Abstract
Objective
The objective of this study was to formulate a practical method for the use of cone beam CT (CBCT) for the verification of sequential and integrated tumour bed boosts for early breast cancer patients.
Methods
Partial arc scan geometries were assessed on a treatment unit. Imaging dose measurements on an Elekta Synergy CBCT system were made in a CT dose phantom for scan parameters 100 kV, 25 mA and 40 ms with an S20 collimator. The protocol was used to verify the setup of a cohort of 38 patients, all of whom had surgical clips inserted in the tumour bed. Setup errors with and without an extended no action level (eNAL) protocol were calculated.
Results
Arcs from 260° to 85° (left breast) and 185° to 15° (right breast) were found sufficient to image fiducial markers and anatomy whilst accounting for the physical limits of the equipment. A single treatment and imaging isocentre was found by applying simple constraints: isocentre <8 cm from midline and isocentre–couch distance <30 cm. Contralateral breast doses were ∼2 mGy per scan (right breast) and ∼12 mGy (left breast). Both mean population systematic error and mean population random error were 3 mm prior to correction. The systematic error reduced to 1.5 mm using an eNAL correction protocol, implying that a 5-mm setup margin could be achieved.
Conclusion
An image-guided verification protocol using CBCT for breast cancer boost plans was implemented successfully. Setup errors were reduced with an acceptable imaging dose to the contralateral breast.
There is much interest in more sophisticated radiotherapy techniques for early breast cancer treatments, particularly for patient cohorts that can be identified as at a high risk of recurrence, where a simultaneous integrated boost technique may be used to increase the dose to the tumour bed [1]. One example of a prospective randomised trial testing this approach is the UK Intensity Modulated and Partial Organ Radiotherapy (IMPORT) High trial, which was given approval by the Cambridgeshire 4 Research Ethics committee on 30 April 2008 [2]. This trial is designed to test dose-escalated intensity-modulated radiotherapy after conservation surgery for early breast cancer in women with higher-than-average local recurrence risk, with a primary end point of palpable induration of the ipsilateral breast. The trial required a sequential conformal photon boost for the control arm, and an integrated boost in the two trial arms. The planning target volume (PTV) margin for the tumour bed boost was 5 mm, so effective verification protocols were necessary, using more complex imaging methods than standard tangential beam treatment portals (with a typical whole-breast PTV margin of 10 mm). The use of fiducial markers (either surgical clips or gold seeds) to mark the tumour bed was mandatory in order to facilitate the complex planning and for verification imaging. Participating centres were required to provide a clearly defined imaging strategy, which was sufficient to reduce setup error so that a 5-mm tumour bed PTV margin could be achieved. A range of image-guided methods were permitted: orthogonal planar imaging (both MV and kV), and volumetric imaging via kV cone beam CT (CBCT) and MV systems (for example, on a TomoTherapy unit; TomoTherapy Inc., Madison, WI). This work reports a practical method of image-guided radiation therapy (IGRT) using a kV cone beam CT system for sequential, and integrated, photon breast boosts.
Methods and materials
Patient data set
The data set used was from the UK IMPORT High trial for patients at high risk of local recurrence [2]. Data from the first 38 patients recruited at one centre which used kV CBCT for verification were used. The patients were immobilised on a commercial breast board (MT-350 carbon fibre breast board; Medtec, Alton, IA) with both arms raised and supported at the upper arms and wrists. The arm, wrist and head supports were adjustable to indexed positions, and patient-specific settings were recorded at the time of CT scanning and used throughout treatment. A cushioned rest at the base of the gluteus maximus muscles and knee supports provided additional immobilisation. The board was inclined at a low angle between 5° and 7° to enable access through the bore of a Philips Brilliance Big Bore CT scanner (Philips Medical Systems, Eindhoven, Netherlands). Patients were randomised to a sequential conformal photon boost (control arm) or an integrated boost (both test arms). All boost plans required an appropriate IGRT verification strategy to ensure that a tumour bed PTV margin of 5 mm was achieved.
