Abstract
The main objective of the current study is to examine the role of the statistical relation between BCL2 gene (Ala43Thr) single nucleotide polymorphism and growth hormone (GH1) levels in Egyptian HCV genotype-4 patients before and after treatment with pegylated interferon plus ribavirin. Eighty patients with HCV genotype-4 and 40 healthy volunteers as controls were enrolled in the prospective study. Gene polymorphism of BCL2 (Ala43Thr) using PCR-RFLP technique and GH1 concentrations using ELISA procedure were measured for all patients and controls. The present study resulted that Responder HCV genotype-4 Patients, with BCL2 43Ala genotype, have high significant increase in pre-treatment GH1 levels (>1 ng/ml); which represent normal levels, as compared to non-responders pre-treatment GH1 levels (<1 ng/ml); which represent low concentrations. We concluded that HCV genotype-4 patients who have normal GH1 concentrations and BCL-2 43Ala genotype can successfully achieve response to interferon based therapy.
Keywords: BCL2, SNP, GH1, HCV, PEG-IFN-α/RBV
Introduction
Hepatitis C virus (HCV), a major health problem worldwide, is one of the main infectious causes of hepatitis [1]. HCV belongs to the genus Hepacivirus of the family Flaviviridae. HCV is a single stranded, positive sense RNA virus with a genome of approximately 9,500 nucleotide [2]. Egypt has one of the world’s highest prevalence of HCV infection [3], estimated nationally at 14.7 %. An estimated 9.8 % are chronically infected [4]. Combination therapy with pegylated interferon (PEG-IFN-α) and ribavirin (RBV) has markedly improved the clinical outcome, but less than half of the patients with chronic HCV can be expected to respond favorably to currently available agents [5].
B cell CLL/lymphoma-2 (BCL2) gene is one of BCL2 family members which considered as key regulators of apoptosis [6], and display anti-apoptotic activity [7]. Apoptosis or programmed cell death is an important mechanism that plays an important role in limiting viral replication in infected cells. Many viruses produce variety of mechanisms to neutralize IFN secretion and block genes participating in apoptosis. HCV has the features of both induction and inhibition of hepatocyte apoptosis [8].
Previous observations discussed mutations/polymorphisms in the BCL2 gene at SNP (Ala43Thr) and its involvement in chemoresistance of acute myeloid leukemia [9], Egyptian HCV genotype-4 response to IFN/RBV combination therapy [10], and autoimmune diseases resistance [11] including; type-1 diabetes mellitus (T1DM), systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA). It was suggested that all patients treated with IFNα2b + RBV showed a significant reduction in BCL2 accumulation in hepatocytes. Moreover, BCL2 expression was also decreased in non-responders (NR), although to a less extent than in responders, under the influence of IFNα2b + RBV therapy [12].
Previous studies suggested that GH1 in HCV German [13] and Egyptian [14] patients was significantly decreased and increased significantly after antiviral therapy with interferon. Growth hormone (GH1) resistance is present in chronic liver disease (CLD) from the early stages. This result could begin new therapeutic lines of attack in the management of CLD [15]. GH1 secretion has been localized to numerous sites such as cells of the immune system including macrophages, B cells, T cells, and natural killer cells [16, 17]. Mitsunaka et al. [18] hypothesized that BCL2 expression was increased by GH1 treatment in lymphocytes.
Overall, these previous observations promoted us to establish a novel statistical meaning which examine the potential role of the relation between Bcl-2 gene single nucleotide polymorphism (SNP) at (Ala43Thr) and circulating GH1 among Egyptian HCV genotype-4 infected patients treated with pegylated interferon-α and RBV (PEG-IFN-α/RBV) combination therapy.
Materials and Methods
Subjects
One hundred and twenty individuals were included in this study and divided into two groups; 80 HCV infected patients group and 40 healthy controls group. HCV chronically infected patients visiting out patient clinics of Tropical Medicine and Hepatology Department, El-Kasr El-Aini Hospital, Cairo University, Egypt were enrolled in our study after signing consent form. HCV Egyptian patients received combination treatment of PEG-IFN/RBV for 24 weeks. The study protocol and informed consent were approved by the Ethics Committee of Cairo University.
