Abstract
Objective
To identify which frontal plane lower limb sensorimotor functions predict gait speed and efficiency (step-width-to-step-length ratio) on an uneven surface.
Design
Cross sectional, observational study.
Setting
Biomechanics research laboratory.
Participants
Thirty-three subjects (14; 42.4% female and 21; 63.6% with diabetic distal symmetric peripheral neuropathy), with a spectrum of lower limb sensorimotor function ranging from normal to marked diabetic neuropathy.
Methods
Independent variables included ankle inversion/eversion proprioceptive thresholds, and normalized measures of maximum voluntary strength and maximum rate of torque development (RTD) of hip abduction/adduction and ankle inversion/eversion. Kinematic data were obtained using an optoelectronic system as subjects walked over an uneven 10m surface.
Main Outcome Measures
Dependent variables included gait speed and efficiency (determined by step-width-to-step-length ratio) on an uneven surface.
Results
Hip adduction RTD, ankle inversion RTD, and hip abduction maximal strength predicted XY% of gait speed, with the first predicting the majority (45%). Ankle inversion RTD was the only significant predictor of gait efficiency, accounting for 46% of its variability. Age predicted neither gait speed nor efficiency.
Conclusions
The rapid generation of strength in the frontal plane at the hip and ankle is responsible for the successful negotiation of irregular surfaces in older persons. Age demonstrated no independent influence. Training regimens in older persons should include maneuvers that develop strength rapidly in hip adductors and ankle invertors if navigation of uneven surfaces is a functional goal.
Keywords: Diabetic neuropathies, sensorimotor gait disorder, proprioception, muscle strength, postural balance
INTRODUCTION
The prevalence of type 2 diabetes mellitus is increasing worldwide [1]. It is therefore anticipated that the prevalence of one of its common complications, a distal symmetric peripheral neuropathy (DSPN), will increase as well. It is broadly recognized that a DSPN leads to a decrement in distal lower limb sensory function; however, there is also a neuropathy-related decrease in distal motor function, even among those with relatively mild disease [2]. The degradation in lower limb sensorimotor function often results in balance and gait impairment among older persons with diabetic DSPN [3–7]. These gait abnormalities are accentuated when subjects with DSPN walk on uneven surfaces [3, 5]. Accordingly, the majority of falls in older subjects with neuropathy occur when they are walking on an irregular surface [5]. Despite this link between walking surface irregularity and falls, the great majority of gait research is performed on smooth surfaces [4], and so the lower limb sensorimotor features that allow safe and effective gait on irregular surfaces have not been determined. The question is of clinical relevance given that exercise, often in the form of walking, is fundamental to the management of type 2 diabetes mellitus [8].
There is increasing recognition of the importance of lateral (or frontal plane) control during ambulation. In addition to the markedly increased injury potential associated with lateral falls [9], there is a growing appreciation of the importance of frontal plane sensory and motor function with respect to one legged balance, fall risk, and fall prevention [10–13]. However the relative importance of lower limb sensory and motor functions involved in lateral control with respect to functional gait, as defined by speed and efficiency, on an uneven surface has not been explored. Speed and efficiency of gait are relevant measures given that a slower gait speed is associated with increased fall risk [14], increased morbidity and mortality [15], reduced overall health status and increased duration of hospital stay [16], and that an efficient gait allows greater ease in daily activities that involve accessing the community. In studies identifying optimal gait efficiency in humans measuring mechanical work as well as metabolic costs, the latter by means of open circuit respirometry, the optimal step-width-to-step-length ratio is 0.14 [17–19], with greater ratios leading to greater energy costs.
Therefore the objective of this research was to identify the lower limb sensory and/or motor functions involved in frontal plane control that most powerfully influence gait speed and efficiency on an uneven surface in older persons. To achieve this objective ankle and hip motor functions, and ankle sensory function, relevant to frontal plane control were measured in a group of older persons with a spectrum of peripheral neurologic function ranging from normal to moderate/severe diabetic DSPN. Subsequent determination of gait speed and efficiency on an uneven surface allowed the exploration of relationships between lower limb sensorimotor functions and these gait characteristics. It was hypothesized that greater hip and ankle motor function, and more precise ankle sensory function, would be associated with increased gait speed and efficiency on the irregular surface.