Cone beam CT protocol and dose measurement
The patient cohort was treated on Elekta Synergy® linear accelerators (Elekta Oncology Systems, Crawley, UK). These have integrated kV CBCT systems with the kV tube positioned at an angle of 90° from the linear accelerator treatment head (i.e. if the linear accelerator head is positioned at 0° on the gantry scale the kV unit is at 90°). Commercial linear accelerator gantries can rotate 360° from −180° to +180° (full clockwise rotation) and vice versa (a full anti-clockwise rotation). They do not rotate continuously through the 180° point. This has the consequence that rotation of the Elekta kV unit in an anti-clockwise direction between −180° and 270° is not possible. Prior to trial commencement, appropriate angles for left and right breast CBCT scans were determined by assessing the clearance of the unit from the breast board and couch for typical patient positions, while accounting for the physical constraints of the linear accelerator. The scan protocol was required to minimise the additional dose to the contralateral breast.
Dose measurements were carried out in a CT dose phantom. The cylindrical phantom was made of poly(methyl methacrylate) and had an external diameter of 320 mm with a length of 140 mm. A central section of the phantom of 160 mm diameter and 140 mm length could be removed. The phantom was offset from the isocentre by 8 cm in the lateral and 8 cm in the vertical direction to mimic a breast setup. Figure 1a is a schematic diagram of the circular face of the phantom. Dose measurements were made in seven positions in the phantom for both left- and right-side imaging protocols; the positions are indicated by the circles and numbers in Figure 1a. Average air kerma was measured within the 100-mm central section of the CBCT scan with a CT pencil chamber [Computer Tomography Dose (CTDI) chamber 20X6-3CT] of 100 mm length from RadCal Corporation (Monrovia, CA) with a RadCal 2026C electrometer in a CT dose phantom. These were calibrated against a secondary diagnostic energy standard traceable to a primary standard. Measurements were made in the positions shown in Figure 1a using a protocol of 100 kV, S20 collimator (=26 cm reconstruction circle), at 25 mA and 40 ms, with no beam shaping filter (F0) and 361 frames. Average air kerma, rather than peak air kerma at the centre of the beam or CT dose index, was measured as we were interested in doses relative to those from the radiotherapy; the average air kerma is therefore more appropriate. Two examples showing how the phantom measurement positions related to patient