Abstract
Cytokinesis shape change occurs through the interfacing of three modules, cell mechanics, myosin II-mediated contractile stress generation and sensing, and a control system of regulatory proteins, which together ensure flexibility and robustness. This integrated system then defines the stereotypical shape changes of successful cytokinesis, which occurs under a diversity of mechanical contexts and environmental conditions.
Keywords: Actin network, Contractility, Control system, Feedback, Mechanosensing, Myosin II
Introduction
Cytokinesis has been appreciated as being a complex mechanical process ever since the early days of Ray Rappaport’s research into cytokinesis mechanisms (Rappaport 1996). Rappaport’s interest in cytokinesis mechanics was shared by several of his contemporaries, such as Hiramoto (Hiramoto 1956; Hiramoto 1963; Hiramoto 1990), Mitchison and Swann (Mitchison and Swann 1955), and Wolpert (Wolpert 1966). Their early experiments focused heavily on defining the changes in the cortex which were associated with furrow formation and ingression (Rappaport 1964; Rappaport 1965; Rappaport and Ebstein 1965). These studies drew upon several state of the art methods of the day including reshaping the cells mechanically, micropipette aspiration, compression between plates, and force measurement by observing the bending of glass needles. Many of these methods appeared to go out of fashion only to return in more recent years as very effective techniques to assess these cortical changes and monitor cortical mechanics, especially when combined with modern imaging methods. Of the many classical experiments that Rappaport performed, a particularly elegant example was his direct measurement of the amount of force that the cleavage furrows of echinoderm eggs could generate during furrow ingression. To make these measurements, Rappaport inserted two glass needles into the dividing egg, one of which held the cell in place and allowed the second, softer needle to deflect in response to applied forces. Then, by examining the bending of the needle, Rappaport determined the amount of force (~20–30 nN) that the cleavage furrow could generate (Rappaport 1967). Rappaport pointed out that these measured forces only revealed what the furrow was capable of generating and that the system was likely to be highly dynamic, adjusting its force generation as needed. Indeed, dynamic adjustment of the cellular force balance has proven to be a key feature by which the cell ensures cytokinesis fidelity. In this review, we will present our current thinking on the mechanics of cytokinesis and how the contractility machinery is regulated, not only through forward biochemical pathways, but also through mechanical feedback loops to ensure that the cleavage furrow generates just the right amount of force to drive cytokinesis shape change, ensuring successful cell division. We will focus much of our discussion on studies from Dictyostelium discoideum, a model organism which provides experimenters with ready access to a broad array of genetic, mechanical, and chemical tools to facilitate the study of metazoan-type cytokinesis. However, we will point out parallels from other systems where similar or related measurements have been made.
Dissecting cortical changes during cytokinesis reveals three major modules whose mechanical features complement each other in order to ensure robust progression through cytokinesis. The first module is the cortical meshwork of cross-linked actin, which defines local mechanics and the energy cost for deformation. The second module is myosin II, which can be distributed around the cell to generate stress and alter local viscoelasticity, and which is itself mechanoresponsive, accumulating to sites of stress. Finally, a control system of regulatory proteins feeds into the other two modules, tuning their outputs to drive cell rounding, position the equator, and then generate and sustain cleavage furrow ingression in a highly stereotypical manner.
First module: The cell cortex, a dynamic cross-linked network of actin filaments
The main goal of cytokinesis is for the cell to remodel its shape until a major topological change occurs in which a mother cell splits into two daughters. The majority of this remodeling occurs at the cell cortex, the cell’s “skin”, which consists of the plasma membrane and the underlying cytoskeletal polymer network (Pesen and Hoh 2005; Reichl et al. 2008). The cortex of a living cell is a complex, dynamic environment, which is called upon to perform a far-reaching array of cellular tasks. The cortex must be strong enough to withstand stresses imposed by mechanical insults, osmotic pressure, and cell-to-cell adhesions, yet flexible enough to accommodate a broad range of cellular morphologies, from spherical oocytes to flattened epithelial cells to elongated neurons, as well as adaptable enough to actively remodel itself in response to signals. To accommodate these numerous mechanical demands, the cortex relies on several basic building blocks, each with its own unique time-scale of association. The actin filaments provide mechanical connections between different parts of the cell, and cross-linking proteins join these actin filaments to each other and to the cell membrane. Actin polymer dynamics along with myosin II interactions aid in network remodeling (Girard et al. 2006; Guha et al. 2005; Murthy and Wadsworth 2005).
The features of the cytoskeletal network define how the cell will deform in response to applied mechanical stress, whether internally generated or externally imposed. The cellular cytoskeleton is described mechanically as being viscoelastic, meaning that the cortex has both elastic and viscous character(Evans and Yeung 1989; Girard et al. 2004; Yang et al. 2008). Rheological measurements reveal that on short time-scales (<0.2 s) the cell cortex has a mechanical phasing of ~15°, which indicates that elasticity dominates at these time-scales (a pure solid has a phasing of 0°, while a pure liquid has a phasing of 90°). On time-scales longer than 0.2 s, super-diffusive behaviors (activities that operate faster than pure thermal motion would allow) become prominent (Girard et al. 2006). The passive mechanical behavior of the cell is largely defined by the actin network, as latrunculin treatments reduce the total polymeric actin levels by 55%, which causes an 85% reduction in cortical viscoelasticity(Girard et al. 2004; Luo et al. 2012). This non-linear relationship between viscoelasticity and polymer concentration is expected, as pure actin networks and crosslinked actin networks typically have viscoelastic moduli that depend on the actin concentration (cAγ) where γ is the power law and typically ranges from >1 to 2.5. The exact value depends on variables such as actin polymer length, the nature of the crosslinkers, and crosslinker density (Gardel et al. 2006; Gardel et al. 2004; Luo and Robinson 2011; MacKintosh et al. 1995).
In cells, the actin polymer length is typically much lower than the persistence length (~10 μm) (Isambert et al. 1995) of the actin filaments. Measured lengths depend on species, ranging from 600–1,000 nm in S. pombe contractile rings to 100 nm in Dictyostelium cleavage furrows to 45–90 nm in the cleavage furrows of HeLa cells (Kamasaki et al. 2007; Maupin and Pollard 1986; Reichl et al. 2008). As a result, the filaments behave as rigid rods in the cell and the actin crosslinkers have an essential role in integrating the polymers into a viscoelastic network that can span the circumference of the cleavage furrow. Indeed, multiple actin crosslinking proteins contribute significantly to the spatial mechanics of dividing cells (e.g. global vs. equatorial) and interphase cells (e.g. pseudopod vs. rear end of cell) (Girard et al. 2004; Mukhina et al. 2007; Octtaviani et al. 2006; Reichl et al. 2008; Simson et al. 1998; Stossel et al. 2001). Crosslinking proteins are far from generic, with each having its own unique time-scale for actin association. This leads to time-scale dependent variations in cell mechanics. For example, fimbrin is a crosslinker that associates only transiently with actin, demonstrating a fluorescence recovery after photobleaching (FRAP) recovery time of 260 ms, which is not much slower than the 150 ms recovery time of freely diffusing GFP. Mechanically, fimbrin null cells have a significant reduction in cortical viscoelasticity on the sub-100 ms time-scale but little change in cortical tension, which is measured on the seconds time-scale. In contrast, other actin crosslinking proteins, such as dynacortin and cortexillin I, show impact on sub-second to seconds-time-scale mechanics, along with recovery times (as determined by FRAP) of >0.5 s (Reichl et al. 2008).