MATERIALS and METHODS
Subjects
Subjects were recruited from the University of Michigan Orthotics and Prosthetics Clinic, Endocrinology Clinic and the Older Americans Independence Center Human Subjects Core. The research protocol was approved by the University of Michigan Institutional Review Board and all subjects provided written informed consent. Inclusion criteria included: age between 50 and 85; mass not greater than 136 kilograms (so as to not exceed the suspension harness safety margin); a history of type 2 diabetes mellitus for subjects with DSPN; the ability to walk household distances without assistance or assistive devices; dorsiflexion ankle strength at least against gravity (grade ≥ 3 by manual muscle testing). The history of type 2 diabetes mellitus was confirmed by medical record review of elevated fasting glucose (> 125 milligram/deciliter) and the ongoing use of oral hypoglycemic agents or insulin. The presence of DSPN was confirmed by: a) symptoms consistent with neuropathy (subject reported altered sensation in the distal lower limbs; b) signs consistent with neuropathy (Michigan Diabetes Neuropathy Score; MDNS) ≥ 10[20]; c) electrodiagnostic evidence of neuropathy (bilaterally abnormal peroneal motor responses recording over the extensor digitorum brevis, defined as amplitude < 2 mV and/or latency > 6.2 milliseconds and/or conduction velocity < 41.0 m/s). The MDNS assigns a numerical score to clinical measures of distal strength, sensation and reflexes, which then summed to provide the MDNS score, with greater score suggesting more severe neuropathy. The MDNS score has been found to strongly correlate with more detailed neuropathy severity measuring systems [20]. Subjects without DSPN had no history of diabetes mellitus, no symptoms or signs of DSPN (MDNS < 10), and normal peroneal nerve conduction studies. Subjects with or without DSPN were excluded if they reported a fall within one month of testing or had history or clinically evident central nervous system dysfunction (for example, hemiparesis, myelopathy or cerebellar ataxia). Additional exclusion criteria included: neuromuscular disorders other than DSPN (for example, myopathy or neuromuscular junction disorders), evidence of vestibular dysfunction, history of angina or angina-equivalent symptoms with exercise, plantar skin sore or joint replacement within the previous year, symptomatic postural hypotension, significant musculoskeletal deformity (for example, amputation or Charcot changes), lower limb or spinal pain that limited standing to less than 10 minutes, or walking to less than one block.
Evaluation of Lower Limb Sensorimotor Function
Ankle Sensory Function
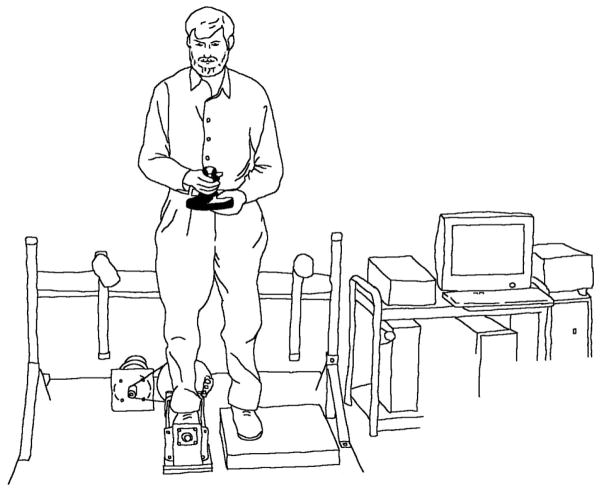
Ankle proprioception thresholds were determined as previously described [21]. In brief, subjects stood with the foot/ankle being tested in a 40 × 25 cm cradle that rotated in the frontal plane (inversion and eversion; Figure 1). The cradle was rotated by a servomotor equipped with an 8,000 line rotary encoder (Aerotech 1000 servomotor, Aerotech, Inc., Pittsburgh, PA, United States). The subject responded to the direction of the rotation with a hand held joystick. Four blocks of 25 trials (randomly 10 eversion, 10 inversion, and 5 dummy trials) were presented. Each block was interspersed with 2 to 5 minutes rest intervals. The ankle proprioception threshold was defined as the smallest rotational displacement of the ankle that a subject could detect with 100% accuracy.
Figure 1.