anatomy are given in Figure 1b,c. Measurements without the central insert were made to simulate the effect of the lung, with the chamber supported in a clamp stand in the region of the central insert. A standard thorax protocol was measured with no phantom offset to provide data for comparison. This protocol used 120 kV, M15 collimator, the F1 (bowtie filter) with 360° rotation, 40 mA, 40 ms and 650 frames.
Figure 1.
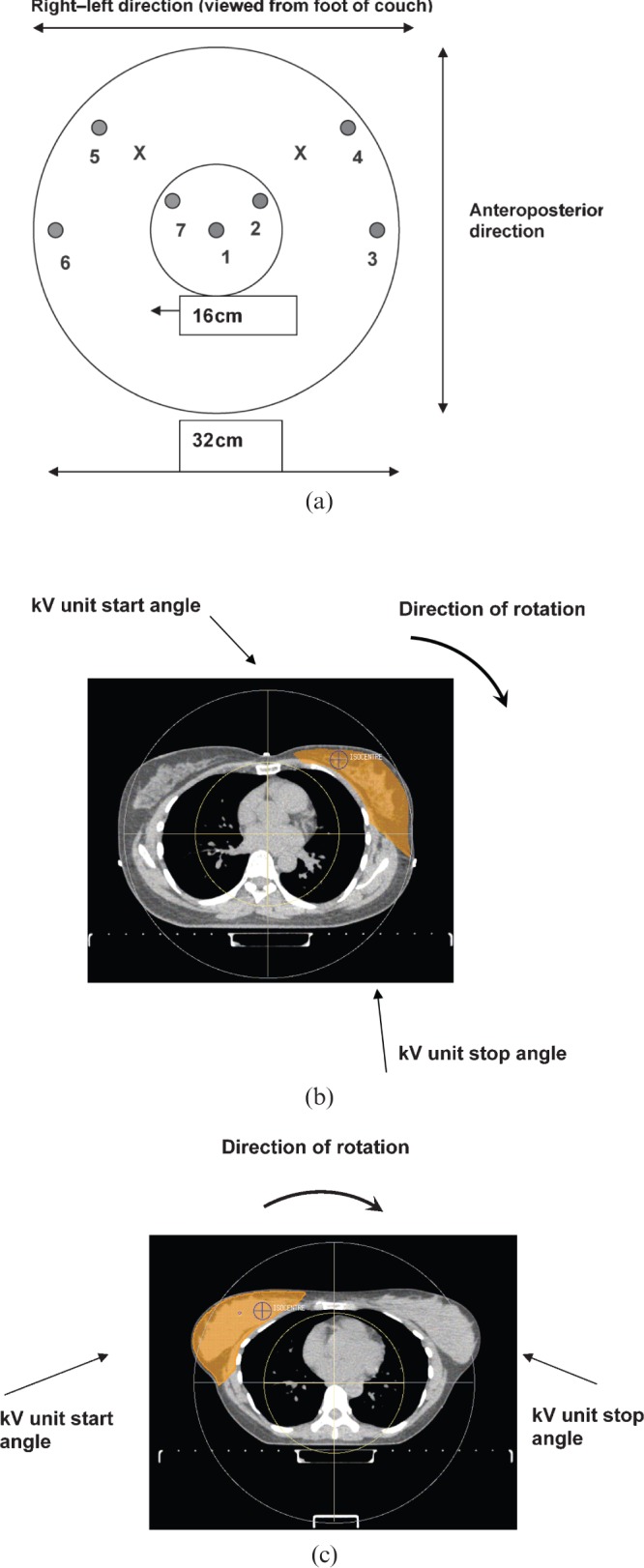
(a) Schematic diagram of the CT dose phantom showing the circular face. The left and right isocentre positions are indicated by crosses. The seven ionisation chamber measurement positions within the phantom are indicated by circles. (b) Schematic diagram of CT dose phantom overlaid on an example CT scan showing a left breast planning target volume (shown in orange). (c) Schematic diagram of CT dose phantom overlaid on an example CT scan showing a right breast planning target volume (shown in orange).
Verification protocol
Daily online imaging, or an offline protocol, were permitted in the trial. Whole-breast radiotherapy in the control arm was given in 15 fractions followed by an 8-fraction photon boost. Only the latter was imaged using CBCT. The test arms were of 15 fractions. The de Boer and Heijmen extended-no-action-level (eNAL) approach [3] was recommended as the off-line protocol, and was implemented by imaging on fractions 1–3, calculating the systematic error and applying the correction prior to fraction 4. One further imaging session occurred on fraction 6 for the sequential boost and two further (fractions 7 and 11) for the integrated boost, after each of which the new systematic error was calculated from all data with previous corrections removed. The new corrections were applied on fractions 8 and 12. de Boer and Heijmen referred to this as eNALavg [3]. The minimum number of imaging sessions planned was four for the sequential boost and five for the simultaneous boost.
Image matching
The Elekta Synergy system enabled matching of CBCT images to a reference three-dimensional (3D) data set from the planning CT scan in right–left (X), superior–inferior (Y) and anteroposterior (Z) directions, and the cardinal axes of rotation. After matching, the rotation errors were resolved in the software into X, Y and Z displacement values to produce the final correction values. An initial automated greyscale match was performed to assess the coincidence of the anatomy with the reference scan, which was followed by manual matching of the titanium clips. Any issues arising from the image matching were recorded and categorised.