Myosin II is also a significant contributor to cell mechanics, but it does so in complex ways. Across the literature, one can find many different observations of how myosin II affects and/or tunes cell mechanics. Various reports have indicated in turn that myosin II has no effect on cell mechanics (Hoffman et al. 2006), increases the fluidity of the cellular network(Marion et al. 2005), increases cell deformability (Feneberg et al. 2001; Merkel et al. 2000), or decreases deformability(Dai et al. 1999; Egelhoff et al. 1996; Pasternak et al. 1989; Reichl et al. 2008). Our observations indicate that these apparently conflicting findings may be partially reconciled by considering which actin crosslinker(s) or linking proteins are working in conjunction with myosin II, and whether the network is under mechanical stress (Girard et al. 2006; Reichl et al. 2008; Ren et al. 2009).
The cell cortex may be deformed by stretching (Sc) or bending (B). The stretch and bending moduli constitute the energy costs for deforming the cortex away from equilibrium. The contribution of each parameter decays over a characteristic length-scale (l). Bending (B) decays as l−3, while stretch (Sc) decays as l−1. Therefore, as the distance between two points on the surface of the cell increases, the energy costs for deformation can transition between a zone dominated by bending (for small-scale structures) to one primarily influenced by stretch (for larger-scale structures). Based on measured values for these parameters, this cross-over distance is on the order of 100 nm for Dictyostelium cells. Since structures such as pseudopods, ruffles, and the cleavage furrow deform cells over several μm2, well beyond the cross-over distance, this implies that the energy cost for stretch will dominate in these cases (Reichl et al. 2008; Robinson et al. 2012; Simson et al. 1998). This dominance is likely to be the case for most cell types and large-scale cortical movements. In fact, the bending modulus has been proposed to decrease at the cleavage furrow of C. elegans embryos (Koyama et al. 2012), perhaps to shift the dominant mechanics to a stretch mode within the extremely narrow furrow region which is typical of these cells.
The combination of elastic, viscous, and super-diffusive elements defines the mechanical context under which stresses act at the cortex to drive and guide shape change. The passive stretch coefficient, Sc, combines with active stresses to give rise to the effective cortical tension (T), which is a major determinant of cytokinesis progression. The cortical tension (T = γ + Sc(A–A0)/A0) is composed of the persistent tension γ (which includes the passive tension in the network counterbalancing the osmotic pressure within the cell, as well as active stresses from myosin motors and actin polymer assembly), and the deformation of the elastic element of the cortex Sc(A–A0)/A0 (Derganc et al. 2000; Robinson et al. 2012). Similar formulations for cortical tension have also been described (Clark and Paluch 2011). However, it is important to note that the elasticity of the cortex depends on time-scale: at short time-scales the cortex is mostly elastic and resists deformation, whereas at longer time-scales the cortex has more viscous behavior and the effective cortical tension approaches the persistent tension γ. Thus, cortical tension is a prominent mechanical feature of cells, which combines with local surface curvature to create fluid pressures that serve to minimize the surface area to volume ratio. During cytokinesis, cortical tension first serves to resist cellular deformation, promoting rounding, and then becomes a major driver of cytokinesis furrow ingression once the cell has passed a critical threshold. Cortical tension, when combined with the longtime-scale viscous character of the cell, accounts for the kinetics of furrow ingression (Poirier et al. 2012; Zhang and Robinson 2005). In addition, cortical tension is predictive of the forces required to drive furrow ingression (Robinson et al. 2002; Yoneda and Dan 1972), the molecular requirements for cytokinesis, including how cells can divide without myosin II (Poirier et al. 2012; Zhang and Robinson 2005), and the consequences of pressure imbalances, which can lead to cellular oscillations during mammalian furrow ingression (Sedzinski et al. 2011).
Rappaport considered whether tension gradients were enough to drive furrow ingression, but concluded that they were insufficient (Rappaport 1999). Anew computational model has also addressed this question by determining the relative contributions of tension gradients to cleavage furrow ingression (Poirier et al. 2012). The authors found that tension differentials could be sufficient for driving cytokinesis; however, the differentials had to be non-physiologically large, consistent with Rappaport’s findings. The model describes the shape changes of cytokinesis furrow ingression by summing the forces acting around the cell (such as protrusive forces, contractility, and cortical tension), and then finding the energetic minimum around the cell perimeter, thus describing the contours of the cell membrane. While this approach relies solely on experimentally determined values for the types and magnitude of forces and the viscoelasticity of the cell, the actual shape of the simulated cell is unconstrained. The modeled cell closely resembles a dividing cell in vivo, and adjustments to the model to eliminate adhesive forces or contractility (in silico analogs to cell division on a non-adherent substrate or the division of cells lacking myosin II) also demonstrate the same quantitative behavior as those observed in vivo (Fig. 1). The model shows that while cortical tension gradients are major drivers of furrow ingression, they do not act alone; force contributions from protrusion coupled with adhesion and/or myosin II contractility are required to initiate furrow ingression.
Figure 1. Cells can complete cytokinesis effectively in the absence of myosin II (adapted from Poirier et al. [2012]).
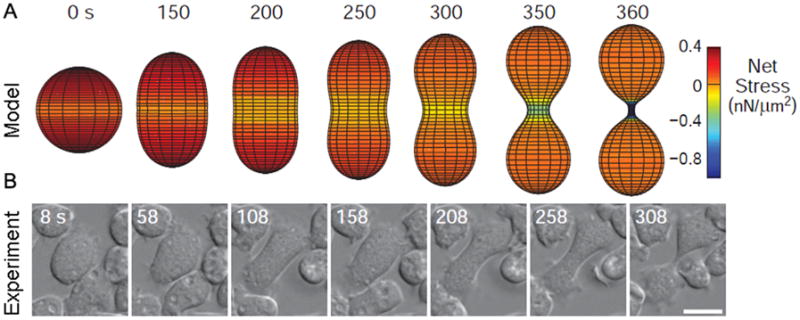
A) The net force balance from protrusive forces, adhesion, and cortical tension leads to cleavage furrow ingression in silico. The color scheme shows net stresses; negative values indicate inward direction.