Apparatus for determining frontal plane ankle proprioceptive thresholds
Ankle Motor Function
During maximum voluntary strength testing, subjects stood on a force plate (Model #OR6-7 Force Plate, AMTI, Inc., Watertown, Massachusetts, United States) touching hand rails on both sides as needed. Subjects then lifted one leg, shifted their center of gravity as far laterally under their foot as possible and lifted their hands from the rails for three seconds. The test was repeated three times for the lateral margin of the foot (maximum voluntary inversion), and repeated for the medial margin of the foot (maximum voluntary eversion). To measure ankle rate of torque development (RTD), subjects stood on the test foot on the force plate and moved the center of ground support reaction from the lateral margin of the foot to the medial margin as quickly as possible, and then back again to the lateral margin, as previously described [2]. Three trials, each trial with five medial-lateral movements, were performed. Subjects were allowed to touch horizontal hand rails as needed.
Hip Motor Function
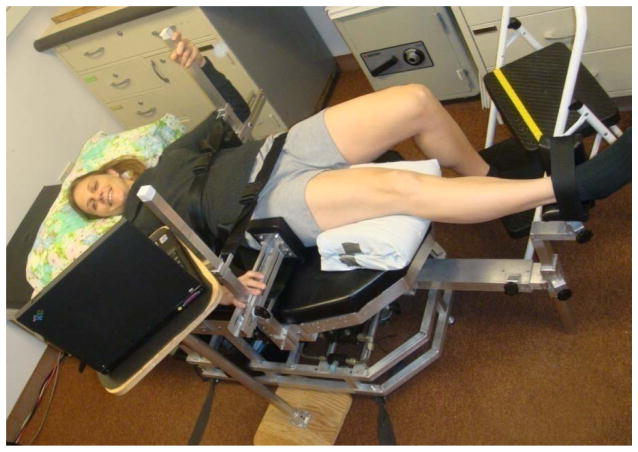
A custom, whole-body dynamometer (BioLogic Engineering, Inc. Dexter, MI.) was used to measure maximum voluntary strength and RTD of frontal plane hip musculature [22]. The subject lay supine on a horizontal bench with the pelvis and upper body immobilized with adjustable harness straps, and the limb being tested secured with straps against a lever, allowing all measurements to be made in a gravity-free plane. During maximum voluntary strength tests subjects progressively increased their isometric effort to their maximum over a count of three seconds, held it for two seconds, and relaxed. To quantify rate of isometric strength development, subjects increased their effort as rapidly as possible for 3 s. Three trials were performed with 1 minute rest between trials. Subjects had a real time visual display of the force generated to allow them to evaluate their efforts. Ankle and hip motor functions were normalized for body size, defined as body height multiplied by weight in units of Newton-m.
Gait Analyses
Gait analyses were performed as previously described [6]. All subjects wore flat-soled athletic shoes (New Balance Athletic Shoe Inc., Boston, Massachusetts) as well as a full-body safety harness which was adjusted so that their knees or other body parts could not touch the floor in the event of a fall. Subjects walked on a 1.5 m*10 m irregular surface which was created by randomly fixing triangular wooden prisms under a strip of industrial carpeting (Figure 2). The position of the prisms was not changed between trials or subjects. Each subject walked across the surface 10 times, two accommodation trials and 8 data acquisition trials. Prior to each trial subjects were instructed: “Please walk at a comfortable pace, as if you were walking to the corner to mail a letter.” Optoelectronic markers were placed over the midline of the trunk at the umbilicus and on the base and top of aluminum tabs which were inserted into the laces of the shoes in midline and flexed to vertical. Step width and step length were defined as the medial-later and anterior-posterior distance, respectively, between foot markers during double support. An optoelectronic camera system measured kinematic data at 100 Hz (Optotrak 3020, Northern Digital Corp., Waterloo, Ontario.) [6]. These data were then processed using a custom MATLAB1 algorithm to quantify walking speed, step width, and step length as previously described [6].
Figure 2.
Subject standing on a force plate inverting and everting the ankle as quickly as possible (a) and as far as possible (b) so as to determine ankle rate of torque generation and maximum voluntary strength, respectively.