Setup error analysis
The X, Y and Z displacement data were collected from all imaging sessions and used to calculate the population systematic and random setup errors with and without the eNAL correction. These were defined as: Σ, standard deviation of the individual patient systematic errors; σ, mean random error. An estimate of the margin resulting from the setup displacements was given by M=2Σ+0.7σ [4]. Data were analysed using the statistical package SPSS® v. 19 (IBM Corporation, New York, NY).
Results
Imaging protocol
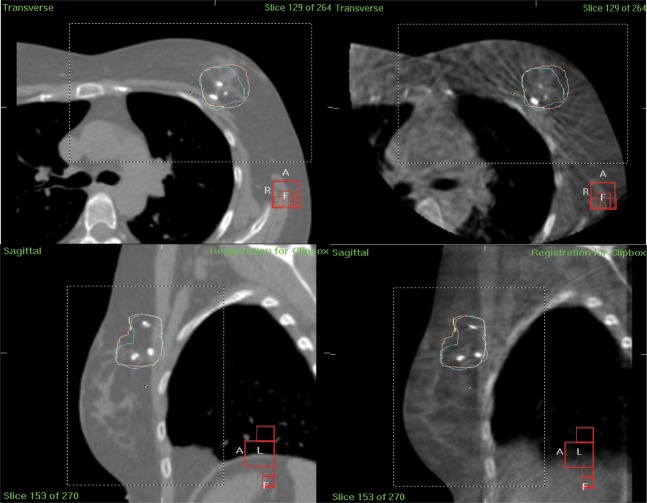
A left breast scanning protocol with a gantry start angle of 260° (kV start angle 350°) and gantry stop angle of 85° (kV stop angle 175°) and a right breast scanning protocol with an acquisition start angle of 185° (kV start angle 275°) and a stop angle of 15° (kV stop angle of 105°), were found to be feasible given the gantry rotation limitations. The effect of the rotation restriction can be seen in Figure 1b,c where the left limits the contralateral breast exposure while the right breast protocol exposes both breasts. An example of CBCT images is given in Figure 2. An isocentre displacement of no more than 8 cm from the midline, and an isocentre-to-couch distance of <30 cm, enabled the system to rotate without collision, but showed sufficient tissue in the image for matching.
Figure 2.
An example of reference and verification images from a patient with left breast disease. Transverse view from reference CT scan (top left); transverse view from verification cone beam CT (CBCT) scan (top right); sagittal view from reference CT scan (bottom left); sagittal view from verification CBCT scan (bottom right). Images show tumour bed planning target volume (blue contour) and 95% isodose structure (pink structure).
Dose
The measured imaging dose data are given in Table 1. Doses changed by no more than 3 mGy when the central phantom insert was removed. As expected, the measured doses from the standard thorax protocol were higher than those from the breast-specific protocol. The effect of the limitation of the gantry rotation is seen in the right breast protocol, where contralateral breast dose is of the order of 12 mGy rather than 1 mGy for the left breast protocol.
Table 1. Imaging doses measured in CT dose phantom.
| Left breast imaging protocol—complete phantom | |||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Dose (mGy) | 2.9 | 6.8 | 19.1 | 17.3 | 1.0 | 0.2 | 2.2 |
| Left breast imaging protocol—central insert removed | |||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Dose (mGy) | 4.0 | 5.1 | 17.1 | 15.4 | 1.2 | 0.6 | 2.8 |
| Right breast imaging protocol—complete phantom | |||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Dose (mGy) | 3.1 | 6.4 | 6.9 | 12.3 | 15.8 | 8.8 | 6.4 |
| Right breast imaging protocol—central insert removed | |||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Dose (mGy) | 4.2 | 4.1 | 6.9 | 10.5 | 14.2 | 8.3 | 5.0 |
| Thorax imaging protocol | |||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Dose (mGy) | 11.0 | 13.9 | 18.7 | 19.8 | 19.8 | 21.8 | 14.4 |
Bold indicates treatment dose area; bold and italics indicate contralateral breast region.