B) An example myosin II null cell dividing in vivo shows similar kinetics and morphological changes as the model cell in panel A.
Second module: The force-generating protein myosin II
Myosin II mechanochemistry and regulation
A very important, yet non-essential enzyme involved in cytokinesis is myosin II. Myosin II acts on the actin network to generate contractile stresses, which can lead to increased cortical tension and/or relative movement between actin filaments. In its active form, non-muscle myosin II assembles into bipolar thick filaments (BTFs), with motor domains at both ends that bind to actin filaments and use the energy from ATP hydrolysis to generate forces within the cytoskeletal network. The dynamics of myosin II regulation are quite elaborate, as its force-generating properties are dependent on its ATPase cycle as well as its assembly state. Non-muscle myosin II consists of a globular head, which contains the actin-binding motif and the ATPase site, a neck region which binds the essential light chains and the regulatory light chains, and a long coiled-coil tail region, which contains the BTF assembly domain (Spudich 2001). Two myosin heavy chains, each with their bound light chains, form the functional myosin monomer, which then assembles into the BTFs. Current models suggest that in mammals, myosin monomers are maintained in an assembly-incompetent state by heavy chain phosphorylation and an association between the unphosphorylated regulatory light chain and the tail (the so-called 10S structure) (Breckenridge et al. 2009). Upon activation, the myosin undergoes a 10S-6S transition, whereupon the 10S monomer uncurls to form the assembly competent, elongated 6S state. In Dictyostelium, myosin II assembly regulation does not appear to involve the 10S-to-6S transition. While regulatory light chain phosphorylation does occur in Dictyostelium, it is not involved in assembly and only results in a slight (3–5-fold) increase in actin-activated ATPase activity (Ostrow et al. 1994; Uyeda et al. 1996). Instead, Dictyostelium BTF assembly is primarily controlled by heavy chain phosphorylation (Egelhoff et al. 1993; Sabry et al. 1997). In both cases, BTF assembly occurs through a nucleation-elongation mechanism, where assembly competent monomers form a parallel dimer and then two dimers form an antiparallel tetramer referred to as the “nucleus” (Mahajan and Pardee 1996; Sinard et al. 1989). Assembly into larger filaments is then thought to proceed by sequential dimer addition, forming bipolar thick filaments (BTFs). These structures contain multiple motor heads on each end of the bundle, all of which are capable of pulling on the actin filaments so that myosin II-generated force may be conducted through the network.
Since myosin force generation requires its assembly into bipolar thick filaments and most myosin II is found in the assembly-incompetent monomeric state (Egelhoff et al. 1993), the cellular distribution of assembly-regulating factors help direct local contractility. For example, myosin heavy chain kinases (MHCKs) are encoded by four genes in Dictyostelium, three of which (isoforms A–C) appear to play important roles in cytokinesis. These isoforms are spatially segregated in the cell with MHCKA enriched at the poles and MHCKB and Clocalized to the cleavage furrow during cytokinesis (Liang et al. 2002; Yumura et al. 2005). In contrast, a myosin II heavy chain phosphatase (a PP2A member) is solely cytoplasmic and requires the Dictyostelium huntingtin protein for full activation (Wang et al. 2011). Mutant cells lacking the huntingtin protein fail to maintain myosin II at the cleavage furrow. These enzymes (the MHCKs and the phosphatase) primarily maintain the steady state of unassembled myosin II. The steady state level of unassembled myosin is ~80–90% as determined by biochemical fractionation (Egelhoff et al. 1993), or 50–70% based on immobile fractions from FRAP analysis (Zhou et al. 2010). Thus, cells maintain a substantial free pool of myosin so that they can assemble BTFs wherever they are needed.
By harnessing such contractility regulators to promote localized assembly of myosin into the BTF form, cells can control their cortical properties to direct spatially regulated shape changes. This effect can be observed at the tail end of migrating cells, as well as in the classic equatorial stimulation model for cytokinesis. There are two groups of contractility regulators important for cytokinesis:the chromosomal passenger complex proteins (including INCENP, survivin, and aurora kinase), and central spindling (including kinesin-6 and MgcRacGAP) (Adams et al. 2000; Chen et al. 2007; Cooke et al. 1987; Li et al. 2008). MgcRacGAP is found in higher metazoans, but not in Dictyostelium (Mishima et al. 2002). The central spindlin complex is thought to activate rhoAin the overlying cortex and lead to the activation of ROCK kinase, which in turn phosphorylates myosin II on its regulatory light chain, promoting the 10S–6S transition. The subsequent assembly of myosin increases local cortical tension and contractility, and thus drives furrow ingression.
Myosin II mechanosensing
Myosin II exhibits enhanced binding to actin filaments under tension (Uyeda et al. 2011), and will accumulate to regions of high stress within the cell (Effler et al. 2006; Kee et al. 2012; Ren et al. 2009). This ability to detect and respond to forces is known as mechanosensation. This property has also been demonstrated in other myosins such as myosin I, V, and VI (Altman et al. 2004; Gebhardt et al. 2006; Laakso et al. 2008). There are several force-dependent cytoskeletal phenomena which, when taken together, can explain this behavior. First, actin filaments are highly allosteric, making them ideal mediators of cooperative interactions between actin-associated proteins along the polymer. This allostery allows point forces exerted on an actin filament to propagate subunit conformational changes locally (Galkin et al. 2012). Second, when myosin II proceeding along an actin filament is subjected to a resistive force, its duty ratio increases, causing it to lock onto the strained filament (Kovacs et al. 2007). The result of these phenomena is that tension on the actin filaments and/or force generated by a bound motor head will stretch the actin filaments, facilitating the binding of additional motor heads nearby (Tokuraku et al. 2009; Uyeda et al. 2011). This localized binding of motors on an actin filament then promotes further BTF assembly, forming a force-dependent positive feedback loop (Luo et al. 2012; Ren et al. 2009). A recent multi-scale model demonstrates quantitatively how myosin motor force-sensing and cooperativity and the BTF assembly mechanism are integrated to promote local mechanosensitive accumulation of myosin II (Luo et al. 2012).
