Abstract
In recent years, many animal models of memory have focused on one or more of the various components of episodic memory. For example, the odor sequence memory task requires subjects to remember individual items and events (the odors) and the temporal aspects of the experience (the sequence of odor presentation). The well-known spatial context coding function of the hippocampus, as exemplified by place cell firing, may reflect the ‘where’ component of episodic memory. In the present study, we added a contextual component to the odor sequence memory task by training rats to choose the earlier odor in one context and the later odor in another context and we compared the effects of temporary hippocampal lesions on performance of the original single context task and the new dual context task. Temporary lesions significantly impaired the single context task, although performance remained significantly above chance levels. In contrast, performance dropped all the way to chance when temporary lesions were used in the dual context task. These results demonstrate that rats can learn a dual context version of the odor sequence learning task which requires the use of contextual information along with the requirement to remember the ‘what’ and ‘when’ components of the odor sequence. Moreover, the additional requirement of context-dependent expression of the ‘what-when’ memory made the task fully dependent on the hippocampus. Moreover, the addition of the contextual component made the task fully dependent on the hippocampus.
Keywords: hippocampus, episodic memory, what-where-when, context, sequence memory
In recent years, much research has been focused on episodic memory in animals (Babb & Crystal, 2006; Clayton & Dickinson, 1998; Eacott & Norman, 2004; Ergorul & Eichenbaum, 2004; Kart-Teke, De Souza Silva, Huston, & Dere, 2006). By definition, episodic memories include memory for the individuals, objects and events that were part of the episode (what), as well as the place where the events occurred (where) and the time of their occurrence (when). The odor sequence memory task requires subjects to remember individual odors and their position in the temporal sequence of events (Fortin, Agster, & Eichenbaum, 2002; Kesner, Gilbert, & Barua, 2002). Thus, this task has become an important model for studying memory for individual events (what) and the temporal sequence in which they occur (when).
Various authors have suggested that hippocampal encoding of the spatial context, as exemplified by place cell firing, reflects the ‘where’ component of episodic memory (Anderson & Jeffery, 2003; Nadel, Willner, & Kurz, 1985; Smith & Mizumori, 2006a) and this is consistent with the well-known role of the hippocampus in processing contextual information (e.g. Hirsh, 1974). Requiring the rats to perform the odor sequence task in a context dependent manner would incorporate a key component of episodic memory. In the present study, we have modified the odor sequence task by training rats to choose the earlier odor in one context (a white box) and to choose the later odor in another context (a black box) and we compared the effects of temporary inactivation of the dorsal hippocampus in the new dual context task and the original single context task.
Previous studies have used lists containing 5 odors (Fortin et al., 2002; Kesner et al., 2002) or 6 odors (Wolff, Gibb, & Dalrymple-Alford, 2006). In the present study, we used 7-item lists so that a greater variety of probes could be constructed for each lag size, which refers to the number of intervening odors during the sequence presentation. One problem with shorter lists is that most of the possible probes contain one (or both) of the first and last items from the list. These items may be easier to remember (e.g. due to recency and primacy effects) and rats could exhibit moderately good performance by remembering the first and last items, even without maintaining memory for the items of the middle of the list. The use of longer lists mitigates this problem by allowing for the construction of many probes that do not contain the first or last odor from the sequence.
Methods
The subjects were eight adult male Long-Evans rats that were food deprived to approximately 85% of their free feeding weight. All of the rats were first trained to a criterion on the single context task, followed by surgery to implant guide cannula for intrahippocampal infusions. All procedures complied with guidelines established by the Cornell University Animal Care and Use Committee. After recovery, the rats were re-trained to the criterion and then tested with saline and muscimol using a within subjects design. The rats were then trained on the dual context version of the task. After reaching the behavioral criterion in this task, the rats were again tested in each of the contexts with saline and muscimol infusions.
Details of the odorants, apparatus and general training procedures have been published elsewhere (Butterly, Petroccione, & Smith, 2012). Briefly, trials consisted of the presentation of a sequence of odor cues, presented one at a time mixed into cups of digging medium with a buried sucrose pellet reward (45 mg, Bioserve, Frenchtown, NJ) in each cup. This was immediately followed by a memory probe which consisted of the simultaneous presentation of two cups containing odor cues from the sequence, but only the cup containing the earlier odor from the sequence had a buried reward (100 mg sucrose pellet). Digging responses in the later odor were not rewarded. The odors for each trial were randomly selected from a set of 20 pure odorants (for details see Butterly et al., 2012). Probes included odors selected from each of the odor positions within the sequence and each of 3 different lag sizes. There were 4 different probes of lag sizes 1 and 2, and 3 probes of lag size 3 (i.e. odors with 1, 2 or 3 intervening odors in the sequence).