Setup errors and estimated margins
The setup displacement data were tested and were found to be normally distributed (Q–Q plots and Shapiro–Wilk test). There were 14 patients in the control arm group (9 in Test Arm 1 and 15 in Test Arm 2). An analysis of variation test was used to compare the mean setup errors between the groups; the null hypothesis of no difference was retained, hence all data have been analysed together. A correction was made to the calculated Σ (standard deviation of the individual patient systematic errors) to reflect the small number of imaging sessions per patient [5]. The median number of imaging sessions for the sequential boost was 5 (range 2–6); the median for the simultaneous boost was 7 (range 3–10).
Table 2 summarises the setup displacements and the estimated margins. Mean population systematic error and mean population random error were 3 mm prior to correction. The mean population systematic error reduced to 1.5 mm using an eNALavg correction protocol; the population random error increased slightly. The estimated margins from these setup errors were 8 and 5 mm, respectively.
Table 2. Setup errors.
| Parameter |
X (left–right) |
Y (superior–inferior) |
Z (anteroposterior) |
All data |
||||
| No correction (cm) | Corrected (cm) | No correction (cm) | Corrected (cm) | No correction (cm) | Corrected (cm) | No correction (cm) | Corrected (cm) | |
| Σ | 0.27 | 0.15 | 0.22 | 0.12 | 0.27 | 0.12 | 0.29 | 0.14 |
| σ | 0.25 | 0.3 | 0.3 | 0.32 | 0.24 | 0.29 | 0.28 | 0.31 |
| aMargin | 0.8 | 0.5 | ||||||
Σ, standard deviation of the individual patient systematic errors; σ, mean random error.
aPlanning target volume margin for setup error. Estimate only based on the highlighted values in the table. Does not include residual errors, or those due to deformation.
Image matching
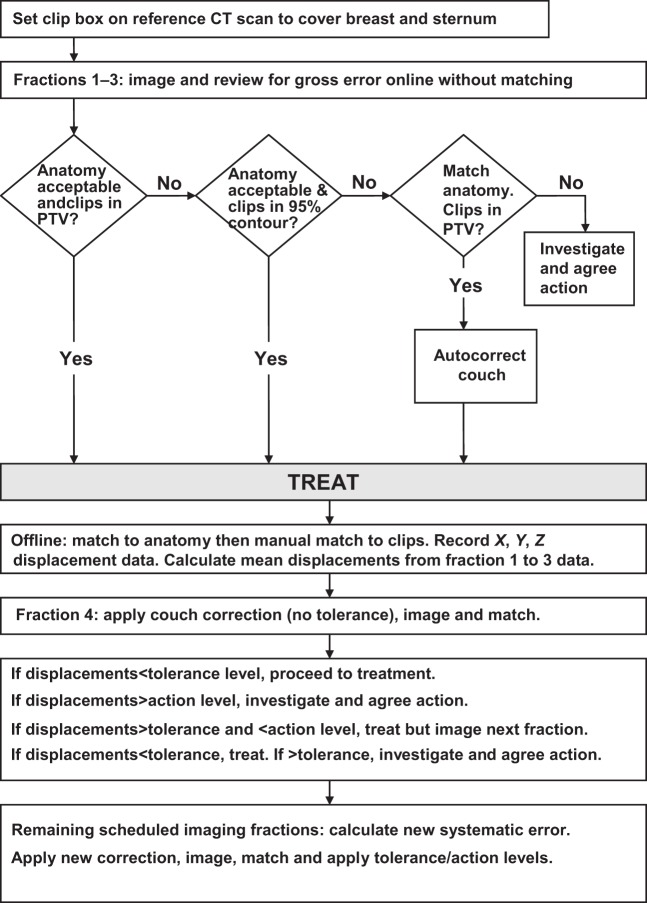
The reported image-matching issues are detailed in Table 3. The clips and anatomical features were visualised on all images; the main difficulties related to decisions on the most appropriate clips to use for matching, possible clip migration and concern that the breast mound had changed in shape and/or size from its reference shape on the planning CT scan (for example, due to swelling of the breast, or the amount of fluid in a seroma). Clip migration was suspected if a clip moved with a different displacement, or direction, from the majority of clips over a series of scans. Contour structures of the tumour bed PTV, and the 95% isodose of the boost plan, were imported from the treatment planning system to assist with image matching. An image-matching flow chart was devised and is summarised in Figure 3. Possible interventions when clips were still positioned outside the PTV and/or the anatomy match which remained unacceptable were: exporting the CBCT scan to the planning system to assess the dosimetric impact; a new CT planning scan and plan; repositioning the patient; and repeating the CBCT scan.