Statistics
Statistical analyses used SPSS (SPSS for Windows, Rel.11.0.1.2001 Chicago). Descriptive statistics were determined for all measures. Data were examined for normality and screened for outliers. Pearson product-correlation coefficients were calculated to assess relationships between lower limb sensorimotor functions, gait speed, and step-width-to-step-length ratio. Multiple regression models were used to determine the independence and relative influence of sensorimotor functions (independent variables) and gait speed and step-width-to-step-length ratio (dependent variables). Sensorimotor functions with significant relationships to gait speed or efficiency were introduced in a stepwise regression model as potential predictors, using age and BMI as co-variates. The significance level for all tests was set at 0.05.
RESULTS
Subjects
Thirty three subjects were enrolled (14; 42.4% female and 21; 63.6% with diabetic DSPN). Means and standard deviations of age, BMI, and MDNS results are presented in Table 1.
Table 1.
Subject information
| Parameter (N=33) | Minimum | Maximum | Mean (SD) |
|---|---|---|---|
| Age (years) | 52.0 | 85.0 | 69.67 (8.89) |
| Height (cm) | 150.0 | 188.0 | 171.72 (10.09) |
| Weight (kg) | 49.5 | 125.0 | 88.74 (19.04) |
| BMI(m2/kg) | 19.2 | 46.5 | 30.11 (6.30) |
| MDNS (0–46) | 0.0 | 31.0 | 9.49 (8.39) |
Univariate Analyses
Correlation coefficients between lower limb sensorimotor functions and gait speed, as well as gait efficiency are listed in Table 2. The data indicate that all of the sensorimotor functions were significantly associated with gait speed with correlations (r) ranging from .412 to .665. Additionally, all sensorimotor functions except ankle eversion RTD were significantly associated with gait efficiency with significant correlations ranging from .385 to .648. Age was indirectly related to speed and efficiency (r = −.447; p = .009; respectively r = .425; p = .014) whereas BMI correlated with neither gait speed nor efficiency.
Table 2a.
Relationships between sensory and motor functions and gait speed
| Variable | N | Correlation coefficient | 95% CI* | p-value |
|---|---|---|---|---|
| Ankle inversion Rate of Torque Development | 32 | 0.665 | 0.490 to 0.802 | <0.001 |
| Hip Add Rate of Torque Development | 33 | 0.626 | 0.422 to 0.796 | <0.001 |
| Hip Abd Rate of Torque Development | 33 | 0.504 | 0.198 to 0.721 | 0.003 |
| Hip Add Maximum Voluntary Strength | 33 | 0.475 | 0.115 to 0.718 | 0.005 |
| Ankle Inversion Maximum Voluntary Strength | 29 | 0.491 | 0.263 to 0.692 | 0.007 |
| Ankle Eversion Maximum Voluntary Strength | 31 | 0.447 | 0.032 to 0.716 | 0.012 |
| Ankle Eversion Rate of Torque Development | 31 | 0.441 | 0.196 to 0.689 | 0.013 |
| Hip Abd Maximum Voluntary Strength | 33 | 0.422 | 0.082 to 0.699 | 0.015 |
| Ankle Proprioceptive Threshold | 33 | −0.412 | −0.639 to −0.092 | 0.017 |
Multivariate Analyses
Regression analyses identified two significant predictors of gait speed on the irregular surface (Table 3.a.). Hip adductor RTD and ankle inversion RTD predicted over half of the variance in gait speed, with hip adductor RTD accounting for over 40%. The only significant predictor of gait efficiency (step-width-to-step-length ratio) was ankle inversion RTD which accounted for 46% of the variance (Table 3.b.).
Table 3a.
Sensory and motor functions predicting gait speed on the irregular surface
| (N= 29) Parameters | R | R2 | Adj. R2 | SE Estimate | R2 Change | F Change | Sig. F Change |
|---|---|---|---|---|---|---|---|
| Hip Add RTD | .661 | .436 | .416 | .129 | .436 | 20.907 | .000 |
| Ankle Inv RTD | .735 | .540 | .504 | .119 | .103 | 5.826 | .023 |
RTD Hip Add = Rate of torque development of Hip Adductors; RTD Ankle Inv = Rate of torque development of Ankle inversion
Table 3b.
