Abstract
Recently, the understanding of dynamic cellular changes that occur in vivo has advanced significantly, both at the extracellular and intracellular levels. These changes might fluctuate with daily, circadian, weekly, or monthly intervals, and the approaches used to understand these changing conditions in vitro should parallel in vivo studies. In addition, the in vitro milieu should be optimized and better defined, so that artefacts due to in vitro culture systems would not pose dangers for the proper interpretation of results. In this article, we discuss some of these issues and propose solutions.
Interpreting in vitro results: messages lost in translation
The past 40 years have seen a great number of breakthrough discoveries using in vitro cellular systems from both normal and transformed tissues. One great contribution to these in vitro approaches has come from the discovery of the critical and beneficial roles of growth factors and hormones in culture media and the supplementation of media with fetal sera (often of bovine origin). The advantageous development of culture media with compositions resembling “normal” or “physiologic” conditions has undoubtedly allowed for important discoveries in cell biology, oncology, endocrinology, immunology, and neurobiology [1]. Today, in vitro cell cultures are a broadly used, relatively low-cost approach to research, are ethically preferable to other methods and significantly reduce the need for experimental animals.
Significant advancements have been achieved in understanding the dynamic changes that occur in vivo, both at the extracellular and intracellular levels. These dynamics can change at daily, circadian, weekly, or monthly intervals, yet they appear to follow defined temporal patterns [2]. This greater understanding of cellular dynamics mandate that if in vitro approaches are to be used to emulate in vivo functions, the contemporary understanding of the continuously changing in vivo milieu must be adapted to in vitro approaches[2].
Numerous aspects and artefacts of in vitro culture systems represent a “clear and present danger” to the insightful interpretation of results, as well as to an accurate determination of their relevance, and some of these issues are discussed below.
Culture media
Culture media are generally supplemented with glucose, amino acids, and lipids. Looking at the general compositions of the most commonly used media (i.e. RPMI, DMEM, IMDM), the glucose concentration ranges from 2- to 4-fold higher than the physiologic plasma concentration: in RPMI it is 200mg/dl, DMEM is 450mg/dl, and IMDM is 450mg/dl, compared with a physiological range in plasma of 60–100mg/dl [1, 3, 4]. Amino acid concentrations differ even more dramatically, ranging from 200- to 500-fold higher than normal plasma levels in humans and mice [1, 3, 4]*. Such is also the case with lipids, in particular the levels of cholesterol, phospholipids, and triglycerides [1, 3, 4]*. Supra-physiologic concentrations will profoundly impact the results and cellular responses when compared with normal physiology. Glucose, amino acids, and lipids activate a series of intracellular responses such as the mammalian target of rapamycin (mTOR) pathway, the nutrient energy sensing systems (ie. AMPK, SIRT1), and nuclear receptors such as the PPARs [5]. Given a series of reports showing that these systems can profoundly affect cell growth and survival as well as having dynamic and oscillatory activities [6], it must be considered that constant supra-physiologic concentrations of these nutrients in culture media will influence the experimental results and their interpretation in light of normal physiology.
Culture sera
The addition of serum to culture media is often crucial for both normal and transformed or immortalized cells. The enormous value of this supplement is undisputed, yet it carries implications that are rarely pondered or questioned. Bovine serum derived from foetal calves (FBS) has a very high concentration of placental hormones including prolactin, chorionic gonadotropin, growth hormone, leptin, oestrogen, progesterone, and cortisol, among others [1, 2]. All of these factors, even when diluted to 10% of their original concentrations into the culture media, remain at such high levels to practically reproduce an in vitro environment of pregnancy rather than a condition of normalcy. Obviously, these conditions affect cells at multiple levels. Consequently, this key aspect has sometimes been dealt with by using autologous sera, which may nonetheless still be used at supra-physiologic concentrations. Finally, little consideration has been given to the possibility that culture media components themselves may have hormonal activity. For example, phenol red, which bears a structural resemblance to some nonsteroidal estrogens and is used ubiquitously as a pH indicator in tissue culture media, has significant estrogenic activity at the concentrations (15–45 mM) typically found in tissue culture media [7].
