Summary
The molecular mechanisms that instruct the formation of synaptic layers are only incompletely understood. In this issue, Timofeev et al. describe an instructive role for the guidance molecule Netrin and its receptor Frazzled in mediating layer-specific targeting of one photoreceptor cell type in the Drosophila visual system.
Of glomeruli, columns and layers
The complex and precise connectivity of the brain is central to neural circuit function. In sensory systems, both the structure of the stimulus and the nature of the computations performed by the brain create architectural constraints. As a result, a small number of morphological themes appear repeatedly in different brain regions. Remarkably, across the animal kingdom, many sensory systems utilize one or more of only three basic architectural elements, namely glomeruli, columns and layers. Understanding the molecular mechanisms by which each of these core features assembles during development therefore represents a focus of considerable current research (Luo and Flanagan, 2007). In this issue, Timofeev et al. describe a new molecular mechanism that instructs layer formation in the Drosophila brain.
The visual systems of both vertebrates and arthropods are typically organized into columns and layers. In both systems, arrays of columns are arranged in topographic maps that preserve spatial relationships between points in visual space. Columns are broadly identical in structure, with each representing a single point in visual space. In addition, columns can be divided into a series of layers that contain different combinations of axons and dendrites. Thus, layers likely represent different neural circuit operations. At the cellular level, layers are units of pre- and postsynaptic specificity, and they form during development by the joint recruitment of specific axons and dendrites. Given this anatomical organization, what are the molecular mechanisms that mediate layer-specific targeting of axons and dendrites?
How are layers built?
A classic challenge in developmental neuroscience is reflected by the fact that nervous systems can contain several orders of magnitude more synaptic connections between specific neurons than the number of guidance and adhesion factors encoded in their genomes. How are so many specific synapses programmed using only limited molecular resources? Layer-specific targeting provides a critical part of the answer to this conundrum, as getting the right axons and dendrites to the correct layer represents a key step in ensuring that the proper synaptic connections form. Work in many experimental systems has uncovered several different mechanisms guiding layer specificity. One hypothesis posits that future synaptic partners express a unique set of adhesion molecules that together form an adhesive code that causes only the right combinations of pre- and post-synaptic processes to come together. This idea is supported by studies in the chick, where four separate homophilic adhesion molecules (DSCAM, DSCAM-L, Sidekick-1 and -2) are expressed and required in non-overlapping pairs of synaptic partners that form distinct layers in the retina (Yamagata and Sanes, 2008). Similarly, in Drosophila expression of the adhesion molecule Capricious in both photoreceptor axons and their target neurons directs layer- specific targeting (Shinza-Kameda et al., 2006). In addition, repulsive cues can be part of combinatorial codes. For example, the repellant Semaphorin-6 and its receptor PlexinA4 are expressed in mutually exclusive layers in the mouse retina, and in either mutant, processes of PlexinA4 positive cell types invade Sema6 territory, likely due to loss of repulsion (Matsuoka et al., 2011).
Combinatorial codes provide one means of expanding the functional repertoire of a limited set of molecules, but other mechanisms have also been described. For example, precise temporal control of a ubiquitously expressed adhesion molecule can cause layers to form when subsets of pre- and post-synaptic cells simultaneously express high levels of the same factor (Petrovic and Hummel, 2008). Finally, gradients of expression of secreted guidance molecules can also be used, allowing different neurites to choose distinct layers based on quantitative differences (Xiao et al., 2011). In their present work, Timofeev et al. (2012) uncover yet another mechanism to increase both the functional range and specificity of a well-characterized guidance molecule. They demonstrate that secreted Netrins can be localized to a specific layer in the Drosophila medulla by ligand- capture, and that this local concentration of Netrin is sensed by a specific photoreceptor type that innervates this layer.