Sensory and motor functions predicting step-width-to-step-length ratio
| (N=29) Parameter | R | R2 | Adj. R2 | SE Estimate | R2 Change | F Change | Sig. F Change |
|---|---|---|---|---|---|---|---|
| RTD Ankle Inv | .678 | .460 | .440 | .061 | .460 | 22.960 | .000 |
RTD Ankle Inv = Rate of torque development of Ankle inversion
DISCUSSION
The most important finding was that frontal plane motor function at the hip and ankle (adduction and inversion, respectively) explained nearly three quarters of the variance in gait speed and nearly half of the variance in gait efficiency among older subjects with varying degrees of peripheral neurologic function walking on an irregular surface. Moreover it was RTD of these muscles, rather than their maximal strength, that was critical to gait speed and efficiency under the experimental conditions. In contrast, ankle proprioceptive function did not appear to influence gait when frontal plane motor function and confidence were taken into account. Finally, age did not independently predict gait speed or efficiency on the irregular surface.
Although the importance of frontal plane motor function relative to that more routinely studied in the sagittal plane (i.e., knee extensors and plantar flexors) was not determined, comparisons with other work are useful. For example in prior work we found that frontal plane hip strength explained about 45% of unipedal balance time [22], whereas a separate study found that knee extensor and flexor strength explained only 10% [23]. Similarly, in a study of older adults with diabetic DSPN, sagittal plane muscle strength predicted about one fifth of gait speed change when subjects transitioned from smooth to uneven terrain [24], substantially less than our study in which frontal plane motor function predicted over half of the variance in gait speed. These comparisons suggest that motor function in the frontal plane, rather than that in the more frequently studied sagittal plane, exerts the dominant influence when balance is challenged. However, we are not aware of any studies that concurrently explored the influences of sagittal and frontal plane lower limb sensorimotor functions on standard or perturbed gait.
Rates of torque generation at the hip and ankle were more important than the maximum strengths of these same muscle groups, a point supported by other research which emphasizes motor response speed for successful recovery from a perturbation. Jumping distance [25] and gluteus medius onset latency [10] in response to a perturbation were the best predictors of prospectively identified falls among community-dwelling older persons. Similarly, jump height was the best clinical measure of ability to recover from an induced trip [26], and subjects routinely recovered from trip durations less than 700 milliseconds, but could not recover from longer trip durations [27]. Our results add to current research suggesting that successful response to a balance challenge, including gait on an irregular surface, is time dependent and so requires short response latencies and rapid generation of force. Similarly, improvement in lower limb power led to increased gait speed in mobility impaired older adults independent of lower limb strength [28]. The greater importance of hip adduction and ankle inversion RTD, rather than abduction and eversion, is likely related to the ability of the former movements to generate a laterally directed ground reaction force which allows control of a laterally displaced center of mass [29].
The absence of a significant, independent age effect implies that aging does not intrinsically contribute to difficulty navigating an irregular surface. It is likely that age is a marker for decrements in RTD in frontal plane muscles, along with reductions in plantar flexor power [30] which are the true sources of apparent age-related gait change. Although a greater number of subjects might reveal an independent age effect, our data suggest that such an effect would be minor in comparison to lower limb motor function.
The apparent lack of influence of frontal plane proprioceptive sensory function at the ankle was not anticipated given their importance to unipedal stance time [21, 22]. However, this finding is consistent with other analyses of these same subjects which found that hip motor function compensates for imprecise ankle proprioception during one-legged balance [22]. The apparent lack of ankle sensory contribution may also be related to subjects seeing the walking surface irregularities which could have allowed visual compensation for somatosensory deficits. In support, lower field visual information is important to adapting to varying walking surfaces [31] and so proprioceptive thresholds might have played a measurable role if lower field vision was occluded.
The study has strengths in comparison with prior work. Among these is the simultaneous quantification of sensory and motor function relevant to frontal plane control within each subject, a novel feature of the study. In addition the subjects were selected to represent a spectrum of peripheral sensorimotor function, and the presence and severity of PN were determined by history, physical examination and nerve conduction studies as has been recommended [32]. In contrast, studies often use a single modality such as vibratory perception threshold or monofilament testing to determine the presence and severity of PN. The measurement of RTD is infrequently determined, but is an advantage given other work finding that diabetic neuropathy is associated with selective dropout of Type II motor neurons and reduced rate of force generation [33, 34]. The use of an uneven walking surface is more functionally relevant than gait analysis on a smooth surface, as is typically used, particularly given the fall risk associated with irregular surfaces [5].
