Abstract
Nuclear reprogramming with stemness factors enables resetting of somatic differentiated tissue back to the pluripotent ground state. Recent evidence implicates mitochondrial restructuring and bioenergetic plasticity as key components underlying execution of orchestrated dedifferentiation and derivation of induced pluripotent stem cells. Aerobic to anaerobic transition of somatic oxidative energy metabolism into a glycolytic metabotype promotes proficient reprogramming, establishing a novel regulator of acquired stemness. Metabolomic profiling has further identified specific metabolic remodeling traits defining lineage redifferentiation of pluripotent cells. Therefore, mitochondrial biogenesis and energy metabolism comprise a vital axis for biomarker discovery, intimately reflecting the molecular dynamics fundamental for the resetting and redirection of cell fate.
Keywords: biomarker, differentiation, glycolysis, induced pluripotent stem cell, metabolomics, metabotype, mitochondria, oxidative metabolism, regenerative medicine, stem cells
Stem cell biology provides an essential cornerstone of regenerative medicine [1,2]. A case in point, embryonic stem cells (ESCs) harbor key stemness properties, namely pluripotency (the ability to give rise to diverse cell lineages) and self-renewal (maintenance of the undifferentiated state with high replication potential). However, the use of ESCs for regenerative applications has been met with concern over the ethics of their derivation, and further limited by immunological mismatch between the donor and host. An alternative to ESCs was introduced with the realization that fibroblasts and, in general, somatic tissue could be reprogrammed back to the pluripotent ground state by transduction of a set of stemness transcription factors [3]. The process of nuclear reprogramming provides an autologous pluripotent cell population – termed induced pluripotent stem (iPS) cells – with potential applications for personalized therapy and diagnosis, without the requirement for an embryo source [4]. The development of novel biomarkers that track nuclear reprogramming to the pluripotent state and subsequent differentiation into defined lineages is, therefore, essential not only to further the understanding of stem cell biology but also to ensure optimal and accelerated translation towards regenerative medicine applications.
Energy metabolism
All cells require a continuous production of energy, in the form of ATP, by intermediary metabolism to support the energy demands of proliferation and homeostasis. The specific metabolic pathways utilized to generate ATP and anabolic precursors are highly dependent upon the cell type and state, and represent a unique landscape for biomarker discovery. In fact, a number of metabolic biomarkers are already widely used in practice for disease diagnosis and prognosis. Adult cells rely heavily on efficient mitochondrial oxidative metabolism to maximize energy production in the form of ATP [5]. However, as oxygen is the final electron acceptor of oxidative phosphorylation, when oxygen availability is limited, such as during hypoxia or anoxia, differentiated cells can switch to anaerobic glycolysis. Although ATP generation from glycolysis is much less efficient than oxidative metabolism, producing only two ATP molecules compared with 36 for the complete oxidation of glucose, it can maintain essential cellular processes until the re-establishment of oxygen availability [6]. By contrast, highly proliferative cells such as cancer cells rely on glycolysis despite sufficient oxygen availability to support oxidative generation of ATP [6]. This metabolic profile is a hallmark of cancer cells, and is routinely utilized as a clinical biomarker by measuring metabolite uptake with the glucose analog fluoro-2-deoxyglucose [7]. Thus, cells have significant metabolic flexibility to match anabolism and catabolism to their state-specific bioenergetic requirements. The monitoring of global cell metabolism can thus be utilized to identify metabolic biomarkers that are informative of the cellular status.
Nuclear reprogramming to pluripotency
Nuclear reprogramming with ectopic over-expression of stemness-related transcription factors, such as Oct4, Sox2, Klf4 and c-Myc (Yamanaka factors), or Oct4, Sox2, Lin28 and Nanog (Thomson factors), is sufficient to reset the parental cell fate [3,8,9]. Research has rapidly progressed since these initial reports, with studies optimizing acquisition of the pluripotent ground state and derivation of iPS cells with greater reprogramming efficiency, using a variety of delivery methods including viruses, RNA, DNA vectors, proteins and small molecules [10]. Although complete characterization of iPS cells is still in progress, extensive gene profile analysis demonstrates that nuclear reprogramming stimulates the reacquisition of an embryonic gene network, allowing adoption of an ESC-like morphology and traits of pluripotent stringency, including a propensity for multilineage embryoid body differentiation, formation of teratomas and contribution to chimeric offspring with germline transmission [3,8,11–19]. This extensive structural and functional reorganization imposes bioenergetic stress on the metamorphosing cell, supported by comprehensive metabolic reprogramming to meet the demands of the iPS cell phenotype [20,21].