Patients were divided into two groups; 40 sustained virological responders (SVR) when the HCV-RNA remains undetected after 6 months of cessation of treatment and 40 NR who were tested positive with detectable HCV-RNA at week 24.
Patients Criteria
Patients participated in the study fulfilled the inclusion criteria included: age 18–54 years, elevated ALT and AST (>37 IU/L); within 6 months prior to entry the study, positive HCV antibodies, detectable HCV-RNA, HCV genotype-4, liver biopsy showing histological evidence of chronic hepatitis and they were never previously treated with interferon.
On the other hand, patients presented with liver cirrhosis or hepatocellular carcinoma (HCC), hemoglobin (<11 g/dL), total leucocytes count (<3,000/mm3), neutrophil (<1,500/mm3), platelets (<100,000/mm3), prothrombin time out of normal range (9.8–13.8 s), presence of antinuclear antibodies (ANA titre <1/160), liver diseases other than hepatitis C were excluded from this study.
Biochemical and Histological Investigations
Liver profile and alpha fetoprotein (AFP) were performed as standard biochemical laboratories tests. GH1 concentrations were measured in Egyptian hepatitis C genotype-4 infected patients and healthy controls using GH1 ELISA kit supplied by (DRG International, Inc., USA). Human GH1 levels (>1 up to 7 ng/ml) represent normal concentration ranges, but GH1 levels (<1 ng/ml) represent low concentrations. HCV patients were subjected to abdominal ultrasound and liver biopsy was taken from each patient before the onset of therapy to estimate the grade of activity and fibrosis according to Metavir Scoring System [19].
HCV Genotyping and Viral Load Determination
RNA was extracted for HCV genotyping and viral load determination from sera using a Qiagen Viral RNA kit (Hilden, Germany). HCV-RNA genotyping was performed using Ohno method. This method depended on nested PCR amplification of HCV core gene using genotype specific primers [20]. HCV-RNA quantification was performed by Real-time PCR using a LightCycler system (Roche Diagnostics GmbH, Mannheim, Germany). HCV-RNA in serum was graded into low, moderate and high levels.
BCL-2 Genotyping at (Ala43Thr) Polymorphism Point
The polymorphism of BCL2 gene at point (Ala43Thr) was studied in the current study to test whether BCL2 gene polymorphism has an impact on pegylated interferon plus RBV treatment response using PCR-RFLP technique. The DNA was extracted from whole blood of all patients and healthy controls using QIAamp DNA blood kit supplied by (Qiagen Inc., Valencia, USA). Nested PCR for amplifying BCL2 gene using specific four primer sequences (5′–3′) and reaction conditions were mentioned in our previous study [10]. PCR reagents were supplied by (Promega, Inc., UK). The nested PCR product (178 bp amplicon) was incubated at 37 °C over night with BglI restriction enzyme using Restriction Fragment Length Polymorphism (RFLP) technique to identify different three BCL2 genotypes. PCR and digested PCR products were electrophoresed on a 2 and 3 % agarose gel respectively. The gel was visualised on a UV gel documentation computerized system and images were manipulated by BioDocAnalyze software program (Biometra®, Goettingen, Germany). PCR reagents, BglI and DNA ladder were supplied by (Fermentas, Life Sciences).
Statistical Analysis
Data were statistically described in terms of range, mean, standard error (±S.E.), frequencies (number of cases) and relative frequencies (percentages) when appropriate. Comparison of variables between the study groups was done using (ANOVA) test. For comparing categorical data, Chi square (χ2) test was performed. A probability value (P value) less than 0.05 was considered statistically significant. Statistical calculations were done using statistical computer program: SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA, 2001).
Results
Interferon Therapy Response
Table 1 illustrated the clinical, virological and histological features of responder and non-responder patients to combination therapy of pegylated interferon and RBV; where there were a high significant differences of post-treatment ALT, AST and GH when comparing non-responder to responder patients treated with pegylated interferon and RBV combination therapy (P = 0.000). However, there were no significant differences (P < 0.05) of gender, age, pre-treatment ALT, AST, AFP, GH1, HCV-RNA levels and metavir (grade of liver activity and fibrosis score) when comparing responder to non-responder patients.