One of the study’s potential limitations is the combining of subjects with diabetic DSPN with subjects who have neither diabetes nor neuropathy. This study population was chosen so that it would include as broad a spectrum of peripheral neurologic function as possible. Subjects with diabetes but without neuropathy would be less likely to represent optimal peripheral neurologic function. Additionally, this concern is mitigated by evidence that declining peripheral neurologic function, rather than the presence of diabetes mellitus, is primarily responsible for impairments in ankle motor function, balance and age-associated mobility loss (for example [2, 35–37]). Another limitation involves the method for determining ankle motor function which assumed the ankle center of rotation to be mid-way between the malleoli. However this technique has been used previously and its validity is supported by the relationship between ankle strength determined in this manner, and the presence of neuropathy and unipedal stance time [2]. The method for determining hip strength applied a varus/valgus stress on the knee. Although no subjects reported pain they may have not given maximal effort for fear of causing discomfort. In addition, it has recently been demonstrated that the knee has frontal plane proprioceptive function [38], something we did not account for. The number of subjects was reasonable given the quantitative nature of the study, but the 33 subjects allow the reliable consideration of just 3 to 4 potential predictors within a multiple regression analysis.
The results of this research have potential clinical application. Specifically, it appears that strengthening regimens in older persons with diabetic DSPN should focus on the capacity to develop strength quickly, rather than on maximal strength generated gradually. In addition, training should involve muscles of frontal plane control, rather than focusing exclusively on muscles involved in sagittal plane control; however, this is not routinely recommended [39, 40]. The dominant effect of the proximally located hip muscles on gait speed on an uneven surface suggests that even patients with more severe PN can benefit from training, given the distal nature of neuropathic disease. It is less clear that patients with PN, particularly those with more severe impairments, can increase ankle inversion rate of torque generation. However some strengthening is likely possible in most cases given that both the anterior and posterior tibialis muscles perform this function, with the latter muscle innervated by the tibial nerve which takes a shorter route to its target, rendering it relatively less affected in a length dependent neuropathic process. However, improvements in tandem stance, unipedal stance and functional reach were noted in older subjects with diabetic DSPN who underwent a three week regimen of ankle strengthening [41].
Our results suggest that a future trial examining the effect of a training program designed to increase rate of torque generation in frontal plane muscles in older persons with diabetic PN is reasonable, using gait speed and the ability to tolerate perturbations while walking as outcomes. It is tempting to suggest that increasing frontal plane rate of torque generation will reduce fall risk in older patients with diabetic PN when walking on an uneven surface. Although this is likely true, that specific hypothesis was not explored and is a question that merits further study. The absence of influence of ankle proprioceptive function is surprising but may, as suggested above, show influence if the lower visual field is obscured. Such a study has special relevance to the diabetic population given the likelihood that some patients will have visual impairment from retinopathy.
Figure 3.
Whole-body dynamometer used to measure maximum voluntary strength and rate of torque development of frontal plane hip musculature.
Figure 4.
Experimental set-up for determining gait speed and step-width-to-step-length ratio on an irregular surface
Table 2b.
Relationships between sensory and motor functions and step-width-to-step-length ratio
| N | Correlation coefficient | 95% CI* | p-value | |
|---|---|---|---|---|
| Hip Add Maximum Voluntary Strength | 33 | −.648 | −0.802 to −0.425 | .000 |
| Ankle Inversion Rate of Torque Development | 31 | −.636 | −0.783 to −0.414 | .000 |
| Ankle Proprioceptive Threshold | 33 | .503 | 0.261 to 0.705 | .003 |
| Ankle Eversion Rate of Torque Development | 31 | −.514 | −0.690 to −0.312 | .003 |
| Hip Abd Rate of Torque Development | 33 | −.486 | −0.722 to −0.183 | .004 |
| Hip Abd Maximum Voluntary Strength | 33 | −.475 | −0.699 to −0.137 | .005 |
| Hip Add Rate of Torque Development | 33 | −.471 | −0.695 to −0.161 | .006 |
| Ankle Inversion Maximum Voluntary Strength | 29 | −.385 | −0.614 to −0.102 | .039 |
| Ankle Eversion Maximum Voluntary Strength | 31 | −.326 | −0.567 to −0.002 | .074 |
Acknowledgments
This work was supported by the National Institutes of Health (RO1 AG026569), PHS Grants (P30AG024824) and the Swiss National Foundation (IZKOZ3 133925).