All of the rats were first trained to a behavioral criterion of 80% correct over 30 trials on the single context task. This ensured that all of the rats were performing the task equivalently well (84.03±3.39% correct, mean ± SEM) and only rats that reached the criterion were included in the experiment. Various training methods were used to bring the rats to this level of performance and the duration of training varied considerably (70-270 trials, mean=146.13±25.21). The best results were achieved by gradually shaping the rats to select the earlier odor from sequences of increasing length (3 odors, 4 odors, etc.) until they were able to perform with 7 item sequences. For each sequence length, the rats were trained until they got 5 consecutive correct choices before advancing to the next longer sequence.
After reaching the criterion, the rats underwent stereotaxic surgery to implant bilateral guide cannulae for the infusion of muscimol (0.6μl of a solution containing 1 μg/μl of muscimol) or saline solution into the dorsal hippocampus (one infusion site per hemisphere in dorsal CA1, 3.6 mm posterior and 2.6 mm lateral to Bregma, 2.2 mm ventral to the cortical surface, Fig 1A). All procedures complied with guidelines established by the Cornell University Animal Care and Use Committee. After recovery, the rats were retrained to the criterion and then given test sessions (9 trials per session) with saline or muscimol infusions given 30 min prior to starting the session. Each rat was given two saline control sessions, followed by two muscimol and then two additional saline control sessions in a within subjects design. Performance did not differ across the two muscimol sessions (t(7)=1.49, p=.18) so the percent correct data were combined across the two sessions of each condition and submitted to a repeated measures ANOVA. One rat died after the test sessions for the single context task, leaving 7 subjects for the second dual context experiment.
Figure 1.
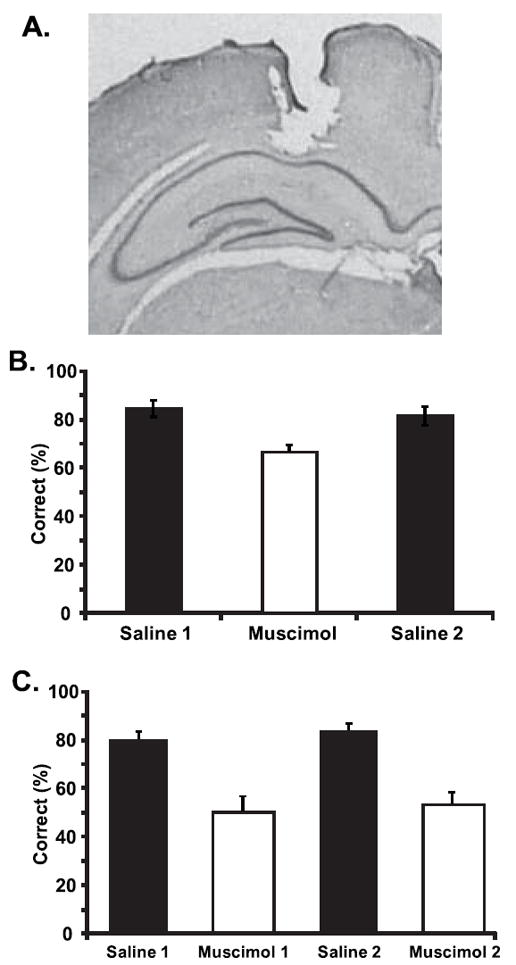
Panel A shows a representative section with the location of the infusion cannula in the dorsal hippocampus. Panel B shows the percentage of trials with a correct choice on the probe in the single context task during saline and muscimol sessions. Panel C shows the percent correct during saline and muscimol sessions of the dual context task. Note that each session (i.e. each bar in the plot) includes trials from each of the two contexts.