Short- range Netrin signaling guides layer- specific targeting
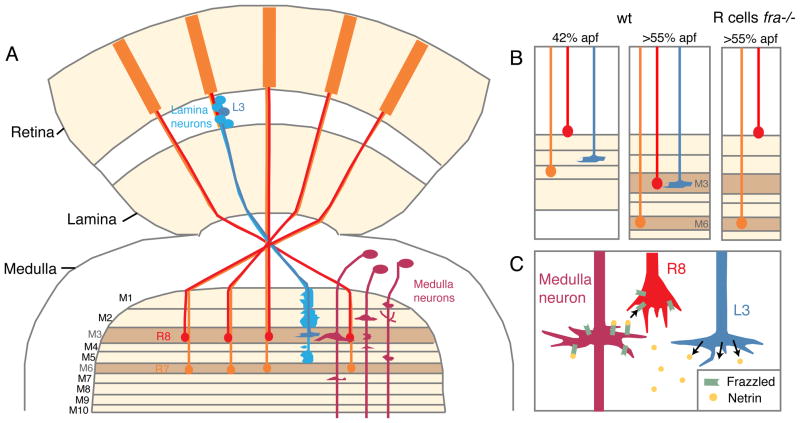
The Drosophila visual system provides a powerful model for dissecting the molecular mechanisms of layer specificity. In this system, photoreceptors, designated R cells, project their axons directly into the brain, with subtypes of R cells targeting to different layers in different neuropils. While photoreceptors R1–R6 project their axons to one brain region, the lamina, a second subset of photoreceptors, designated R7 and R8, extend their axons into a different brain region, the medulla. The medulla neuropil is organized into both columns and layers, comprising roughly 800 columnar elements, each divided into ten distinct layers (designated M1–M10; Figure 1A). Each layer contains a specific combination of processes from projection neurons originating in the lamina, ascending neurons from deeper brain centers, and a wealth of processes from many types of medulla neurons. In aggregate, this structure is arguably the most complex neuropil in the Drosophila brain, and yet incoming R7 and R8 axons manage to invariably terminate in two specific layers, M6 and M3, respectively. Targeting occurs in two sequential steps. First, during larval development R7 and R8 innervate specific, “temporary” layers. Later, during mid-pupal stages, R7 and R8 extend deeper into the medulla, innervating their “recipient” layers, after which they form synapses with their target neurons. Several cell surface molecules, including Flamingo, Golden Goal and N-cadherin, are expressed in R7 and/or R8 and play critical roles in layer-specific targeting of these cells (Senti et al., 2003, Tomasi et al., 2008, Ting et al., 2005, Lee et al., 2001). However, exactly how these molecules catalyze assembly of a layer remains unclear.
Figure 1.
(A) Schemata of the adult Drosophila visual system. (B) Summary of the main findings of Timofeev et al. (2000). Wildtype R7 and R8 axons target to distinct temporary layers at 42%, and to their respective target layers, M3 and M6, after 55% of pupal development. When all R cells are Frazzled mutant, R8 axons will fail to reach their final layer, while targeting of R7 is unaffected. (C) Model of Netrin/Fra signaling; only layer M3 is shown. Axons of Lamina neuron L3 secrete Netrin, which is captured by Fra expressed on unknown medulla neurons. Fra on R8 then binds Netrin, which guides its axon towards M3.
The study by Timofeev et al. (2012) in this issue of Neuron identifies a novel strategy to achieve layer-specific targeting in the fly visual system (Figure 1B, C). They demonstrate that the guidance cue Netrin localizes to the R8 target layer, and that R8 axons detect Netrin by expressing the attractive Netrin receptor Frazzled (Fra). R8 axons that have lost Fra stall at their temporary layer, and fail to extend towards their final target. Conversely, removing Netrin from the R8 target area precisely phenocopies these defects, demonstrating that target-derived Netrin attracts R8 axons by activating Fra. Intriguingly, Netrin protein is highly enriched in the R8 target layer M3, where it is deposited by the axonal processes of a specific lamina neuron, designated L3. But how can Netrin, which is a secreted molecule, be restricted to a single layer? The authors hypothesize that Netrin can be bound to Fra on surfaces of cells in the target area, and is presented as an active complex to incoming R8 axons. To test this idea, the authors deleted Fra only from neurons in the R8 target area and observed the loss of the layer-specific localization of Netrin. Furthermore, expression of membrane-tethered Netrin can completely rescue the Netrin mutant phenotype, demonstrating that Netrin acts locally rather than as a long- range diffusible molecule in this context. In line with these findings, several previous studies demonstrated that Netrin can act as a “membrane-captured” protein. For example, in the fly embryonic nervous system, Fra binds to and redistributes Netrin, which instructs the guidance both of pioneer neurons and commissural axons (Hiramoto et al., 2000, Brankatschk and Dickson, 2006). Furthermore, Unc-40/Fra- captured Netrin mediates dendrite self- avoidance in C. elegans (Smith et al., 2012), suggesting that this mode of Netrin function is widely used for different tasks in various species.