Table 3. Verification issues.
| Issue | Number of patients affected | Comment |
| None reported | 21 | |
| Clip matching | 7 | Spatial distribution of clips changed; uncertainty as to best match |
| Cone beam CT image imported to planning system | 4 | Breast swelling seen |
| Concern over possible clip migration | ||
| Breast swelling | 2 | Cone beam CT image imported to planning system to assess if new plan required |
| Seroma made matching difficult | 1 | Replan after seroma drained |
| Patient arm rotated | 1 | Resolved by fraction 2 |
| Patient body rotated | 1 | Resolved by fraction 3 |
| Patient very anxious | 1 |
Figure 3.
A flowchart of the image matching workflow and the use of the eNALavg correction protocol of de Boer and Heijmen [3].
Discussion
A practical CBCT method for verification of a photon breast boost has been shown. The method has been demonstrated on a patient cohort of 38. The overall systematic and random errors without correction are 3 mm, which is similar to other reports such as Leonard et al [6] and Penninkhof et al [7], both of whom analyse data based on the use of fiducial markers in the tumour bed. Leonard et al give mean ± standard deviation values of 2.7±1.8 mm for superior–inferior, 2.5±2.6 mm for right–left and 3.7±5.9 mm for anteroposterior setup errors. Table 2 shows our uncorrected data were similar to these. In our cohort the measured population mean systematic error reduced to 1.5 mm with the use of an eNAL approach. Penninkhof et al show a reduction to 1 mm in each direction with the 3D value reducing to 1.5 mm when an eNAL method is applied to their data [7].
We have not applied the eNAL method strictly as described by de Boer and Heijmen [3] but used the eNALavg method as this is simpler to implement in practice. In the modelling study of de Boer and Heijmen [3] the eNALavg method still resulted in the magnitude of the population systematic error halving compared with no correction, even in the presence of time trends and large changes in the data. The application of the eNAL protocol (in either form) means that the magnitude of the correction for systematic error changes with time (three potential corrections in our application). This has the effect of broadening the error distribution, and this is seen in the small increase in the random error in Table 2 between uncorrected and corrected data. While the margin recipe of Stroom et al [4] was derived for displacement distributions with no setup correction, we have used it to calculate a margin value with the correction protocol, to estimate the magnitude of the change. Our values of population systematic and random errors when using the eNALavg protocol gave a tumour bed PTV margin estimate of 5 mm (the trial requirement).
The gantry start and stop angles for both right and left breasts have been satisfactory for all patients in the cohort. There have been no collision issues, although clearance is checked prior to each treatment. The angles avoid undue exposure of the contralateral breast, and measurements of dose indicate the contralateral breast receives a dose of 1–2 mGy from a left breast protocol and ∼12 mGy from a right breast protocol. These compare with a standard thorax protocol that may expose these organs to around 20 mGy. Although the mechanical restrictions mean that the exposures from the right breast protocol are six times higher than the left, the dose is less than in the thorax protocol, and this demonstrates the importance of a breast-specific protocol. Roberts et al [8] present a modification to an Elekta linear accelerator to use a low Z target to image through the treatment beam portal. Using an imaging system via the treatment portal would enable better optimisation of the imaging arcs, and hence a potential reduction of the contralateral breast dose compared with that received from the right breast imaging protocol presented here.