Abbreviations
- DSPN
Distal Symmetric Peripheral Neuropathy
- MDNS
Michigan Diabetes Neuropathy Score
- RTD
Rate of Torque Development
Footnotes
Contribution statement
Lara Allet: Recruitment and screening of subjects, data acquisition, analysis and interpretation of data, initial draft and obtaining funding; James K. Richardson: Study conception and design, recruitment and screening of subjects, interpretation of data, critical revision of manuscript, and obtaining funding; James A. Ashton-Miller: Design of measurement apparatus and methods, accuracy of data analysis, interpretation of data, critical revision of manuscript; Hogene Kim: Data acquisition, data processing and revising the manuscript. All authors gave final approval of manuscript.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez EM, Helber MD, Dealva D, Ashton-Miller JA, Richardson JK. Mild diabetic neuropathy affects ankle motor function. Clin Biomech (Bristol, Avon) 2001;16:522–8. doi: 10.1016/s0268-0033(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 3.Allet L, Armand S, de Bie RA, et al. Gait alterations of diabetic patients while walking on different surfaces. Gait Posture. 2009;29:488–93. doi: 10.1016/j.gaitpost.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24:173–91. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 5.DeMott TK, Richardson JK, Thies SB, Ashton-Miller JA. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med Rehabil. 2007;86:125–32. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 6.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc. 2004;52:1532–7. doi: 10.1111/j.1532-5415.2004.52418.x. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil. 1996;77:1152–6. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 8.Matheson GO, Klugl M, Dvorak J, et al. Responsibility of sport and exercise medicine in preventingand managing chronic disease: applying our knowledge and skill is overdue. Br J Sports Med. 2011;45:1272–82. doi: 10.1136/bjsports-2011-090328. [DOI] [PubMed] [Google Scholar]
- 9.Greenspan SL, Myers ER, Kiel DP, Parker RA, Hayes WC, Resnick NM. Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am J Med. 1998;104:539–45. doi: 10.1016/s0002-9343(98)00115-6. [DOI] [PubMed] [Google Scholar]
- 10.Brauer SG, Burns YR, Galley P. A prospective study of laboratory and clinical measures of postural stability to predict community-dwelling fallers. J Gerontol A Biol Sci Med Sci. 2000;55:M469–76. doi: 10.1093/gerona/55.8.m469. [DOI] [PubMed] [Google Scholar]
- 11.Hilliard MJ, Martinez KM, Janssen I, et al. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89:1708–13. doi: 10.1016/j.apmr.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Lockhart TE. Age-related joint moment characteristics during normal gait and successful reactive-recovery from unexpected slip perturbations. Gait Posture. 2009;30:276–81. doi: 10.1016/j.gaitpost.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers MW, Mille ML. Lateral stability and falls in older people. Exerc Sport Sci Rev. 2003;31:182–7. doi: 10.1097/00003677-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Espy DD, Yang F, Bhatt T, Pai YC. Independent influence of gait speed and step length on stability and fall risk. Gait Posture. 2010;32:378–82. doi: 10.1016/j.gaitpost.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afilalo J, Eisenberg M, Morin J, et al. Gait Speed as an Incremental Predictor of Mortality and Major Morbidity in Elderly Patients Undergoing Cardiac Surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42:535–46. doi: 10.1682/jrrd.2004.07.0087. [DOI] [PubMed] [Google Scholar]
- 17.Kuo AD. A simple model of bipedal walking predicts the preferred speed-step length relationship. J Biomech Eng. 2001;123:264–9. doi: 10.1115/1.1372322. [DOI] [PubMed] [Google Scholar]
- 18.Kuo AD. Energetics of actively powered locomotion using the simplest walking model. J Biomech Eng. 2002;124:113–20. doi: 10.1115/1.1427703. [DOI] [PubMed] [Google Scholar]