In summary, Timofeev et al., (2012) provide the first evidence that Netrin play an important role in layer specific targeting, serving to trap incoming photoreceptor axons in the correct layer. Moreover, they extend previous work by showing that Netrin acts not only as a graded signal over long distances, but can be locally captured and presented by Fra to function over short distances. Thus, by identifying a new role for these proteins, as well as a new mechanism for their action, these studies significantly extend our understanding of the versatility of these molecules. Future studies should address how the Netrin/Fra system interacts with other molecules that are also required for R8 targeting, such as Flamingo, Golden Goal, and Capricious. At a higher level, a critical question concerns the relationship between layer-specific targeting and synaptic specificity. R8 makes only a subset of its synaptic connections within the M3 layer; thus, layer-specific targeting is clearly only part of the story (Takemura et al., 2008). However, it remains possible that the synapses that do form in M3 are promoted by Net-Fra interactions, and hence it becomes important to know which cell types in the M3 layer capture Netrin. Could these cells be synaptic targets of R8? The answer to this question will address whether layer- specific targeting and synapse- specificity are always two molecularly distinct processes, or whether they can be achieved by the same set of molecules.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- BRANKATSCHK M, DICKSON BJ. Netrins guide Drosophila commissural axons at short range. Nature neuroscience. 2006;9:188–94. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- HIRAMOTO M, HIROMI Y, GINIGER E, HOTTA Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2000;406:886–9. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- LEE CH, HERMAN T, CLANDININ TR, LEE R, ZIPURSKY SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–50. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- LUO L, FLANAGAN JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- MATSUOKA RL, NGUYEN-BA-CHARVET KT, PARRAY A, BADEA TC, CHEDOTAL A, KOLODKIN AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–63. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETROVIC M, HUMMEL T. Temporal identity in axonal target layer recognition. Nature. 2008;456:800–3. doi: 10.1038/nature07407. [DOI] [PubMed] [Google Scholar]
- SENTI KA, USUI T, BOUCKE K, GREBER U, UEMURA T, DICKSON BJ. Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Curr Biol. 2003;13:828–32. doi: 10.1016/s0960-9822(03)00291-4. [DOI] [PubMed] [Google Scholar]
- SHINZA-KAMEDA M, TAKASU E, SAKURAI K, HAYASHI S, NOSE A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron. 2006;49:205–13. doi: 10.1016/j.neuron.2005.11.013. [DOI] [PubMed] [Google Scholar]
- SMITH CJ, WATSON JD, VANHOVEN MK, COLON-RAMOS DA, MILLER DM., 3RD Netrin (UNC-6) mediates dendritic self-avoidance. Nature neuroscience. 2012;15:731–7. doi: 10.1038/nn.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMURA SY, LU Z, MEINERTZHAGEN IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. The Journal of comparative neurology. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIMOFEEV K, JOLY W, HADJIECONOMOU D, SALECKER I. Localized Netrins Act as Positional Cues to Control Layer-specific Targeting of Photoreceptor Axons in Drosophila. Neuron. 2012;XX:XX–XX. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TING CY, YONEKURA S, CHUNG P, HSU SN, ROBERTSON HM, CHIBA A, LEE CH. Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development. 2005;132:953–63. doi: 10.1242/dev.01661. [DOI] [PubMed] [Google Scholar]
- TOMASI T, HAKEDA-SUZUKI S, OHLER S, SCHLEIFFER A, SUZUKI T. The transmembrane protein Golden goal regulates R8 photoreceptor axon-axon and axon-target interactions. Neuron. 2008;57:691–704. doi: 10.1016/j.neuron.2008.01.012. [DOI] [PubMed] [Google Scholar]
- XIAO T, STAUB W, ROBLES E, GOSSE NJ, COLE GJ, BAIER H. Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell. 2011;146:164–76. doi: 10.1016/j.cell.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGATA M, SANES JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–9. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]