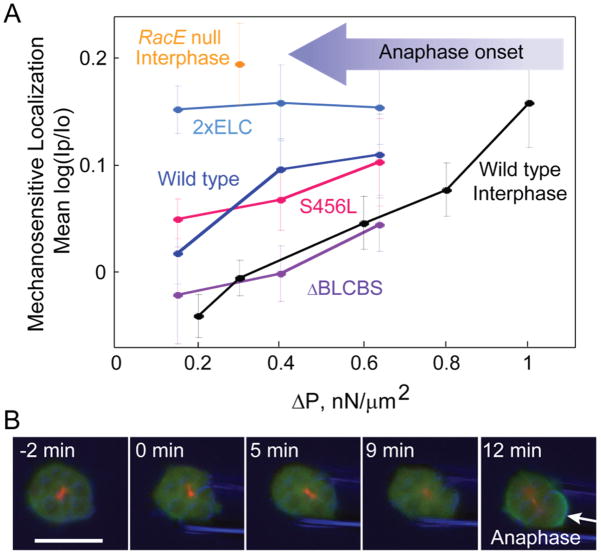
When sliding actin filaments, myosin motor proteins harness the energy of ATP hydrolysis (~100 pN•nm) to perform work over the ~8 nm step size of the myosin lever arm (Murphy et al. 2001). As a longer lever arm will increase the myosin II step size (consequently increasing the unloaded motor speed), conservation of energy dictates that the maximum force generated will be proportionately lower. Therefore, a longer lever arm stalls more easily under load, whereas a shorter lever arm will produce more force and be less sensitive to stalling. Stalling is thought to occur when a bound myosin head is in the isometric state between the pre-and the post-stroke configurations. Several lever arm mutants of Dictyostelium myosin II have been cloned and characterized, and they all retain full motor activity with the expected changes in step size and unloaded motor speed (Uyeda et al. 1996). Furthermore, when these mutant motors are expressed in cells lacking endogenous myosin II, they demonstrate the expected trends in mechanosensitive accumulation; all mutants show a linear relationship between accumulation and applied force, but the longer lever arm 2xELC mutant is more mechanosensitive at all pressures, and the short lever arm ΔBLCBS mutant is less mechanosensitive at all pressures (Fig. 2A). Additional support for the isometric stalling model is provided by the S456L uncoupler mutant, which has the same lever arm length as WT myosin II, but which has a defect in the ATPase region that reduces its step size and unloaded filament sliding velocity below the level of the short lever arm (ΔBLCBS) mutant (Murphy et al. 2001). If myosin’s mechanosensitive accumulation to sites of stress was dependent on its motility, the S456L mutant would be expected to have similar characteristics to ΔBLCBS. However, its mechanosensitive behavior is similar to WT myosin, indicating that lever arm length is the critical parameter, not motor speed or step size.
Figure 2. Myosin mechanosensation is dependent on the lever arm length and cell cycle phase (Effler et al. 2006; Luo et al. 2012; Ren et al. 2009).
A) Myosin localization to the pipette in dividing cells was measured under a range of aspiration pressures for various lever arm mutants, as well as for WT interphase cells. Upon anaphase onset (large arrow), the pressure regime (ΔP) required to trigger myosin II accumulation is shifted to the left. Mean log(Ip/Io) is the average log of the ratios of GFP-myosin II intensity (I) of the cortex inside the micropipette (Ip) to the opposite cortex (Io).
B) A continuously aspirated mitotic cell becomes mechanosensitive upon anaphase onset. The arrow identifies the mechanosensitive accumulation of myosin II. Green-GFP labeled myosin II, Red-RFP labeled tubulin, Blue-DIC.
While stalled myosin thus functions as an effective force sensor, other factors must also be present in order to drive the productive accumulation of myosin to sites of stress. A reservoir of soluble, assembly-competent myosin feeds the growth of stalled BTFs, and the long-lived actin crosslinker cortexillin I forms stable connections between actin filaments, conducting forces through the stressed network. Myosin also demonstrates hetero-cooperativity with cortexillin I through actin, amplifying the accumulation of both proteins; disruption of either protein abolishes the mechanosensitive accumulation of the other. This complex behavior allows the three proteins (actin, cortexillin I, and myosin II) to form a force-dependent feedback controller, which actively stiffens and contracts to counteract external stresses. The mechanosensitive response is independent of the spindle signaling pathway, but requires proper regulation of myosin bipolar thick filament assembly and regulatory light chain phosphorylation (Ren et al. 2009).
Third module: The control system that tunes the structural elements of the cytoskeleton
Interestingly, the mechanosensitive behavior of myosin II is heavily attenuated during interphase through the action of the small GTPase racE. If WT cells are aspirated at any stage of the cell cycle prior to the onset of anaphase, the mechanosensitive response is undetectable at low pressures (Fig. 2A), but upon the transition to anaphase myosin will begin to accumulate to the pipette (Fig. 2B). The exact mechanism of this attenuation is unknown, but racE is known to play a fundamental role in the regulation of actin crosslinker distribution and in the modulation of cleavage furrow ingression (Robinson and Spudich 2000; Zhang and Robinson 2005). By shifting cytoskeletal stresses onto alternate actin crosslinkers, such as dynacortin and enlazin, the effective force experienced by the mechanosensitive cortexillin I crosslinker would be diminished, thus suppressing mechanosensation. Indeed, cells lacking racE are highly mechanosensitive at all stages of the cell cycle (Fig. 2A), and also exhibit much lower cortical stiffness, with a decrease in the cortical localization of dynacortin and coronin (Ren et al. 2009; Robinson and Spudich 2000). This evidence suggests that a post-metaphase transition in the regulation of racE allows cells to harness the mechanosensitive abilities of myosin to create a force gradient, leading to furrow ingression and the successful completion of cytokinesis.
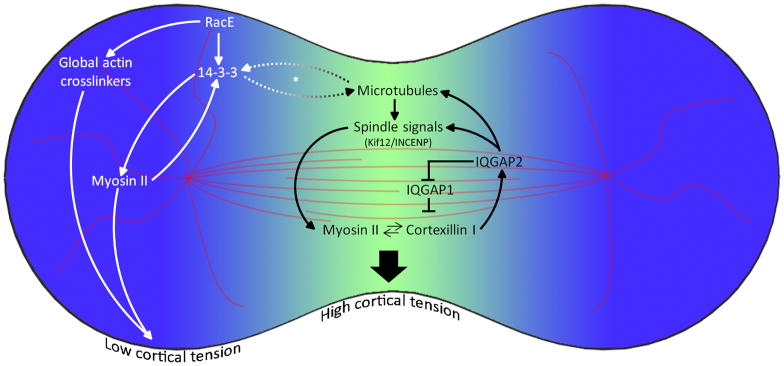
RacE also interacts with myosin II through the essential protein14-3-3 (Zhou et al. 2010) (Fig. 3). The 14-3-3 protein serves as an intermediary between microtubules, racE, and myosin, promoting myosin BTF remodeling and helping to maintain proper cortical tension under the regulation of racE. RacE is responsible for the cortical localization of 14-3-3, and 14-3-3 in turn directly associates with myosin to promote BTF turnover. 14-3-3 also tunes overall microtubule length and is required for normal microtubule-cortex interactions. 14-3-3’s localization to the polar cortex and its interaction with myosin, along with its ability to rescue the cytokinesis defects of racEnull cells, suggest that it is involved in establishing the tension gradient between the cleavage furrow and the poles (Zhou et al. 2010). Importantly, 14-3-3 proteins are known in other systems to play many roles in cell division such as modulating mitotic translation and central spindlin signaling (Douglas et al. 2010; Wilker et al. 2007).