The International Commission on Radiological Protection recommend that for radiotherapy, doses are assessed per organ rather than as a global value [9]. If the measured values were assumed mean doses to the organ, then the contribution of the imaging to the contralateral breast dose is ∼0.014 Gy (right breast) or ∼0.08 Gy (left breast) for seven imaging sessions of a 15-fraction integrated boost (i.e. the test arms of IMPORT High, or ∼0.01 Gy for the right breast and ∼0.06 Gy for the left breast for five imaging sessions of an 8-fraction sequential boost control arm treatment). These may be compared with 0.4–1.5 Gy from the radiotherapy exit and scattered dose to contralateral breast [10,11]. Harrison [12] suggested a practical limit of 0.5 for the ratio of imaging dose to total (imaging, scattered and leakage) dose in a non-target organ; our measured values are less than that limit and would remain so even if an online protocol had been used. The additional concomitant imaging dose to the treated breast is <1% of the radiotherapy dose and hence at the level of the daily output tolerance of the linear accelerator.
A similar approach using a reduced-arc, offset isocentre imaging protocol for a Varian CBCT system (OBI v. 1.4; Varian Medical Systems, Palo Alto, CA) was reported by Ueltzhoffer et al [13], who investigated a number of protocols in a breast and thorax phantom. For an arc with kV unit angles of 180° to 16° with isocentre on the right of a phantom they reported dose as 7.2 mGy to the ipsilateral breast and 0.2 mGy to the contralateral breast. The scan parameters used were 100 kVp, 20 mA, 20 ms, 353 projections, 141 mAs, half bow-tie filter, half-fan. While pointing out the difficulty of minimising contralateral breast exposure for a scan with the isocentre on the left, they do not give any dose data, although they comment that the doses will be higher for a similar scan arc (as shown in this work). While it is difficult to compare dose values without matching all imaging parameters in the scans, the measurement methods and the image quality, our values show a similar pattern of dose, with that in the contralateral breast region lower that of the ispilateral breast region (in a CT dose phantom). It would be possible to reduce the doses from the CBCT scan protocol we describe by reducing mA and/or ms; however, assessing the impact on image quality is not trivial. The images in Figure 2 show the reduction of image quality from the CT planning scan to the verification CBCT with our current protocol. However, the purpose of this work is to present a practical method for using CBCT with an Elekta system for this application rather than a detailed analysis of the consequences of changing the imaging settings.
Specifying a limit on the position of the plan isocentre enabled the same centre for both planning and imaging. This limit (<8 cm from midline and <30 cm isocentre–couch distance) did not compromise the treatment plans, and was efficient and safe at the time of treatment, as it avoided the need for an imaging-specific centre with the associated risk of errors in moving the couch more than a few millimetres between imaging and treatment. The adequacy of the isocentre position was checked at the time of planning by creating a structure with diameter 26 cm (the CBCT reconstruction size) and using this to ensure an appropriate amount of anatomy would be encompassed by the CBCT scan.
The most frequent image-matching issue was related to decisions about the most appropriate way of matching the clips. The movement of the breast mound, and possible deformation of the excision cavity, meant that it was unlikely all six pairs of clips would be matched at each session, or for each patient. The use of the 95% isodose, in addition to the tumour bed PTV, as structures on the reference images enabled decisions about matching to be made more efficiently. Where concerns were raised about possible clip migration, seroma changes or breast swelling the CBCT images were imported into the treatment planning system for image registration and dosimetric assessment. Only one replan was required in this cohort, and that was for a patient with a seroma, which was drained. Few seromas were seen in this patient cohort because of the type of surgical technique used for the breast conservation surgery. In other cohorts it may be necessary to replan more frequently, depending on the evolution of the seroma with time.
Conclusion
A clinically feasible, image-guided verification protocol using cone beam CT for sequential, or integrated, tumour bed boost plans for breast radiotherapy has been demonstrated. It enables a PTV margin of the order of 5 mm to be achieved while restricting the imaging dose per scan to the contralateral breast to 2 mGy (left) and 12 mGy (right).