Table 1.
Clinical, virological and histological features of responder (SVR) and NR HCV patients pre- (A) and post-treatment (B) of PEG-IFN/RBV combination therapy
| Characteristics | SVR (n = 40) | NR (n = 40) | P value |
|---|---|---|---|
| Gender (M/F) | 32/8 | 33/7 | >0.05 |
| Age (years) | |||
| Mean ± S.E. | 39.05 ± 1.32 | 39.77 ± 1.35 | >0.05 |
| Range | (18–53) | (21–54) | |
| Liver enzymes (Mean + S.E.) | |||
| ALT, “A” (IU/L) | 62.27 ± 3.82 | 68.85 ± 6.74 | >0.05 |
| ALT, “B” (IU/L) | 32.85 ± 2.08 | 57.00 ± 4.21 | 0.000* |
| AST, “A” (IU/L) | 55.37 ± 5.09 | 54.35 ± 3.96 | >0.05 |
| AST, “B” (IU/L) | 31.35 ± 2.26 | 54.52 ± 5.53 | 0.000* |
| AFP, “A” (U/L) (Mean ± S.E.) | 5.77 ± 0.36 | 6.02 ± 0.41 | >0.05 |
| AFP, “B” (U/L) (Mean ± S.E.) | 4.64 ± 0.28 | 5.34 ± 0.26 | >0.05 |
| GH, “A” (ng/ml) (Mean ± S.E.) | 0.61 ± 0.26 | 0.85 ± 0.16 | >0.05 |
| GH, “B” (ng/ml) (Mean ± S.E.) | 2.66 ± 0.02 | 0.70 ± 0.16 | 0.000* |
| HCV-RNA level, “A” | |||
| Low | 22 (55 %) | 14 (35 %) | >0.05 |
| Moderate | 16 (40 %) | 21 (52.5 %) | |
| High | 2 (5 %) | 5 (12.5 %) | |
| Metavir, “A” | |||
| Activity score | |||
| A1 | 34 (85 %) | 29 (72.5 %) | >0.05 |
| A2 | 6 (15 %) | 11 (32.5 %) | |
| Fibrosis score | |||
| F1 | 22 (55 %) | 15 (37.5 %) | 0.05 |
| F2 | 12 (30 %) | 13 (32.5 %) | |
| F3 | 6 (15 %) | 12 (30 %) | |
The bold numbers represent significant differences (P > 0.05)
SE standard error
(n)—size of samples; (%)—frequency; (A)—before IFN/RBV therapy or pre-treatment; (B)—after IFN/RBV therapy or post-treatment; GH >1 up to 7 ng/ml—normal ranges; GH <1 ng/ml—low concentrations
* Significant difference (P < 0.05)
BCL-2 Gene Polymorphism at Point (Ala43Thr)
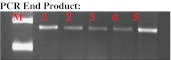
As illustrated in Fig. 1, the end product of the nested PCR of BCL2 gene was identified at amplicon size (178 bp). The BCL2 genotypes were detected using RFLP technique as described previously. BglI restriction enzyme cuts 43Ala PCR products but does not cut 43Thr fragments and the end products were the wild type homozygote, i.e. the 43Ala genotype (157 + 21 bp), the mutant type homozygote, i.e., the 43Thr genotype undigested (178 bp), and the heterozygote, i.e., the Ala43Thr genotype (178 + 157 + 21 bp) as illustrated in Fig. 2.
Fig. 1.
Detection of PCR product size of BCL-2 gene using PCR technique. Lane M represents 100 bp DNA Marker and lanes 1–5 represent BCL-2 gene nested PCR product size (178 bp amplicon)
Fig. 2.