After completing the test sessions for the single context task, the rats were trained on the dual context version of the task. All previous training for the single context task took place in a white chamber. For the dual context task, the same white chamber was used and a second black chamber was introduced. The two contexts also differed in terms of the color of the surrounding area (black walls or white curtains), the substrate in the chamber (uncovered Plexiglass floor or a black rubber mat), the 65 dB continuous background masking noise (white noise or pink noise) and the ambient odor left by wiping out the chamber with baby wipes prior to each training session (unscented or scented, Rite Aid, Inc).
For the dual context task, the rats were given training trials as described above, except that they took place in the black box and the rats were required to select the odor that had been presented later on the list during the probe. In order to ensure that performance on the first task remained high, continuing trials in the original (white box) context with the ‘select the earlier odor’ rule were interleaved with training in the new context. After reaching the criterion on the dual context task (80% correct over 30 trials in each context), the rats were given 2 saline and 2 muscimol test sessions in an ABAB design. The four sessions were needed to give an adequate number of test trials in each of the two contexts and for counterbalancing. Each session included trials in each of the two contexts (9 trials of each injection condition and each context for a total of 36 trials).
Results
Muscimol infusions significantly impaired task performance in the single context version of the task (repeated measures ANOVA of the three conditions: saline 1, muscimol and saline 2, F[2,14]=16.89, p<.001, Fig 1B). The temporary lesions impaired performance on all probes, regardless of lag size. A repeated measures ANOVA with lesion condition and lag size as within subjects factors confirmed a main effect of the temporary lesions (F[1,14]=62.26, p<.001), but no effect of lag size (F[2,14]=0.80, p=.45) and no interaction of lag size and lesion condition (F[2,14]=0.91, p=.42). Interestingly, performance during the muscimol session remained significantly above chance (65.97±3.30% compared to chance performance of 50% correct, t(7)=6.00, p<.005).
We compared performance on probe trials that did and did not contain either the first or last odor from the list. For example, the two kinds of probe trials did not differ during the saline sessions (t(7)=0.06, p=.95) or during the muscimol sessions (t(7)=0.21, p=.84) described above. Indeed, the average percent correct for both kinds of probes was nearly identical. The equivalent performance on the two kinds of probes confirms that with our training procedures, the rats did not adopt a strategy of remembering the first or last odors without attending to the odors in the middle of the list. The same pattern of results was seen in the following dual context experiment.
During testing in the dual context task, there were no differences in performance across the two contexts (t(6)=0.46, p=.66) or across the two muscimol sessions (t(6)=0.44, p=.67), so the percent correct data were combined to form saline and muscimol conditions which were compared with a paired samples t-test. The average data for each test session are shown in figure 1C. Muscimol infusions significantly impaired performance on the dual context task (t(6)=6.06, p<.001). In contrast to the single context task, performance on the dual context task dropped all the way to chance levels during the muscimol sessions (50.79±4.27% correct, which did not differ from chance, t(6)=0.19, p=.86), suggesting that the temporary muscimol lesions caused a greater impairment than in the previous single context task. This was confirmed by a significantly greater lesion-induced decrement in performance in the dual context task than in the single context task (comparison of difference scores computed for each subject by subtracting performance during the muscimol sessions from performance during the saline sessions, for the two tasks, t(6)=2.89, p<.05).
Although previous studies with these procedures have shown that rats can’t directly detect the buried reward (Butterly et al., 2012), the rats of the present study were tested after the completion of training by presenting them 20 trials involving two randomly selected odors from the training list, but with only one of the cups baited. If the rats could detect the buried reward, they would be expected to choose the baited cup at a rate that was greater than chance. The rats chose the baited cup 49.0±4.19% of the time, which did not differ significantly from chance performance (t(4)=0.54, p=.62).
Discussion
These results demonstrate that rats can learn a dual context version of the odor sequence task which involved learning a 7-item odor list and learning to follow different rules (pick the earlier or later odor) in separate contexts, within the same testing session. Thus, this task adds a context processing requirement to the well-known odor sequence learning task (Fortin et al., 2002; Kesner et al., 2002). Since episodic memory involves memory for the spatial context in which events occurred, these results are relevant to animal models of episodic memory and they join a growing literature indicating that the component memory processes that contribute to episodic memory are present in a variety of species (Babb & Crystal, 2006; Clayton & Dickinson, 1998; Eacott & Norman, 2004; Ergorul & Eichenbaum, 2004).