- 19.Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc Biol Sci. 2001;268:1985–92. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 21.Son J, Ashton-Miller JA, Richardson JK. Do ankle orthoses improve ankle proprioceptive thresholds or unipedal balance in older persons with peripheral neuropathy? Am J Phys Med Rehabil. 2010;89:369–75. doi: 10.1097/PHM.0b013e3181d89861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allet LKH, Ashton-Miller J, DeMott T, Richardson JK. Frontal plane hip and ankle sensorimotor function, not age, predicts unipedal stance time. Muscle&Nerve. 2011 doi: 10.1002/mus.22325. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Citaker S, Kaya D, Yuksel I, et al. Static balance in patients with patellofemoral pain syndrome. Sports Health: A Multidisciplinary Approach. 2011;3:524–527. doi: 10.1177/1941738111420803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allet L, Armand S, de Bie RA, et al. Clinical factors associated with gait alterations in diabetic patients. Diabet Med. 2009;26:1003–9. doi: 10.1111/j.1464-5491.2009.02811.x. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez D, Rose DJ. Predicting which older adults will or will not fall using the Fullerton Advanced Balance scale. Arch Phys Med Rehabil. 2008;89:2309–15. doi: 10.1016/j.apmr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Pijnappels M, van der Burg PJ, Reeves ND, van Dieen JH. Identification of elderly fallers by muscle strength measures. Eur J Appl Physiol. 2008;102:585–92. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeesters C, Hayes WC, McMahon TA. The threshold trip duration for which recovery is no longer possible is associated with strength and reaction time. J Biomech. 2001;34:589–95. doi: 10.1016/s0021-9290(01)00005-7. [DOI] [PubMed] [Google Scholar]
- 28.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J Am Geriatr Soc. 2010;58:2363–8. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otten E. Balancing on a narrow ridge: biomechanics and control. Philos Trans R Soc Lond B Biol Sci. 1999;354:869–75. doi: 10.1098/rstb.1999.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graf A, Judge JO, Ounpuu S, Thelen DG. The effect of walking speed on lower-extremity joint powers among elderly adults who exhibit low physical performance. Arch Phys Med Rehabil. 2005;86:2177–83. doi: 10.1016/j.apmr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Marigold DS, Patla AE. Visual information from the lower visual field is important for walking across multi-surface terrain. Exp Brain Res. 2008;188:23–31. doi: 10.1007/s00221-008-1335-7. [DOI] [PubMed] [Google Scholar]
- 32.England JD, Gronseth GS, Franklin G, et al. Distal symmetrical polyneuropathy: a definition for clinical research. A report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Arch Phys Med Rehabil. 2005;86:167–74. doi: 10.1016/j.apmr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Andersen H, Mogensen PH. Disordered mobility of large joints in association with neuropathy in patients with long-standing insulin-dependent diabetes mellitus. Diabet Med. 1997;14:221–7. doi: 10.1002/(SICI)1096-9136(199703)14:3<221::AID-DIA338>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005;15:392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 35.Turcot K, Allet L, Golay A, Hoffmeyer P, Armand S. Investigation of standing balance in diabetic patients with and without peripheral neuropathy using accelerometers. Clin Biomech (Bristol, Avon) 2009;24:716–21. doi: 10.1016/j.clinbiomech.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white andblack adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–72. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the health, aging and body composition study. J Am Geriatr Soc. 2009;57:2004–10. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cammarata ML, Schnitzer TJ, Dhaher YY. Does knee osteoarthritis differentially modulate proprioceptive acuity in the frontal and sagittal planes of the knee? Arthritis Rheum. 2011;63:2681–9. doi: 10.1002/art.30436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo J, Hong A, Lau E, Lynn H. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing. 2007;36:262–8. doi: 10.1093/ageing/afm005. [DOI] [PubMed] [Google Scholar]
- 40.Korpelainen R, Keinanen-Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J. Effect of exercise on extraskeletal risk factors for hip fractures in elderly women with low BMD: a population-based randomized controlled trial. J Bone Miner Res. 2006;21:772–9. doi: 10.1359/jbmr.060116. [DOI] [PubMed] [Google Scholar]
- 41.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–9. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]