Figure 3. Different regulatory regimes in the cleavage furrow and poles create a force imbalance that drives furrow ingression.
The myosin II-cortexillin I-IQGAP-kif12-INCENP control system regulates the equatorial cortex. In contrast, the microtubule-racE-14-3-3-myosin II pathway and global crosslinking proteins control the polar mechanics. Note: the dashed arrows and asterisk (*) denote that the interaction between 14-3-3 and microtubules has been characterized in interphase cells, but due to the sparse nature of astral microtubules in Dictyostelium cells, this interaction has not been fully characterized in mitotic cells. Data are compiled from Kee et al. [2012]; Reichl et al. [2008]; Robinson and Spudich [2000]; Surcel et al. [2010]; [Zhou et al. 2010].
Several proteins traditionally associated with the mitotic spindle also show accumulation at the cleavage furrow at later stages of cytokinesis, including the kinesin-6 protein kif12, inner centromeric protein (INCENP), and Aurora kinase (Chen et al. 2007; Li et al. 2008) (Fig. 3). Interestingly, kif12 and INCENP also demonstrate mechanosensitive accumulation to an aspirating micropipette, even when the spindle is ablated with nocodazole, a microtubule depolymerizing drug (Kee et al. 2012). This cortical localization of kif12 could involve a similar mechanism as the cortical localization of mammalian kinesin-6 (MKLP1), which localizes to the stem-body region of the midbody independently of its microtubule association (Hu et al. 2012). Extensive analysis of mutant Dictyosteliumstrains demonstrated that the cortical localization of kif12 is dependent on the central myosin II-cortexillin I mechanosensor. This ability of the mechanosensor to feed information about the mechanical state of the cell to upstream regulators is referred to as mechanotransduction, and in Dictyostelium is mediated through the protein IQGAP2. Dictyostelium has three IQGAP proteins, IQGAP1 (a.k.a. DGap1), IQGAP2 (a.k.a. GapA), and a more distantly related IQGAP3. IQGAP1 and IQGAP2 are known to bind to cortexillin I (Adachi et al. 1997; Faix et al. 2001; Mondal et al. 2010). The IQGAP1-cortexillin I complex suppresses mechanosensation, whereas IQGAP2 relieves this suppression as well as mediates mechanotransduction to kif12 and INCENP (Fig. 3). Aniqgap1/iqgap2double mutant cell preserves normal myosin-cortexillin mechanosensing, yet kif12 and INCENP no longer accumulate to the cleavage furrow or to an aspirating pipette (Kee et al. 2012). These double mutant cells still accumulate myosin to the cleavage furrow, but have reduced cytokinesis fidelity. However, when the cells experience additional mechanical stress, the lack of mechanotransduction compromises their ability to accumulate myosin to the cleavage furrow as compared to WT cells. Thus, mechanosensation and mechanotransduction combine to tune the level of myosin accumulation at the cleavage furrow under a wide range of force regimes. This allows cells precise, robust control over their cortical mechanics, ensuring cytokinesis fidelity under a variety of conditions.
Connecting the modules into a robust morphological engine
The three modules described above are the drivers of cell shape change. The cortical network of cross-linked actin defines the deformability of the cell, myosin II alters local stiffness and tension and accumulates to sites of stress, and the control system tunes cortical mechanics and directs myosin II localization and mechanosensitivity to carry out various cellular programs. During cytokinesis, the output of these modules is furrow ingression. Many descriptions of cytokinesis have explained furrow ingression as being driven by myosin contractility through a sarcomeric-like mechanism involving a “purse-string” of actin and myosin II filaments. However, we believe that it is more accurate to describe furrow ingression as being driven by multiple force-generating systems where cortical tension is a prominent one, and myosin II’s function is to contributecontractile stress and an increase in cortical tension at the furrow (Poirier et al. 2012; Zhang and Robinson 2005). Because cortical stresses are not only driven by myosin but also depend on factors such as actin polymerization, actin crosslinkers, membrane anchoring, surface curvature, and osmotic pressure (which must be resisted by the cortex), the cell can achieve force differentials in many different ways. This principle explains the ability of cells lacking myosin II to perform cytokinesis (Neujahr et al. 1997; Poirier et al. 2012; Zang and Spudich 1998; Zhang and Robinson 2005), as well as the ability of certain mammalian cells to perform cytokinesis in the presence of blebbistatin, a myosin inhibitor (Kanada et al. 2005; Straight et al. 2003). It also accounts for why myosin II mechanochemistry is not rate-limiting for furrow ingression and why in Dictyostelium, furrow ingression rates are inversely related to the length of the myosin II lever arm (if filament sliding were the primary mechanism for furrow constriction, the longer lever arm mutants would be expected to ingress faster) (Kee et al. 2012; Reichl et al. 2008). This fundamental principle has also been implicated in mammalian cells, where a mutant myosin II that has mechanosensitivity but no actin-filament sliding ability is able to support cytokinesis in cultured cells as well as during embryonic development (Ma et al. 2012).
The various modules, connected through feedback loops, ensure that the appropriate magnitude of mechanical stresses are distributed around the cortex to generate shape changes, which allows furrow ingression to happen under a wide range of contexts. This robust system allows cells to divide with and without adhesion, in the absence of genes which contribute to cortical mechanics, and in the presence of external stresses such as those experienced by cells in tissues. Inputs that emanate from the spindle trigger initial myosin II accumulation and symmetry breaking, and the force-sensitive feedback loop then tunes the accumulation of myosin II as cytokinesis proceeds. Thus, we can now begin to understand how the same cellular machinery can generate sufficient forces for furrow ingression under diverse mechanical contexts.
Conclusions
Even after decades of research, the process of cytokinesis continues to amaze and puzzle biologists with its flexibility and complex behaviors. Under normal conditions, cytokinesis appears to be a vertically organized process. Signals from the mitotic spindle lead to the asymmetrical distribution of cytoskeletal proteins around the cortex, which in turn shift the force distribution around the cell, causing constriction at the furrow and protrusion at the poles. However, when cells are stressed mechanically, this seemingly hierarchal process automatically compensates by drawing upon a mechanical feedback system to adjust the force-generating system to complete cytokinesis successfully. This adaptability allows cells to achieve the same cell shape changes in widely divergent mechanical environments and might explain how some asymmetric cell divisions can occur in the apparent absence of the spindle (Cabernard et al. 2010; Ou et al. 2010). Cells may also automatically “wire around” proteins that play central roles in normal cell division when these components fail to perform their roles, with multiple feedback loops from downstream proteins ensuring that the cell continues to carry out the necessary shape changes for proper division. Cytokinesis is thus a highly robust cellular program, which can use combinatorial synthesis to achieve the same end result through several different cellular modules, many of which are not immediately apparent in unstressed cells.