Consistent with previous studies that used permanent lesions (Fortin et al., 2002; Kesner et al., 2002), temporary inactivation of the dorsal hippocampus with muscimol caused a significant impairment in the single context version of the task. Interestingly, the rats with temporary lesions performed significantly above chance levels in the single context task, but the lesions completely abolished performance of the dual context version of the task in the same subjects. These results suggest that hippocampal lesions may cause significant deficits in tasks that require some of the components of episodic memory (e.g. what and where in the odor sequence task). However, the additional requirement of context-dependent expression of the ‘what-when’ memory made the task fully dependent on the hippocampus. The increased hippocampal role with the addition of episodic memory components supports the well documented hippocampal role in episodic memory (e.g. Rosenbaum et al., 2005; Vargha-Khadem et al., 1997).
These results are also consistent with accounts of hippocampal function that emphasize its role in processing contextual information (e.g. Smith, 2008). As mentioned, episodic memories involve memory for the spatial context in which events occurred (e.g. at the office, in a restaurant, etc.) even when the details of the spatial geometry and the precise locations of events are lost. Context is therefore a natural way to construe the ‘where’ component of what-where-when models of episodic memory. However, we are cautious about suggesting that the present task constitutes a clear case of “what-where-when” memory. Because the contextual information was present at the time of the probe trials, the rats were not explicitly required to remember the context. Instead, the context may have served as a discriminative cue which was used to retrieve the appropriate rule (i.e. pick the earlier or later odor). Nevertheless, the rats did have to process and encode the context sufficiently for recognition and discrimination. Thus, the task involves the encoding and discrimination, if not the un-cued recall, of the spatial context component of episodic memory in addition to the ‘what’ and ‘when’ components of the sequence. The use of different contexts as a component of episodic memory models is advantageous because associating specific memories with different contexts provides a means for subjects to minimize interference (Butterly et al., 2012) and may therefore be a more manageable way for rodents to associate items and temporal aspects of experience with the location where they occurred.
Interestingly, our pattern of results bears some similarity to previous studies of spontaneous object investigation. These studies have shown that rats with lesions of the hippocampus or fornix exhibit impaired memory for object-place-context associations, but memory for associations involving fewer components (e.g. object-place or object-context) is not impaired (Eacott & Gaffan, 2005; Easton & Eacott, 2010; Langston & Wood, 2010). Easton and Eacott (2010) suggest that the differential involvement of the hippocampus in these tasks may occur because the two component tasks can be solved by non-hippocampal dependent familiarity processes whereas the additional memory requirements of the three component task may require hippocampal dependent recollection processes (for a discussion of familiarity and recollection, see Yonelinas, 1994). Consistent with this idea, it is possible that our dual context task depends on recollection processes to a greater degree than the single context task.
Our dual context task can also be thought of as a special kind of conditional discrimination task, in which the predictive value of discriminative cues depends on the presence of another cue (e.g. in the presence of X: A+/B-, in the presence of Y: B+/A-). The conditional cues (X and Y) can be individual stimuli, locations within an environment or different contexts. Interestingly, the role of the hippocampal system in these tasks has not been entirely clear, with some studies finding a lesion induced impairment (Lee & Solivan, 2010; Rajji, Chapman, Eichenbaum, & Greene, 2006; Smith, Wakeman, Patel, & Gabriel, 2004) and others finding mild impairment or none at all (McDonald et al., 1997; Sanderson, Pearce, Kyd, & Aggleton, 2006). Most of these tasks involve a single pair of discriminative cues (A and B) which have reversed predictive values depending on the conditional cue. In contrast, the present task requires that the rats use the conditional cue (the context) in order to retrieve the correct rule (pick the earlier or later odor) and apply it to the probe odors drawn from a sequence of seven odors. This added complexity, with the requirement to hold the ‘what’ and ‘when’ information in memory, in addition to using the context as a conditional cue, may account for the fact that performance dropped all the way to chance in the dual context task.
