA r2 = 0.564), SS with steady state (FARP + CRP r2 = 0.389; FARP + MDA r2 = 0.389) and in AS patients (FARP + CRP r2 = 0.262; FARP + MDA r2 = 0.388) (Table 3). In the sickle cell trait patients, CRP concentration was not significantly correlated with thiol level.
Fig. 2.
The graphs show the correlations of FARP and total thiol with MDA level among SCD, sickle cell trait. a SS with painful episode, b SS in steady state, and c AS
Table 3.
Correlation among inflammatory biomarker, oxidative marker, total antioxidant power (FARP) and total thiol in sickle cell trait, SCD and control individuals
| Condition | Correlation (r) CRP and MDA |
Correlation (r) T-Sh and CRP |
Correlation (r) CRP and FARP |
Correlation (r) FARP and MDA |
Correlation (r) T-Sh and MDA |
Correlation (r) FARP + thiol |
|---|---|---|---|---|---|---|
| Control (AA) | 0.014 | 0.016 | −0.032 | −0.114 | −0.027 | 0.757* |
| AS patients | 0.711* | −0.189 | −0.512* | −0.623* | −0.293 | 0.532* |
| SS steady state | 0.845* | −0.684* | −0.624* | −0.624* | −0.518* | 0.680* |
| SS (painful episode) | 0.727* | −0.603* | −0.648* | −0.751* | −0.638* | 0.716* |
r Pearson correlation coefficient
* p < 0.0001
However, there were no significant association in the levels of either total Antioxidant with CRP and MDA concentrations or between CRP and MDA with total thiol levels in control individuals. As expected, there was a significant positive (p < 0.0001) correlation between total thiol and FARP among AS, SS patients and control individuals (Table 3).
Discussion
Chronic hemolysis and painful episode (vaso-occlusive crisis) in SCD stimulates vascular tissue to counteract the pro-oxidative and pro-inflammatory environment created by free heme or haemoglobin. In the present study data showed that there were increases in oxidative stress and inflammatory markers in SCD patients with painful episode (VOC) and in steady state. Some previous studies among sickle cell patients found similar results with steady state [18–20], and in sickle cell crises [21–23]. No information to the best of our knowledge is available on the level of inflammatory and oxidative stress markers in SCD patients in Chattisgarh. In this study, we found a significant positive correlation between CRP and MDA level and their negative association with HbF levels among AS and SS patients in Chattisgarh. In addition, it was found that higher level of HbF were associated with mild disease presentation.
Elevated levels of CRP, as a general marker of inflammation, have been previously reported in patients with SCD [3, 24, 25]. In this study, we found that CRP concentration was higher in sickle cell patients than in controls individuals, suggesting a covert inflammatory response despite the absence of crisis. Intracellular HbS polymerization is a major factor in the abnormality of sickle erythrocytes. It was proposed that this abnormality may contribute to oxidative damage of the lipid bilayer and membrane integrity in SCD [6].Thus, the elevated CRP in SS patients may be in response to endothelium damage due to the blockage of the vascular endothelium by sickle erythrocytes.
MDA, a product of lipid peroxidation and protein carbonyls, representing oxidation of the circulating proteins, is elevated in SCD. High level of lipid peroxidation in SCA patients were reported [19, 22, 26], but we demonstrated in this study that the high HbF level provide decrease in the lipid peroxidation level in AS and SS patients with painful episode and steady state, confirming its antioxidant property. Moreover, the SS patients presented higher MDA levels when compared to the control group. Accumulation of MDA disturbs the organization of phospholipids in the human erythrocyte membrane bilayer. Membrane damage is considered as an important factor contributing towards pathophysiology due to the formation of irreversible sickle cells [27, 28]. In SCD, oxidative stress is involved in multiple pathophysiologic mechanisms such as accelerated hemolysis, endothelial damage, reduced NO bioavailability [27] and hypercoagulability potentially contributing to SCD related vaso-occlusion and organ damage. With mounting evidence about oxidative stress as a factor of importance in the pathophysiology of SCD, the potential therapeutic effect of new and established anti-oxidative and anti-inflammatory agent in sickle cell patients should be explored.
In addition, we also noted a significant (p < 0.0001) reduced level of total antioxidant power (FARP) and total thiol in SCD patients, compared to the control individuals, supporting the previous studies [29, 30]. Although oxidative stress and alteration in the activities of antioxidant enzyme have been extensively described in SCA, the results are sometimes contradictory. We therefore examined the correlation between lipid peroxidation level with total antioxidant power (FARP) and total thiol (Fig. 2a–c). The statistical correlation analysis between FARP and total thiol levels showed a negative correlation with MDA level (p < 0.0001), in which the increase in MDA levels is associated with the decrease of FARP and total thiol activity that may be due to its consumption by ROS generated by the chronic inflammatory process in SCD.