Detection of the BCL-2 polymorphism by PCR-BglI digestion. BglI cuts 43Ala PCR products but does not cut 43Thr fragments. Lane M represents DNA Marker (100 bp), lanes1 and 4 represent the 43Ala genotype (157 + 21 bp), lane 2 represents the 43Thr genotype (178 bp), and lanes 3 and 5 represent the Ala43Thr genotype (178 + 157 + 21 bp)
As shown in Table 2, HCV patients showed a non-significant higher frequency of BCL-2 43Thr genotype and allele when compared to healthy control individuals. Whereas, the frequencies of BCL2 gene SNP at (Ala43Thr) in HCV patients treated with PEG-IFN/RBV are summarized in Table 3. This table showed that non-responder patients have a high frequency (about double) and significant increase of BCL2 43Thr genotype and allele as compared to responder patients. Moreover, responders showed a higher frequency of BCL2 43Ala genotype and allele when compared to NR.
Table 2.
BCL-2 genotypes at (Ala43Thr) in HCV patients as compared to healthy controls
| BCL-2 genotypes and alleles | Healthy controls (n = 40) | HCV patients (n = 80) | χ2 test | P value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | Calc. | Tab. | ||
| BCL-2 genotypes | |||||||
| Ala 43 Ala | 33 | 82.5 | 57 | 71.25 | 0.82 | 3.84 | NS |
| Ala 43 Thr | 7 | 17.5 | 21 | 26.25 | 1.75 | 3.84 | NS |
| Thr 43 Thr | 0 | 0 | 2 | 2.5 | – | – | – |
| 43 Thr | 7 | 17.5 | 23 | 28.75 | 2.74 | 3.84 | NS |
| BCL-2 alleles | |||||||
| 43 Ala | 73 | 91.25 | 135 | 84.4 | 0.27 | 3.84 | NS |
| 43 Thr | 7 | 8.75 | 25 | 15.6 | 1.93 | 3.84 | NS |
χ2 calc. and tab—χ2 calculated and tabulated; (NS)—non significant difference (P > 0.05)
Table 3.
BCL-2 genotypes at (Ala43Thr) in HCV responders and NR
| BCL-2 genotypes and alleles | SVR (n = 40) | NR (n = 40) | χ2 test | P value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | Calc. | Tab. | ||
| BCL-2 genotypes | |||||||
| Ala 43 Ala | 32 | 80 | 25 | 62.5 | 2.15 | 3.84 | NS |
| Ala 43 Thr | 8 | 20.0 | 13 | 32.5 | 2.98 | 3.84 | NS |
| Thr 43 Thr | 0 | 0 | 2 | 5 | – | – | – |
| 43 Thr | 8 | 20.0 | 15 | 37.5* | 5.33 | 3.84 | S |
| BCL-2 alleles | |||||||
| 43 Ala | 72 | 90 | 63 | 78.75 | 0.75 | 3.84 | NS |
| 43 Thr | 8 | 10 | 17 | 21.25* | 4.05 | 3.84 | S |
The bold numbers represent significant differences (P > 0.05)
χ2 calc. and tab—χ2 calculated and tabulated; * Significant difference; NS non significant difference (P > 0.05); S—significant difference (P < 0.05)
BCL-2 Polymorphism, GH Levels and HCV Response
The GH1 levels of eighty patients infected with chronic HCV infection and forty healthy controls showing different BCL2 genotypes are summarized in Table 4. This table showed that HCV genotype-4 patients have a high significant decrease in GH1 levels regardless of their BCL2 genotypes as compared to healthy control individuals (P = 0.000).
Table 4.