Conflict of interest
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgments
We would like to acknowledge Dr Helen McNair, Professor John Yarnold and Dr Charlotte Coles, for useful discussions and review of the manuscript.
We are very grateful to all of the radiographers in the Radiotherapy Department for their assistance with this project, and for all their hard work for, and dedication to, our patients.
Footnotes
This report is independent research arising from a Healthcare Scientist Post Doctoral award supported by the National Institute for Health Research (NIHR) and the Chief Scientific Officer. EH is supported by the Efficacy and Mechanism Evaluation programme, which is funded by the Medical Research Council (MRC) and managed by the NIHR. We also acknowledge NHS funding of the NIHR Biomedical Research Centre at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust.
References
- 1.Hurkmans C. Radiation therapy using a simultaneously integrated boost for early-stage breast cancer. Future Oncol 2007;3:509–13 [DOI] [PubMed] [Google Scholar]
- 2.Coles C, Yarnold J. The IMPORT trials are launched (September 2006). Clin Oncol (R Coll Radiol) 2006;18:587–90 [DOI] [PubMed] [Google Scholar]
- 3.de Boer HCJ, Heijmen BJM. eNAL: an extension of the NAL setup correction protocol for effective use of weekly follow-up measurements. Int J Rad Oncol Biol Phys 2007;67:1586–95 [DOI] [PubMed] [Google Scholar]
- 4.Stroom JC, de Boer HCJ, Huizenga H, Visser AG. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol Biol Phys 1999;43:905–19 [DOI] [PubMed] [Google Scholar]
- 5.de Boer HCJ, Heijmen BJM. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys 2001;50:1350–65 [DOI] [PubMed] [Google Scholar]
- 6.Leonard C, Harlow CL, Coffin C, Dross J, Norton L, Kinzie J. Use of ultrasound to guide radiation boost planning following lumpectomy for carcinoma of the breast. Int J Radiat Oncol Biol Phys 1993;27:1193–7 [DOI] [PubMed] [Google Scholar]
- 7.Penninkhof J, Quint S, Boer H, Mens JW, Heijmen B, Dirkx M. Surgical clips for position verification and correction of non-rigid breast tissue. Radiother Oncol 2009;90:110–15 [DOI] [PubMed] [Google Scholar]
- 8.Roberts D, Hansen VH, Niven AC, Thompson MG, Seco J, Evans PM. A low Z linear accelerator and flat panel imager: comparison with the conventional imaging approach. Phys Med Biol 2008;53:6305–19 [DOI] [PubMed] [Google Scholar]
- 9.ICRP The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1–332 [DOI] [PubMed] [Google Scholar]
- 10.Donovan EM, Cuirlionis L, Fairfoul J, James H, Mayles H, Manktelow S, et al. Planning with intensity modulated radiotherapy and tomotherapy to modulate dose across the breast to reflect recurrence risk (IMPORT High Trial). Int J Radiat Oncol Biol Phys 2011;79:1064–72 [DOI] [PubMed] [Google Scholar]
- 11.Harrison RM, Wilkinson M, Rawlings DJ, Moore M. Doses to critical organs following radiotherapy and concomitant imaging of the larynx and breast. Br J Radiol 2007;80:989–95 [DOI] [PubMed] [Google Scholar]
- 12.Harrison RM. Doses to organs and tissues from concomitant imaging in radiotherapy: a suggested framework for clinical justification. Br J Radiol 2008;81:970–4 [DOI] [PubMed] [Google Scholar]
- 13.Ueltzhoffer S, Zygmanski P, Hesser J, Hogele W, Wonf J, Bellon JR, et al. Clinical application of Varian OBI CBCT system and dose reduction techniques in breast cancer patients setup. Med Phys 2010;37:2985–98 [DOI] [PubMed] [Google Scholar]