The critical role of the hippocampus in memory for individual items and events, when they occurred and the context in which they occurred is supported by neurophysiological data showing that hippocampal neurons respond to each of these components. Hippocampal neurons fire in response to a variety of task relevant events, including responses to various kinds of cues and reinforcers (e.g. Kang & Gabriel, 1998; Smith & Mizumori, 2006b; Solomon, Vander Schaaf, Thompson, & Weisz, 1986; Wood, Dudchenko, & Eichenbaum, 1999). Spatially localized firing patterns (i.e. place fields) are well known and, as discussed above, could serve as a neural representation of the context (Anderson & Jeffery, 2003; Nadel et al., 1985; Smith & Mizumori, 2006a). Finally, recent data suggest that hippocampal firing is also sensitive to temporal aspects of experience, since hippocampal neurons fire in a temporally determined pattern (Gill, Mizumori, & Smith, 2011; Macdonald, Lepage, Eden, & Eichenbaum, 2011; Pastalkova, Itskov, Amarasingham, & Buzsaki, 2008) and hippocampal neuronal population responses evolve over time in a manner that could encode the temporal aspects of memory (Manns, Howard, & Eichenbaum, 2007). The present results suggest that better than chance performance can be maintained in the absence of hippocampal coding of some components (e.g. what and when) but that hippocampal processing is critical when the additional requirement of contextual discrimination is added.
Acknowledgments
This work was supported by NIH grant MH083809 to D. Smith.
References
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. Journal of Neuroscience. 2003;23(26):8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16(13):1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Butterly DA, Petroccione MA, Smith DM. Hippocampal context processing is critical for interference free recall of odor memories in rats. Hippocampus. 2012;22:906–913. doi: 10.1002/hipo.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395(6699):272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan EA. The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q J Exp Psychol B. 2005;58(3-4):202–217. doi: 10.1080/02724990444000203. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24(8):1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Eacott MJ. Recollection of episodic memory within the medial temporal lobe: behavioural dissociations from other types of memory. Behav Brain Res. 2010;215(2):310–317. doi: 10.1016/j.bbr.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11(4):397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PR, Mizumori SJY, Smith DM. Hippocampal episode fields develop with learning. Hippocampus. 2011 doi: 10.1002/hipo.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behavioral Biology. 1974;12(4):421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Kang E, Gabriel M. Hippocampal modulation of cingulo-thalamic neuronal activity and discriminative avoidance learning in rabbits. Hippocampus. 1998;8(5):491–510. doi: 10.1002/(SICI)1098-1063(1998)8:5<491::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, Dere E. Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem. 2006;85(2):173–182. doi: 10.1016/j.nlm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116(2):286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20(10):1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Lee I, Solivan F. Dentate gyrus is necessary for disambiguating similar object-place representations. Learn Mem. 2010;17(5):252–258. doi: 10.1101/lm.1678210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal "time cells" bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56(3):530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, Murphy RA, Guarraci FA, Gortler JR, White NM, Baker AG. Systematic comparison of the effects of hippocampal and fornix-fimbria lesions on acquisition of three configural discriminations. Hippocampus. 1997;7(4):371–388. doi: 10.1002/(SICI)1098-1063(1997)7:4<371::AID-HIPO3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam P, Tomie A, editors. Context and Learning. Hillsdale, NJ: Erlbaum; 1985. pp. 385–406. [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321(5894):1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26(3):908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum RS, Kohler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, Gao F, Tulving E. The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43(7):989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Pearce JM, Kyd RJ, Aggleton JP. The importance of the rat hippocampus for learning the structure of visual arrays. Eur J Neurosci. 2006;24(6):1781–1788. doi: 10.1111/j.1460-9568.2006.05035.x. [DOI] [PubMed] [Google Scholar]
- Smith DM. The hippocampus, context processing and episodic memory. In: Huston JP, editor. Handbook of Behavioral Neuroscience,Vol 18, Handbook of Episodic Memory, Ekrem Dere, Alexander Easton, Lynn Nadel and Joseph P Huston. The Netherlands: Elsevier; 2008. pp. 465–481. [Google Scholar]
- Smith DM, Mizumori SJY. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006a;16(9):716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY. Learning-Related Development of Context-Specific Neuronal Responses to Places and Events: The Hippocampal Role in Context Processing. Journal of Neuroscience. 2006b;26(12):3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix Lesions Impair Context-Related Cingulothalamic Neuronal Patterns and Concurrent Discrimination Learning. Behavioral Neuroscience. 2004;118(6):1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100(5):729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Dalrymple-Alford JC. Beyond spatial memory: the anterior thalamus and memory for the temporal order of a sequence of odor cues. J Neurosci. 2006;26(11):2907–2913. doi: 10.1523/JNEUROSCI.5481-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. [see comments] Nature. 1999;397(6720):613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]