Relation between growth hormone and BCL-2 genotypes in HCV patients and control individuals
| Characteristic | BCL-2 genotypes | HCV patients (n = 80) | Healthy controls (n = 40) | P value |
|---|---|---|---|---|
| GH, (ng/ml) | Ala 43 Ala | 0.74 ± 0.03 | 3.42 ± 0.26 | 0.000* |
| Mean ± S.E. | ||||
| Ala 43 Thr | 0.75 ± 0.49 | 2.63 ± 0.21 | 0.000* | |
| Mean ± S.E. | ||||
| Thr 43 Thr | 0.76 ± 0.12 | – | – | |
| Mean ± S.E. | ||||
| 43 Thr | 0.72 ± 0.31 | 2.63 ± 0.21 | 0.000* | |
| Mean ± S.E. |
The bold numbers represent significant differences (P > 0.05)
SE standard error; * Significant difference (P < 0.05); GH >1 up to 7 ng/ml—normal range; GH <1 ng/ml—low concentrations
The present study added that, in the level of BCL2 Ala43Ala genotype, Table 5 showed that responder HCV genotype-4 patients have a high significant increase (P = 0.000) in the pre-treatment GH1 levels (>1 ng/ml), which represent normal concentration ranges, as compared to NR GH1 levels (<1 ng/ml), which represent low concentration ranges. Whereas, there were no significant differences of pre-treatment GH1 levels of responder HCV patients treated with combination therapy as compared to NR in the levels of BCL2 Ala43Thr and 43Thr genotypes. Moreover, non-responder patients have a high significant decrease in post-treatment GH1 levels regardless of their BCL2 genotypes as compared to responders (P = 0.000) as illustrated in Table 5.
Table 5.
Relation between GH1 and BCL-2 genotypes in responders and NR before and after treatment
| Characteristic | BCL-2 genotypes | NR (n = 40) | SVR (n = 40) | P value |
|---|---|---|---|---|
| GH (A), (ng/ml) | Ala 43 Ala | 0.39 ± 0.03 | 1.09 ± 0.02 | 0.000* |
| Mean ± S.E. | ||||
| Ala 43 Thr | 0.88 ± 0.84 | 0.61 ± 0.14 | >0.05 | |
| Mean ± S.E. | ||||
| Thr 43 Thr | 0.76 ± 0.12 | – | – | |
| Mean ± S.E. | ||||
| 43 Thr | 0.82 ± 0.48 | 0.61 ± 0.14 | >0.05 | |
| Mean ± S.E. | ||||
| GH (B), (ng/ml) | Ala 43 Ala | 0.78 ± 0.19 | 2.84 ± 0.15 | 0.000* |
| Mean ± S.E. | ||||
| Ala 43 Thr | 0.63 ± 0.24 | 2.48 ± 0.01 | 0.000* | |
| Mean ± S.E. | ||||
| Thr 43 Thr | 0.61 ± 0.02 | – | – | |
| Mean ± S.E. | ||||
| 43 Thr | 0.62 ± 0.13 | 2.48 ± 0.01 | 0.000* | |
| Mean ± S.E. |
The bold numbers represent significant differences (P > 0.05)
SE standard error; * significant difference (P < 0.05)l; (A)—before IFN/RBV therapy or pre-treatment; (B)—represents after IFN/RBV therapy or post-treatment; GH >1 up to 7 ng/ml—normal range; GH <1 ng/ml—low concentrations
Discussion
In our previous studies, we observed that response to interferon plus RBV combination therapy has an impact on GH1 levels [14] and that there was a significant difference between responders and NR as regards 43Thr genotype [10]. These two findings promoted us to conduct the current study to investigate whether the relation between Bcl-2 gene SNP at (Ala43Thr) and the level of circulating GH1 can affect the Egyptian HCV genotype-4 response to pegylated interferon-α and RBV combination treatment.
The present study main finding is that responder HCV genotype-4 patients have a high significant increase in the pre-treatment GH1 levels (>1 ng/ml), which represent normal ranges, as compared to NR GH1 levels (<1 ng/ml), which represent low concentrations; in the level of BCL2 (Ala43Ala genotype); as compared to NR. Thus, we can hypothesized that HCV genotype-4 infected patients who have BCL2 Ala43Ala genotype and normal GH1 levels may successfully achieve response after combination treatment with PEG-IFN/RBV.
The current study finding may be clarified by the following mechanism of action of the GH1 levels and BCL2 Ala43Thr genotypes in HCV patients treated with pegylated interferon plus RBV combination therapy. This mechanism was illustrated as two pathways in Fig. 3. BCL2 family members are key regulators of apoptosis, which is an important mechanism that plays a critical role in limiting viral replication in infected cells. BCL2 gene which is a member of the BCL2 family has anti-apoptotic activity and BCL2 gene has three genotypes; the Ala43Ala genotype, the Thr43Thr genotype, and the Ala43Thr genotype. The Thr43Thr and Ala43Thr genotypes of BCL2 gene were summed in 43Thr genotype. There are two possible pathways of GH1 and BCL2 genotypes were illustrated in Fig. 3.