Administration of HU in SCD reduces the number of painful vaso-occlusive crises and appears to prolong the life span [31]. The effectiveness of HU in the management of SCD is attributed primarily to its ability to increase the synthesis of HbF, which inhibits the polymerization of HbS. HU is oxidized by heme groups to produce NO, which activates soluble guanylyl cyclase to increase the production of cGMP and the consequent transcription of the HbF (gamma) genes [32]. HbF expression also has been observed to reduce oxidant stress in the sickle cell mouse [33]. In this study HbF level was significantly negatively (p ≤ 0.0001) associated with CRP and MDA level in SCD patients. The antioxidant (FARP) and total thiol levels were significantly positively (p ≤ 0.0001) associated with HbF level in SCD patients. Recent studies have revealed that the use of HU promotes higher levels of HbF in SCA patients and is useful in protecting against the sickling of red blood cells, vaso-occlusion and oxidative stress [34, 35]. High HbF expression has ameliorating effects on SCD severity therefore HU treatment may be correlated to lower oxidative status.
In conclusion, we observed that the high HbF level significantly reduce inflammation and lipid peroxidation levels in sickle cell patients. These finding suggest that inflammation and oxidant formed by sickle erythrocytes are less likely to be removed effectively with the endogenous mechanism. ROS and the end-product of their oxidative reactions are potential marker of disease severity and could be target for antioxidant therapies. With these results, we suggest that HU treatment and the antioxidant supplementation is required to reduce the oxidative stress and inflammation levels, improve the clinical course in sickle cell patients in Chattisgarh.
Acknowledgments
The authors are thankful to all the donors of blood samples for the present study.
References
- 1.Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P, Chui DH, Steinberg MH. Fetal haemoglobin in sickle cell anemia. Blood. 2011;118:19–27. doi: 10.1182/blood-2011-03-325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sebastiani P, Wang L, Nolan VG, Melista E, Ma Q, Baldwin CT, Steinberg MH. Fetal hemoglobin in sickle cell anemia: Bayesian modelling of genetic associations. Am J Hematol. 2008;83:189–195. doi: 10.1002/ajh.21048. [DOI] [PubMed] [Google Scholar]
- 3.Schnog JB, Mac Gillavry MR, Zanten AP, Meijers JC, Rojer RA, Duits AJ, Cate H, Brandjes DP. Protein C and S and inflammation in sickle cell disease. Am J Hematol. 2004;76:26–32. doi: 10.1002/ajh.20052. [DOI] [PubMed] [Google Scholar]
- 4.Chaves MAF, Leonart MSS, Nascimento AJD. Oxidative process in erythrocytes of individuals with haemoglobin S. Hematology. 2008;13:187–192. doi: 10.1179/102453308X343356. [DOI] [PubMed] [Google Scholar]
- 5.Katz MA. The expanding role of oxygen free radicals in clinical medicine. West J Med. 1986;144:441–446. [PMC free article] [PubMed] [Google Scholar]
- 6.Asian M, Thomley BD, Freeman BA. Reactive species in sickle cell disease. Ann N Y Acad Sci. 2000;899:375–391. doi: 10.1111/j.1749-6632.2000.tb06201.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RM, Goyette G, Ravindranath Y, Ho YS. Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic Biol Med. 2005;39:1407–1417. doi: 10.1016/j.freeradbiomed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc Natl Acad Sci USA. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng K, Shariff M, Hebbel RP. Comparative oxidation of hemoglobins A and S. Blood. 1998;91:3467–3470. [PubMed] [Google Scholar]
- 10.Dumaswala UJ, Zhuo L, Mahajan S, Nair PNM, Shertzer HG, Dibello P, Jacobsen DW. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280:C867–C873. doi: 10.1152/ajpcell.2001.280.4.C867. [DOI] [PubMed] [Google Scholar]
- 11.Manfredimi V, Lazzaretti LL, Griebeler IH, Santin AP, Brandao VD, Wagner S. Blood antioxidant parameter in sickle cell anaemia patients in steady state. J Natl Med Assoc. 2008;100:897–902. doi: 10.1016/s0027-9684(15)31402-4. [DOI] [PubMed] [Google Scholar]