The feedback control system may account for much of the historical difficulty in determining which proteins are strictly essential for cytokinesis, and which appear to play a role but are not absolutely necessary. It also explains why certain features that are essential, such as the mitotic spindle, are dispensable after a certain point in the division process—once these components have signaled to downstream modules, force-dependent feedback loops ensure that cytokinesis proceeds smoothly without further signaling. Similarly, a feedback mechanism may also explain why furrow formation can be induced by monopolar spindles (Hu et al. 2008). Finally, by moving beyond the simplistic designators of “essential” or “non-essential”, placing genes within related modules should greatly simplify the process of reconciling data from different model systems. Indeed, what at first may appear to be a highly organism-specific mechanism is likely to be simply the result of directing the same cellular modules in a novel way to achieve the necessary results—dependable, consistent division under the environmental conditions particular to that cell.
Two areas of this modular view of cytokinesis remain unclear: the mechanism of symmetry breaking which initiates the process, and how stresses are propagated through the cortical cytoskeletal network to the mitotic spindle, forming the mechanical linkage that completes the mechanotransduction circuit. These two questions are intertwined, as the presence of feedback loops between the cortex and the spindle suggests that the spindle itself might not be the obligate “top-down” driver of symmetry breaking. This concept is also implied by the “long axis rule”; the fact that the spindle elongates along the long axis of the cell strongly suggests that mechanical elements influence division plane specification (Gibson et al. 2011; Minc et al. 2011). Thus, mechanical feedback and stress propagation through the cortex to the spindle may be critical players in this process. Longer term, determining how the mechanical modules described in this review (cross-linked cortical actin, myosin II, and the control system) operate in the context of complex environments, such as tissues, will become particularly important. Tissues offer a radically different mechanical environment from single cells, including additional adhesions to the substrate and to other cells, a more complex mechanical stress environment, and the possibility of mechanical heterogeneity among otherwise identical cells, particularly within tumors. Determining how these features interact with the core modules described in this review will have significant impact on cytokinesis research, as well as implications for health-related biology.
Acknowledgments
We thank the members of the Robinson lab for discussions and providing feedback on the manuscript. Our work is supported by the NIH (GM066817 and GM86704).
References
- Adachi H, Takahashi Y, Hasebe T, Shirouzu M, Yokoyama S, Sutoh K. Dictyostelium IQGAP-related protein specifically involved in the completion of cytokinesis. J Cell Biol. 1997;137(4):891–898. doi: 10.1083/jcb.137.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10(17):1075–8. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Altman D, Sweeney HL, Spudich JA. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–749. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- Breckenridge MT, Dulyaninova NG, Egelhoff TT. Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells. Mol Biol Cell. 2009;20(1):338–47. doi: 10.1091/mbc.E08-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard C, Prehoda KE, Doe CQ. A spindle-independent cleavage furrow positioning pathway. Nature. 2010;467(7311):91–4. doi: 10.1038/nature09334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lakshmikanth GS, Spudich JA, DeLozanne A. The localization of inner centromeric protein (INCENP) at the cleavage furrow is dependent on Kif12 and involves interactions of the N terminus of INCENP with the actin cytoskeleton. Mol Biol Cell. 2007;18(9):3366–3374. doi: 10.1091/mbc.E06-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Paluch E. Mechanics and regulation of cell shape during the cell cycle. Results Probl Cell Differ. 2011;53:31–73. doi: 10.1007/978-3-642-19065-0_3. [DOI] [PubMed] [Google Scholar]
- Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105(5):2053–67. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Ting-Beall HP, Hockmuth RM, Sheetz MP, Titus MA. Myosin I contributes to the generation of resting cortical tension. Biophys J. 1999;77:1168–1176. doi: 10.1016/s0006-3495(99)76968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derganc J, Božic B, Svetina S, Źekš B. Stability analysis of micropipette aspiration of neutrophils. Biophys J. 2000;79:153–162. doi: 10.1016/S0006-3495(00)76280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas ME, Davies T, Joseph N, Mishima M. Aurora B and 14-3-3 coordinately regulate clustering of centralspindlin during cytokinesis. Curr Biol. 2010;20:927–933. doi: 10.1016/j.cub.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler JC, Kee Y-S, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile protein redistribution control cell shape. Curr Biol. 2006;16(19):1962–1967. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff TT, Lee RJ, Spudich JA. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993;75:363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- Egelhoff TT, Naismith TV, Brozovich FV. Myosin-based cortical tension in Dictyostelium resolved into heavy and light chain-regulated components. J Muscle Res Cell Motil. 1996;17(2):269–74. doi: 10.1007/BF00124248. [DOI] [PubMed] [Google Scholar]
- Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix J, Weber I, Mintert U, Köhler J, Lottspeich F, Marriott G. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 2001;20(14):3705–3715. doi: 10.1093/emboj/20.14.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feneberg W, Westphal M, Sackmann E. Dictyostelium cells’ cytoplasm as an active viscoplastic body. Eur Biophys J. 2001;30:284–294. doi: 10.1007/s002490100135. [DOI] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, Egelman EH. Actin filaments as tension sensors. Curr Biol. 2012;22(3):R96–101. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz D. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA. 2006;103(6):1762–1767. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- Gebhardt JC, Clemen AE, Jaud J, Rief M. Myosin-V is a mechanical ratchet. Proc Natl Acad Sci USA. 2006;103(23):8680–8685. doi: 10.1073/pnas.0510191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson WT, Veldhuis JH, Rubinstein B, Cartwright HN, Perrimon N, Brodland GW, Nagpal R, Gibson MC. Control of the mitotic cleavage plane by local epithelial topology. Cell. 2011;144(3):427–38. doi: 10.1016/j.cell.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard KD, Chaney C, Delannoy M, Kuo SC, Robinson DN. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004;23(7):1536–46. doi: 10.1038/sj.emboj.7600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard KD, Kuo SC, Robinson DN. Dictyostelium myosin II mechanochemistry promotes active behavior of the cortex on long time scales. Proc Natl Acad Sci U S A. 2006;103(7):2103–8. doi: 10.1073/pnas.0508819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Zhou M, Wang Y-l. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Cell division without mitotic apparatus in sea urchin eggs. Exp Cell Res. 1956;11(3):630–6. doi: 10.1016/0014-4827(56)90171-9. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of sea urchin eggs II. Changes in mechanical properties from fertilization to cleavage. Exp Cell Res. 1963;32:76–88. doi: 10.1016/0014-4827(63)90070-3. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of the cortex before and during cleavage. Ann NY Acad Sci. 1990;582:22–30. doi: 10.1111/j.1749-6632.1990.tb21664.x. [DOI] [PubMed] [Google Scholar]