Fig. 3.
The mechanism of action of the GH1 levels and BCL2 genotypes in HCV patients
Pathway “A”
Responder HCV patients, with BCL2 Ala43Ala genotype, have high significant increase in pre-treatment GH1 levels (>1 ng/ml); which represent normal levels, as compared to NR pre-treatment GH1 levels (<1 ng/ml); which represent low concentrations. This genotype of BCL2 gene may induce apoptosis which leads to cells homeostasis and induction in immune system. This mechanism sequence leads to HCV elimination and inhibition of viral replication. Pituitary gland activates cells of the immune system including macrophages, B cells, T cells, and natural killer cells to secrete GH1. Then, GH1 stimulates, through the JAK-STAT signaling pathway, the production of insulin-like growth factor 1 (IGF-1). The liver is a major target organ of GH for this process and is the principal site of IGF-1 production. BCL2 expression was increased by GH1. IGF-1 also leads to induction of cells regeneration and immune system and tissue homeostasis. This mechanism is lead to inhibition of HCV replication and after IFN based therapy HCV patient can achieve response. Finally, this mechanism proves that HCV patients who have 43Ala genotype and normal GH levels are more susceptible to achieve response after combination therapy with interferon and RBV.
Pathway “B”
The summed 43Thr genotype was more frequent and statistically significant in non-responder HCV patients as compared to responders. This genotype of BCL2 gene may inhibit the programmed cell death which leads to disturbance in tissue and cells homeostasis and reduction in immune regulation. This result surly lead to viral replication and HCV persistence. Moreover, virus produces variety of mechanisms to neutralize interferon and block genes participated in apoptosis. This mechanism proves that HCV patients who have 43Thr genotype are more susceptible to be NR after combination therapy with interferon and RBV.
Pennisi et al. [21] suggested that GH1 plays a critical role in liver regeneration by which the liver responds to injury. The liver is a major target organ of GH1 and is the principal site of IGF-I production. Moreover, all the previous data showed the critical role of GH1 in body as a fundamental core for human health and any defect in its secretion may be lead to dangerous results.
As we mentioned in the present study introduction that authors discussed polymorphism in the human BCL2 gene focusing on (Ala43Thr) SNP in autoimmune diseases resistance such as; T1DM and SLE [11]. On the contrary, previous study revealed that the reported BCL2 SNP in familial T1DM was not present in the Danish, the Finnish, or the Basque collections at all, i.e. 1,371 individuals were homozygous for the wild-type allele [22]. Scientists added that SNP in the BCL2 gene, using a panel of Mexican Mestizos and Swedish control subjects, did not find the Ala43Thr variation in these populations either [23]. It is likely that the Ala43Thr polymorphism arose spontaneously in Asians/Japanese after the human ancestor population separated into Caucasians and Asians in the early history of Man.
The current study found that NR have higher viral load and higher grade of liver activity and fibrosis than in responders but with no significant differences which agree with previous studies on HCV genotype-4 [24, 25]. On the contrary, several studies have shown that high viral load and high grade of liver activity and fibrosis are negatively related with sustained virological response to interferon treatment [26, 27].
Liver enzymes and AFP were elevated in our Egyptian HCV patients and it is almost same as other patient groups as previously reported [28–32]. The current study was demonstrated that AFP was negatively associated with treatment response in our HCV patients as reported in the previous study which included 100 Egyptian patients with chronic hepatitis C [33]. The current results added that there were high significant differences of post-treatment ALT and AST on response to treatment. These results confirm that early normalization of an abnormal ALT level may indicate response to antiviral treatment which agree with previous studies [34, 35].
In conclusion, we can suggest from this study that the statistical relation between normal GH1 level and BCL2 Ala43Ala genotype were involved in HCV genotype-4 response to interferon based therapy and HCV genotype-4 patients who have normal GH1 concentrations and BCL2 43Ala genotype can successfully achieve response to PEG-IFN/RBV combination therapy.