Incubators
Commonly, cell incubators have an internally controlled and static atmosphere that is supplemented with gases such as carbon dioxide (CO2; 5%) and atmospheric air. CO2 supplementation is necessary to maintain a physiologic pH in the culture media because of its buffering capacity (carbonate/bicarbonate buffering system; HCO3−/H2CO3) [1, 2, 8]*. The pressure of O2 is basically at equilibrium within the atmosphere and is dissolved to approximately 160 mm/Hg in culture media (about 20% in the air). However, both CO2 and O2 concentrations in the incubator chambers do not change dynamically, as they do in the blood stream (i.e., the arterial versus venous blood), but remain static. Although O2 pressure in the atmosphere is at 160 mm/Hg, when at equilibrium in the culture media the concentration of O2 in cultures becomes non physiologic [1, 2, 8]*. Indeed, in normal blood and peripheral tissues, the concentration of O2 is approximately 100 mm/Hg (about 13%) in the arterial blood and approximately 40 mm/Hg (about 8%) in venous blood [8]. These substantial differences in the concentration of O2 between incubators and normal blood (160 mm/Hg versus 40–100 mm/Hg, respectively) are capable of modifying the respiratory and glucose/fatty acid oxidative processes and mitochondrial respiration in a very powerful manner [5]. Although CO2 may be less influential on in vitro studies because the arterial concentration of this gas is approximately 40 mm/Hg (as in the incubators at 5%), and its level in the venous blood averages 46 mm/Hg (about 8%), the above considerations suggest that what is observed in cells kept in culture can differ significantly from what occurs in the whole organism.
Dynamic changes in the extracellular milieu
Culture plates do not provide the dynamic changes in temperature, nutrients, hormones, and growth factors that are present in vivo. Because it is generally assumed that the internal human body temperature is 37°C, tissue culture incubators are set to this constant temperature. However, although the core body temperature is maintained constant, dynamic changes still occur due to the external temperature (of the environment), and owing to variations between vital organs (truncular tissues) and the periphery (i.e. limbs), where the temperature is generally 2–3°C lower [9]. Another critical variation in the circadian body temperature takes place during the sleep–wake cycle, with significantly lower temperatures during the period of sleep compared with the period of wakefulness. These subtle differences in body temperature have a profound impact on cellular and tissue metabolism and functions [9]. It must be reiterated that these dynamic changes cannot be excluded as possible factors affecting in vitro cellular responses. Like body temperature, variations in nutrients exist in the whole body, as do hormonal fluctuations that can occur hourly, intradiurnally, or through circadian cycles (e.g., cortisol, leptin, prolactin, GH). These dynamic changes are not present within in vitro cultures and cannot be reproduced in these systems.
Dynamic changes in the intracellular milieu
The use of immortalized or transformed cells has provided unique possibilities in the molecular dissection of intracellular mechanisms that determine the different cellular responses. Non-immortalized cell lines from normal tissues and primary cultures provide a more physiologic condition to study the processes leading to intracellular signalling. Immortalized cell lines or transformed cells have high basal levels of nutrient sensing factors such as mTOR and AMPK, which do not reflect normal physiology [5]. Conversely, non-immortalized cells have several “fuel sensing systems” that are sensitive to changes in the extracellular and intracellular milieu [5]. The switch in fuel utilization is common and can profoundly affect metabolic responses depending on the intracellular milieu (i.e., glycolysis to fatty-acid oxidation). For example, in both hypothalamic and immune responses, the cyclic and dynamic changes in intracellular activities of multiple enzymes and kinases can differentially affect outcomes and experimental results.
In vitrobias in immunology and neurobiology
Two fields of study, immunology and neurobiology, may be particularly affected by a reliance on in vitro experimentation. Both fields study complex systems that are difficult, or impossible, to replicate in vitro, but for that reason it is even more important to consider biases that may be introduced during in vitro experiments and the consequence of these bias on the models predicted. In addition, these two fields of investigations have been chosen because they display the unique characteristics of utilizing generally primary non-immortalized cell cultures which are more sensitive to possible artificia conditioning of the in vitro systems.
“Ninety-six-well plate immunology” has been instrumental in shedding light on fundamental mechanisms of immune cell function including antigen recognition, cytokine secretion, and immune tolerance, to name just a few. A vast majority of in vitro data in immunology comes from cultures in the presence of bovine sera, as discussed above. In addition, cell isolation techniques based on bead separation or flow cytometry (FACS) employ antibodies that bind to cell surface antigens [10–11]. Antibody binding to cells significantly affects cell signalling and immune responses. In the case of positive selection of cells via magnetic beads or flow cytometers, the binding of antibodies to the cell surface followed by passage through columns or FACS-sorters with high levels of pressure (60 psi, pounds per square inch; 15 psi = 1 atmosphere, therefore 4 Atm), speed (90 km/h), electric shock (varying from 2000–4000 V), and laser lights at various wavelengths (i.e. 488nm, 640nm, and 405nm) can significantly alter the intracellular signalling events that may be under investigation [10–11].
Neurobiological experimental approaches also utilize isolated cells, immortalized cell lines, and organotypic cultures [12]. The pitfalls described above regarding immunobiological approaches apply to these techniques as well [12]. Over the past 50 years, important insights have been gained into neuronal functions through the use of slice electrophysiology. This tool, which remains widely used and respected by the field, hinges on decapitation of animals followed by labor-intensive and time-sensitive preparation of slices in a an artificial cerebrospinal fluid as a medium to maintain “a living state” [12]. After hours of preparation subsequent to decapitation, electrical recordings are made from the slices and inferences are drawn regarding neuronal characteristics of synapses, circuits, and overall brain functions. Undoubtedly, this tool has generated extremely useful information on fine parameters of neuronal functions. By now, very few laboratories rely exclusively on slice electrophysiology, although numerous high impact publications can still be found revolving around this approach. However, the extent to which that information is transferable to the better understanding of in vivo functioning of healthy and diseased brain remains unknown.