- Hoffman BD, Massiera G, Van Citters KM, Crocker JC. The consensus mechanics of cultured mammalian cells. Proc Natl Acad Sci USA. 2006;103(27):10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CK, Coughlin M, Field CM, Mitchison TJ. Cell polarization during monopolar cytokinesis. J Cell Biol. 2008;181(2):195–202. doi: 10.1083/jcb.200711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CK, Coughlin M, Mitchison TJ. Midbody assembly and its regulation during cytokinesis. Mol Biol Cell. 2012;23(6):1024–34. doi: 10.1091/mbc.E11-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isambert H, Venier P, Maggs AC, Fattoum A, Kassab R, Pantaloni D, Carlier MF. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J Biol Chem. 1995;270(19):11437–44. doi: 10.1074/jbc.270.19.11437. [DOI] [PubMed] [Google Scholar]
- Kamasaki T, Osumi M, Mabuchi I. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J Cell Biol. 2007;178(5):765–771. doi: 10.1083/jcb.200612018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanada M, Nagasaki A, Uyeda TQ. Adhesion-dependent and contractile ring-independent equatorial furrowing during cytokinesis in mammalian cells. Mol Biol Cell. 2005;16(8):3865–72. doi: 10.1091/mbc.E05-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y-S, Ren Y, Dorfman D, Iijima M, Firtel RA, Iglesias PA, Robinson DN. A mechanosensory system governs myosin II accumulation in dividing cells. Mol Biol Cell. 2012;23(8):1510–1523. doi: 10.1091/mbc.E11-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA. 2007;104(24):9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Umeda T, Nakamura K, Higuchi T, Kimura A. A High-Resolution Shape Fitting and Simulation Demonstrated Equatorial Cell Surface Softening during Cytokinesis and Its Promotive Role in Cytokinesis. PLoS ONE. 2012;7(2):e31607. doi: 10.1371/journal.pone.0031607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso JM, Lewis JH, Shuman H, Ostap EM. Myosin I can act as a molecular force sensor. Science. 2008;321(5885):133–136. doi: 10.1126/science.1159419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen Q, Kaller M, Nellen W, Graf R, De Lozanne A. Dictyostelium Aurora kinase has properties of both Aurora A and Aurora B kinases. Eukaryot Cell. 2008;7(5):894–905. doi: 10.1128/EC.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Licate LS, Warrick HM, Spudich JA, Egelhoff TT. Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biology. 2002;3:19. doi: 10.1186/1471-2121-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Mohan K, Srivastava V, Ren Y, Iglesias PA, Robinson DN. Understanding the cooperative interaction between myosin II and actin crosslinkers mediated by actin filaments during mechanosensation. Biophys J. 2012;102(2):238–247. doi: 10.1016/j.bpj.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Robinson DN. The role of the actin cytoskeleton in mechanosensation. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues: Mechanosensitivity and Mechanotransduction. New York: Springer-Verlag; 2011. pp. 25–65. [Google Scholar]
- Ma X, Kovacs M, Conti MA, Wang A, Zhang Y, Sellers JR, Adelstein RS. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc Natl Acad Sci U S A. 2012;109(12):4509–14. doi: 10.1073/pnas.1116268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh FC, Kas J, Janmey PA. Elasticity of semiflexible biopolymer networks. Phys Rev Lett. 1995;75:4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- Mahajan RK, Pardee JD. Assembly mechanism of Dictyostelium myosin II: Regulation by K+, Mg2+, and actin filaments. Biochemistry. 1996;35:15504–15514. doi: 10.1021/bi9618981. [DOI] [PubMed] [Google Scholar]
- Marion S, Guillen N, Bacri J-C, Wilhelm C. Acto-myosin cytoskeleton dependent viscosity and shear-thinning behavior of the amoeba cytoplasm. Eur Biophys J. 2005;34:262–272. doi: 10.1007/s00249-004-0449-5. [DOI] [PubMed] [Google Scholar]
- Maupin P, Pollard TD. Arrangement of actin filaments and myosin-like filaments in the contractile ring and of actin-like filaments in the mitotic spindle of dividing HeLa cells. J Ultrastruct Res. 1986;94:92–103. doi: 10.1016/0889-1605(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Merkel R, Simson R, Simson DA, Hohenadl M, Boulbitch A, Wallraff E, Sackmann E. A micromechanic study of cell polarity and plasma membrane cell body coupling in Dictyostelium. Biophys J. 2000;79:707–719. doi: 10.1016/S0006-3495(00)76329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc N, Burgess D, Chang F. Influence of cell geometry on division-plane positioning. Cell. 2011;144(3):414–26. doi: 10.1016/j.cell.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2(1):41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- Mitchison JM, Swann MM. The mechanical properties of the cell surface: III. The sea-urchin egg from fertilization to cleavage. J Exp Biol. 1955;32:734–750. [Google Scholar]
- Mondal S, Burgute B, Rieger D, Muller R, Rivero F, Faix J, Schleicher M, Noegel AA. Regulation of the actin cytoskeleton by an interaction of IQGAP related protein GAPA with filamin and cortexillin I. PLoS ONE. 2010;5(11):e15440. doi: 10.1371/journal.pone.0015440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhina S, Wang YL, Murata-Hori M. alpha-Actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev Cell. 2007;13(4):554–565. doi: 10.1016/j.devcel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, Rock RS, Spudich JA. A myosin II mutation uncouples ATPase activity from motility and shortens step size. Nat Cell Biol. 2001;3:311–315. doi: 10.1038/35060110. [DOI] [PubMed] [Google Scholar]
- Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Neujahr R, Heizer C, Gerisch G. Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: redistribution of the nuclei, re-arrangement of the actin system and formation of the cleavage furrow. J Cell Sci. 1997;110 ( Pt 2):123–37. doi: 10.1242/jcs.110.2.123. [DOI] [PubMed] [Google Scholar]