Contributor Information
Emad F. Eskander, Phone: 02-33335966, FAX: 02-33370931, Email: gysi_pharma@yahoo.com
Ahmed A. Abd-Rabou, Email: ahmedchemia87@yahoo.com
References
- 1.Crawford JM. The liver and the biliary tract- Robbins pathologic basis of disease. 6th ed. Philadelphia: WB Saunders Company; 1999. pp. 845–901. [Google Scholar]
- 2.Choo Q, Richman K, Han J, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr P. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1989;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Hamid M, El-Daly M, Molnegren V, El-Kafrawy S, Abdel-Latif S, Esmat G, Strickland T, Loffredo C, Albert J, Widell A. Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J Gen Virol. 2007;88:1526–1531. doi: 10.1099/vir.0.82626-0. [DOI] [PubMed] [Google Scholar]
- 4.Miller FM, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. PNAS. 2010;107(33):14757–14762. doi: 10.1073/pnas.1008877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65 [DOI] [PubMed]
- 6.Tsujimoto Y, Cossman E, Croce C. Involvement of the bcl-2 gene in human follicular lymphomas. Science (Wash. DC) 1985;228:1440. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 7.Teodoro J, Branton P. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang D, Adams J, Cory S. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 1998;17:1029–1039. doi: 10.1093/emboj/17.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon J, Sohn S, Lee M, Jang J, Kim K, Jung C, Kim D. BCL2 gene polymorphism could predict the treatment outcomes in acute myeloid leukemia patients. Leukemia Res. 2009; LR-3493. [DOI] [PubMed]
- 10.Shaker OG, Eskander EF, Yahya SM, Mohamed MS, Abd-Rabou AA. Genetic variation in BCL-2 and response to interferon in hepatitis C virus type 4 patients. Clin Chim Acta. 2011;412:593–598. doi: 10.1016/j.cca.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Komaki S, Kohno M, Matsuura N, Shimadzu M, Adachi N, Hoshide R, Nishiyama S, Matsuda I. The polymorphic 43Thr bcl-2 protein confers relative resistance to autoimmunity: an analytical evaluation. Hum Genet. 1998;103(4):435–440. doi: 10.1007/s004390050847. [DOI] [PubMed] [Google Scholar]
- 12.Anatol P, Danuta P, Janusz D, Bozena P. Expression of bcl-2 protein in chronic hepatitis C: effect of interferon alpha 2b with ribavirin therapy. World J Gastroent. 2005;11(19):2949–2952. doi: 10.3748/wjg.v11.i19.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plockinger U, Kruger D, Bergk A, Weich V, Wiedenmann B, Berg T. Hepatitis C patients have reduced growth hormone secretion which improves during long term therapy with pegylated interferon alpha. Am J Gastroent. 2007;102(12):2724–2731. doi: 10.1111/j.1572-0241.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- 14.Eskander EF, Abd-Rabou AA, Yahya SM, Shaker OG, Mohamed MS. Does interferon and ribavirin combination therapy ameliorate growth hormone deficiency in HCV genotype-4 infected patients?. Clin Biochem J. 2011 (in press). [DOI] [PubMed]
- 15.Gentilucci UV, Zardi EM, Caccavo D, Petitti T, Manfrini S, Pozzilli P, Afeltra A. TNF-α and growth hormone resistance in patients with chronic liver disease. J Interferon Cytokine Res. 2004;23(5):229–235. doi: 10.1089/107999003321829944. [DOI] [PubMed] [Google Scholar]
- 16.Liu N, Mertani HC, Norstedt G, Tornell J, Lobie PE. Mode of the autocrine/paracrine mechanism of growth hormone action. Exp Cell Res. 1997;237:196–206. doi: 10.1006/excr.1997.3789. [DOI] [PubMed] [Google Scholar]
- 17.Harvey S, Hull KL. Growth hormone. A paracrine growth factor? Endocrine. 1997;7:267–279. doi: 10.1007/BF02801319. [DOI] [PubMed] [Google Scholar]