On the other side of the spectrum of contemporary neurobiological tools are recently developed techniques for the functional imaging of the intact human brain. These remarkable approaches suggest that there may come a time when rudimentary and ambiguous techniques (e.g., slice electrophysiology or electron microscopy) for the examination of the central nervous system become obsolete in the quest to understand the human brain and mind. Until then, however, much needs to be improved regarding functional imaging. Despite efforts to suggest otherwise, all of these tools provide descriptive information that can in no way explain mechanism, circuit involvement, or aetiology of altered function. These different techniques, such as fMRI and PET, rely on detecting specific molecular shifts as an indication of altered neuronal function in a given area. The fact that these arbitrary measurements and changes can be derived in a precise area during a task compared to other regions does not confirm the specific involvement of that region to that task. Beyond the fact that no experimental data exists to directly connect specific neuronal activity to the altered signal in any of these approaches, the complicated and cumbersome nature of data analyses makes the current imaging techniques more like art than an objective scientific tool. Of course, these methods have the potential to revolutionize human neurobiology as they improve and evolve into a quantifiable descriptive tool but one that can test the role of various brain regions and neuronal populations in brain functions.
Concluding remarks
In vitro approaches have limitations that are often underestimated or not considered. The scientific community should be well aware of these aspects when interpreting data and should place a greater emphasis on developing in vitro new approaches that take into consideration the dynamic environmental and intracellular processes that are typical characteristics of normal physiology.
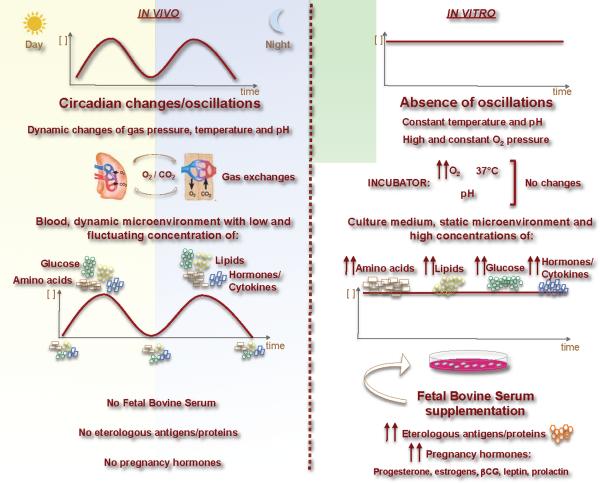
Figure 1. Schematic representation of the main differences between in vivo and in vitro systems.
Typically, in vitro systems are static and not dynamic like in vivo systems. This phenomenon occurs for amino acids, glucose and lipid concentrations; hormones and cytokines; pH and temperature. These differences can dramatically affect experimental results that are often underestimated.
Agknowledgements
The authors with to thank Fortunata Carbone for artwork. G.M. is supported by grants from the EU Ideas Programme, ERC-Starting Independent Grant “LeptinMS” n. 202579, Telethon-JDRF Grant n. GJT08004 and FISM Grant n. 2009/R/26. T.L.H. is supported by the NIH Director's Pioneer Award n. DP1OD006850 and A.L.C is supported by the NIH Grants n. AR53239 and n. AI095921.
Footnotes
References
- 1.Freshney RI. Culture of Animal Cells – A Manual of Basic Technique. 3rd edition Wiley-Liss; New York: 1994. [Google Scholar]
- 2.Huang W, et al. Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 2011;121:2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tcherkas YV, et al. Analysis of amino acids in human serum by isocratic reversed-phase high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A. 2001;913:303–308. doi: 10.1016/s0021-9673(00)01206-1. [DOI] [PubMed] [Google Scholar]
- 4.Diomede L, et al. The effect of culture medium composition on ether lipid cytotoxic activity. Lipids. 1993;28:189–192. doi: 10.1007/BF02536638. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta S, et al. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Procaccini C, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthois Y, et al. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc. Natl. Acad. Sci. U S A. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartzstein RM, Parker MJ. Respiratory physiology: a clinical approach. Lippincot-Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 9.Johnson JM, Kellogg DL., Jr. Local thermal control of the human cutaneous circulation. J. Appl. Physiol. 2010;109:1229–1238. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shevach EM. Current Protocols in Immunology. John Wiley & Sons; 2009. Immunofluorescence and cell sorting. [Google Scholar]
- 11.Wulf S. Flow cytometry educational guide. 2nd Edition Dako, Fort Collins: 2006. [Google Scholar]
- 12.Stoppini L, et al. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]