- 12.Tatum VL, Chow CK. Antioxidant status and susceptibility of sickle erythrocytes to oxidative and osmotic stress. Free Radic Res. 1996;25:133–139. doi: 10.3109/10715769609149918. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta T, Fabry ME, Kaul DK. Antisickling property of fetal haemoglobin enhances nitric oxide bioavailability and ameliorates organ oxidative stress in transgenic-knockout sickle mice. Am J Physiol Regul Integr Comp Physiol. 2010;298:R394–R402. doi: 10.1152/ajpregu.00611.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Campos JL, Arellano E, Calero C, Delgado A, Marquez E, Cejudo P, Ortega F, Rodriguez-Panadero F, Montes-Worboys A. Determination of inflammatory biomarkers in patients with COPD: a comparison of different assays. BMC Med Res Methodol. 2012. doi:10.1186/1471-2288-12-40. [DOI] [PMC free article] [PubMed]
- 16.Benzie FFI, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 17.Hu ML, Dillard CJ. Plasma SH and GSH measurement. Methods Enzymol. 1994;233:385–387. doi: 10.1016/S0076-6879(94)33045-X. [DOI] [PubMed] [Google Scholar]
- 18.Walter BP, Fung BE, Killilea WD, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with β-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akohoue SA, Shankar S, Milne GL, Morrow J, Chen KY, Ajayi WU, Buchowski MS. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr Res. 2007;61:233–238. doi: 10.1203/pdr.0b013e31802d7754. [DOI] [PubMed] [Google Scholar]
- 20.Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol. 1998;61:49–54. doi: 10.1111/j.1600-0609.1998.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 21.Hedo CC, Aken’ova YA, Okpala IE, Durojaiye AO, Salimonu LS. Acute phase reactants and severity of homozygous sickle cell disease. J Intern Med. 1993;233:467–470. doi: 10.1111/j.1365-2796.1993.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 22.Hebbel RP, Eaton IW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Investig. 1982;70:1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titus J, Chari S, Gupta M, Prakash N. Pro-oxidant and anti-oxidant status in patients of sickle cell anaemia. Indian J Clin Biochem. 2004;19:168–172. doi: 10.1007/BF02894279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Investig. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammed FA, Mahdi N, Sater MA, Al-Ola K, Almawi WY. The relation of C-reactive protein to vasoocclusive crisis in children with sickle cell disease. Blood Cells Mol Dis. 2010;45:293–296. doi: 10.1016/j.bcmd.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Das SK, Nair R. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br J Haematol. 1980;44:87–91. doi: 10.1111/j.1365-2141.1980.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 27.Jain SK. The accumulation of malondialdehyde, a product of fatty acid peroxidation can disturb amino-phospholipid organization in the membrane bilayer of human erythrocytes. J Biol Chem. 1984;259:3391–3394. [PubMed] [Google Scholar]
- 28.Capo C, Bongrand P, Benoliel AM, Depieds R. Non-specific recognition in phagocytosis: ingestion of aldehyde treated erythrocytes by rat peritoneal macrophages. Immunology. 1979;36:501–508. [PMC free article] [PubMed] [Google Scholar]
- 29.Hundekar PS, Suryakar AN, Karnik AC, Valvi R, Ghone RA, Bhagat SS. The effect of antioxidant supplementation on the oxidant and antioxidant status in sickle cell anaemia. J Clin Diagn Res. 2011;5:1339–1342. [Google Scholar]
- 30.Fasola F, Adedapo K, Anetor J, Kuti M. Total antioxidants status and some haematological values in sickle cell disease patients in steady state. J Natl Med Assoc. 2007;99:891–894. [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 32.Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol. 1997;34:15–21. [PubMed] [Google Scholar]
- 33.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Investig. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva DG, Belini Junior E, Torres Lde S, Ricci Júnior O, Lobo Cde C, Bonini-Domingos CR, Almeida EA. Relationship between oxidative stress, glutathione S-transferase polymorphisms and hydroxyurea treatment in sickle cell anemia. Blood Cells Mol Dis. 2011;47:23–28. doi: 10.1016/j.bcmd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira Neto PF, Goncalves RP, Elias DB, Araujo CP, Magalhaes HI. Analysis of oxidative status and biochemical parameters in adult patients with sickle cell anemia treated with hydroxyurea, Ceara, Brazil. Rev Bras Hematol Hemoter. 2011;33:207–210. doi: 10.5581/1516-8484.20110055. [DOI] [PMC free article] [PubMed] [Google Scholar]