- 18.Mitsunaka H, Dobashi H, Sato M, Tanaka T, Kitanaka A, Ishida T. Growth hormone prevents Fas induced apoptosis in lymphocytes through modulation of BCL-2 and caspase-3. NeuroImmunoModulation. 2001;9:256–262. doi: 10.1159/000054288. [DOI] [PubMed] [Google Scholar]
- 19.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 20.Ohno O, Mizokami M, Wu RR, et al. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35(1):201–207. doi: 10.1128/jcm.35.1.201-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S. Role of growth hormone (GH) in liver regeneration. J Endocrinol. 2004;145(10):4748–4755. doi: 10.1210/en.2004-0655. [DOI] [PubMed] [Google Scholar]
- 22.Heding P, Karlsen A, Veijola R, Nerup J, Pociot F. No evidence of a functionally significant polymorphism of the BCL2 gene in Danish, Finnish and Basque type 1 diabetes families. Genes Immun. 2001;2:398–400. doi: 10.1038/sj.gene.6363788. [DOI] [PubMed] [Google Scholar]
- 23.Johansson C, Castillejo-Lopez C, Johanneson B. Association analysis with microsatellite and SNP markers does not support the involvement of BCL-2 in systemic lupus erythematosus in Mexican and Swedish patients and their families. Genes Immun. 2000;1:380–385. doi: 10.1038/sj.gene.6363688. [DOI] [PubMed] [Google Scholar]
- 24.Derbala M, Amer A, Bener A, Lopez A, Omar M, El-Ghannam M. Pegylated interferon-alpha 2b-ribavirin combination in Egyptian patients with genotype 4 chronic hepatitis. J Viral Hepat. 2005;12:380–385. doi: 10.1111/j.1365-2893.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- 25.El Makhzangy H, Esmat G, Said M, El Raziky M, Shouman S, Refai S, Rekacewicz C, Raafat R, Vignier N, Abdel Hamid M, Zalata K, Bedossa P, Pol S, Fontanet A, and Mohamed M. Response to pegylated interferon alfa-2a ribavirin in chronic hepatitis C genotype 4. Med. Virol. 2009;1576-1583. [DOI] [PubMed]
- 26.Kamal S, Tawil A, Nakano T, He Q, Rasenack J, Hakam S, Saleh W, Ismail A, Aziz A, Madwar M. Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut. 2005;54:858–866. doi: 10.1136/gut.2004.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akuta N, Suzuki F, Sezaki H, Hosaka T, Someya T, Kobayashi M, Saitoh S, Sato J, Arase Y, Ikeda K, Kumada H. Predective factors of virologic response to interferon-ribavirin combination therpy for patients infected with hepatitis C virus of genotype 1b and high viral load. J Med Virol. 2006;78(1):83–90. doi: 10.1002/jmv.20507. [DOI] [PubMed] [Google Scholar]
- 28.Seeff LB.Natl Hist Hepat C Hepatology 2002361S35–S46. 10.1002/hep.184036070612407575 [DOI] [Google Scholar]
- 29.Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31:9–16. doi: 10.1016/S0168-8278(99)80368-7. [DOI] [PubMed] [Google Scholar]
- 30.Roudot-Thoraval F, Bastie A, Pawlotsky J-M, Dhumeaux D, et al. Epidemiological factors affecting the severity of hepatitis C virus-related disease: a French survey of 6,664 patients. Hepatology. 1997;26:485–490. doi: 10.1002/hep.510260233. [DOI] [PubMed] [Google Scholar]
- 31.Niederau C, Lange S, Heintges T, Erhars A, Buschkamp M, Hueter D, Nawrocki M, et al. Prognosis of chronic hepatitis C: results of a large prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 32.Taketa K. Alfafetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 33.Males S, Raafat Gad R, Esmat G, Abobakr H, Anwar M, et al. Serum alpha foetoprotein level predicts treatment outcome in chronic hepatitis C. Antivir Therapy. 2007;12:797–803. [PubMed] [Google Scholar]
- 34.Davis GL, Lau JY. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology. 1997;26:1225–1275. doi: 10.1002/hep.510260721. [DOI] [PubMed] [Google Scholar]
- 35.Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. N Engl J Med. 1989;321:1501–1506. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]