- Octtaviani E, Effler JC, Robinson DN. Enlazin, a natural fusion of two classes of canonical cytoskeletal proteins, contributes to cytokinesis dynamics. Mol Biol Cell. 2006;17(12):5275–5286. doi: 10.1091/mbc.E06-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow BD, Chen P, Chisholm RL. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. J Cell Biol. 1994;127(6 Pt 2):1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G, Stuurman N, D’Ambrosio M, Vale RD. Polarized myosin produces unequal-size daughters during asymmetric cell division. Science. 2010;330(6004):677–80. doi: 10.1126/science.1196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341(6242):549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Pesen D, Hoh JH. Micromechanical architecture of the endothelial cell cortex. Biophys J. 2005;88(1):670–9. doi: 10.1529/biophysj.104.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier CC, Ng WP, Robinson DN, Iglesias PA. Deconvolution of the cellular force-generating subsystems that govern cytokinesis furrow ingression. PLoS Comput Biol. 2012;8(4):e1002467. doi: 10.1371/journal.pcbi.1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. Geometrical relations of the cleavage stimulus in constricted sand dollar eggs. J Exp Zool. 1964;155:225–230. doi: 10.1002/jez.1401550209. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Geometrical relations of the cleavage stimulus in invertebrate eggs. J Theor Biol. 1965;9:51–66. doi: 10.1016/0022-5193(65)90056-1. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Cell division: Direct measurement of maximum tension exerted by furrow of echinoderm eggs. Science. 1967;156:1241–1243. doi: 10.1126/science.156.3779.1241. [DOI] [PubMed] [Google Scholar]
- Rappaport R. In: Cytokinesis in Animal Cells. Barlow PW, Bard JBL, Green PB, Kirk DL, editors. Cambridge: Cambridge University Press; 1996. p. 386. [Google Scholar]
- Rappaport R. Absence of furrowing activity following regional cortical tension reduction in sand dollar blastomere and fertilized egg fragment surfaces. Development, Growth & Differentiation. 1999;41(4):441–447. doi: 10.1046/j.1440-169x.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- Rappaport R, Ebstein RP. Duration of stimulus and latent periods preceding furrow formation in sand dollar eggs. J Exp Zool. 1965;158:373–382. doi: 10.1002/jez.1401580311. [DOI] [PubMed] [Google Scholar]
- Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, Divi S, Iglesias PA, Kuo SC, Robinson DN. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr Biol. 2008;18(7):471–480. doi: 10.1016/j.cub.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, Rock RS, Robinson DN. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol. 2009;19(17):1421–1428. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN, Cavet G, Warrick HM, Spudich JA. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biol. 2002;3:4. doi: 10.1186/1471-2121-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN, Kee Y-S, Luo T, Surcel A. Understanding how dividing cells change shape. In: Egelman EH, editor. Comprehensive Biophysics. Oxford: Academic Press; 2012. pp. 48–72. [Google Scholar]
- Robinson DN, Spudich JA. Dynacortin, a genetic link between equatorial contractility and global shape control discovered by library complementation of a Dictyostelium discoideum cytokinesis mutant. J Cell Biol. 2000;150(4):823–838. doi: 10.1083/jcb.150.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry JH, Moores SL, Ryan S, Zang J-H, Spudich JA. Myosin heavy chain phosphorylation sites regulate myosin localization during cytokinesis in live cells. Mol Biol Cell. 1997;8:2647–2657. doi: 10.1091/mbc.8.12.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzinski J, Biro M, Oswald A, Tinevez JY, Salbreux G, Paluch E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature. 2011;476(7361):462–6. doi: 10.1038/nature10286. [DOI] [PubMed] [Google Scholar]
- Simson R, Wallraff E, Faix J, Niewöhner J, Gerisch G, Sackmann E. Membrane bending modulus and adhesion energy of wild-type and mutant cells of Dictyostelium lacking talin or cortexillins. Biophys J. 1998;74:514–522. doi: 10.1016/S0006-3495(98)77808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinard JH, Stafford WF, Pollard TD. The mechanism of assembly of Acanthamoeba myosin-II minifilaments: minifilaments assemble by three successive dimerization steps. J Cell Biol. 1989;109(4 Pt 1):1537–47. doi: 10.1083/jcb.109.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA. The myosin swinging cross-bridge model. Nat Rev Mol Cell Biol. 2001;2(5):387–92. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2(2):138–45. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Surcel A, Kee Y-S, Luo T, Robinson DN. Cytokinesis through biochemical-mechanical feedback loops. Semin Cell Dev Biol. 2010;21:866–873. doi: 10.1016/j.semcdb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuraku K, Kurogi R, Toya R, Uyeda TQP. Novel mode of cooperative binding between myosin and Mg2+-actin filaments in the presence of low concentrations of ATP. J Mol Biol. 2009;386(1):149–162. doi: 10.1016/j.jmb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Uyeda TQ, Abramson PD, Spudich JA. The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc Natl Acad Sci USA. 1996;93(9):4459–4464. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda TQ, Iwadate Y, Umeki N, Nagasaki A, Yumura S. Stretching actin filaments within cells enhances their affinity for the myosin II motor domain. PLoS ONE. 2011;6(10):e26200. doi: 10.1371/journal.pone.0026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Steimle PA, Ren Y, Ross CA, Robinson DN, Egelhoff TT, Sesaki H, Iijima M. Dictyostelium huntingtin controls chemotaxis and cytokinesis through regulation of myosin II phosphorylation. Mol Biol Cell. 2011 doi: 10.1091/mbc.E10-11-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EW, van Vugt MA, Artim SA, Huang PH, Petersen CP, Reinhardt HC, Feng Y, Sharp PA, Sonenberg N, White FM, et al. 14-3-3sigma controls mitotic translation to facilitate cytokinesis. Nature. 2007;446(7133):329–332. doi: 10.1038/nature05584. [DOI] [PubMed] [Google Scholar]
- Wolpert L. The mechanical properties of the membrane of the sea urchin egg during cleavage. Exp Cell Res. 1966;41:385–396. doi: 10.1016/s0014-4827(66)80146-5. [DOI] [PubMed] [Google Scholar]
- Yang L, Effler JC, Kutscher BL, Sullivan SP, Robinson DN, Iglesias PA. Modeling cellular deformations using the level set formalism. BMC Syst Biol. 2008;2:68. doi: 10.1186/1752-0509-2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M, Dan K. Tension at the surface of the dividing sea-urchin egg. J Exp Biol. 1972;57:575–587. doi: 10.1242/jeb.57.3.575. [DOI] [PubMed] [Google Scholar]
- Yumura S, Yoshida M, Betapudi V, Licate LS, Iwadate Y, Nagasaki A, Uyeda TQ, Egelhoff TT. Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium. Mol Biol Cell. 2005;16(9):4256–66. doi: 10.1091/mbc.E05-03-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang J-H, Spudich JA. Myosin II localization during cytokinesis occurs by a mechanism that does not require its motor domain. Proc Natl Acad Sci USA. 1998;95:13652–13657. doi: 10.1073/pnas.95.23.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc Natl Acad Sci USA. 2005;102(20):7186–91. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kee Y-S, Poirier CC, Jelinek C, Osborne J, Divi S, Surcel A, Will ME, Eggert US, Müller-Taubenberger A, et al. 14-3-3 Coordinates Microtubules, Rac, and Myosin II to Control Cell Mechanics and Cytokinesis. Current Biology : CB. 2010;20(21):1881–1889. doi: 10.1016/j.cub.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]