Mitochondria dynamics during nuclear reprogramming
Mitochondria are intracellular organelles that function as the cellular power station, with the central role of mediating aerobic respiration by oxidizing metabolic substrates to yield energy. Mitochondria are also essential for other vital functions including regulating reactive oxygen species (ROS), calcium homeostasis and apoptosis. Mitochondria are well known for their ability to undergo fusion/fission and have one of the most dynamic life cycles amongst mammalian cellular organelles [22]. In fact, cells undergoing the dramatic process of nuclear reprogramming display extensive mitochondrial restructuring associated with cellular structural and functional remodeling [20,21]. Somatic or differentiated cells, such as fibroblasts, have characteristically elongated tubular-shaped and branched mitochondria that display well-developed electron-dense cristae (Figure 1). It has recently been demonstrated that nuclear reprogramming reliably induces remodeling of the parental mitochondrial infrastructure of both mouse [20,23] and human fibroblasts [24–26], indicating mitochondrial dynamics as a conserved feature of pluripotency induction. Compared with the abundant mitochondria observed in parental fibroblasts, the resultant iPS cells have reduced mitochondria density, as observed in electron micrographs or quantified using fluorescent mitochondrial probes [20,23–27]. Reprogramming also induces mitochondrial DNA (mtDNA) remodeling, including a reduction in copy number [24,27] and potential induction of homoplasic and heteroplasic point mutations, the majority of which are common variants within the general population or are not annotated in databases [28]. More globally, genetic and epigenetic abnormalities have been reported during reprogramming and subsequent cell culture, despite having a lower mutation rate than mtDNA [29–31]. The future utility of the cells is thus greatly dependent on whether these small changes have any functional consequence [32]. Levels of mitochondrial heteroplasmy, the mixture of mitochondria with different point mutations, also varied among cell lines derived from the same parental source, suggesting that uneven mitochondrial segregation may occur during reprogramming. This genetic bottleneck may have therapeutic potential for the derivation of mutation-free stem cell lines from patients with mtDNA-dependent mitochondrial disorders. iPS cells have immature mitochondrial morphology consisting of globular or spherical structures with similar minimal and maximal axes lengths and small, undeveloped cristae [20,23,24,26]; although one study demonstrated retention of a small number of elongated mitochondria [26]. The iPS cell mitochondria has been interpreted to reflect a more condensed configuration (dark condensed matrix and expanded translucent cristae) compared with a predominately orthodox configuration (tightly packed thin cristae within an expanded translucent matrix) in the differentiated progeny [25]. Nuclear reprogramming also transitions the mitochondrial localization from extensive cytoplasmic networks that ensure a cellular compartment-specific energy supply, to a predominately perinuclear and bipolar localization, potentially to energetically support the ongoing genetic and epigenetic remodeling [20,23,24,26]; although this localization may reflect the small cytosolic space of stem cells. Nuclear reprogramming thereby induces mitochondrial regression to re-establish the mitochondrial prevalence and anatomy analogous to that observed in the inner cell mass of the blastocyst during early mammalian embryo development, the source of natural pluripotent stem cells [33]. Indeed, iPS cells share common mitochondrial features with ESCs, the gold standard of pluripotency [34–36]. Low copy numbers of mtDNA and numbers of spherical mitochondria with poorly developed cristae characterize ESCs, consistent with these observed in iPS cells [24,26,37–43]. Those immature mitochondria do not form well-developed mitochondrial networks in undifferentiated ESCs and are found predominately in a perinuclear location [38–41,44]. This perinuclear localization is also observed in human hematopoietic and mesenchymal stem cells, indicating that this may be a consistent feature of stemness [45,46].
Figure 1. Nuclear reprogramming transforms the mitochondrial infrastructure.
Nuclear reprogramming induces reorganization of cytosolic mitochondrial network of fibroblasts (A) into few perinuclear-localized mitochondria in mouse-derived iPS cells (B). This organization is consistent with natural pluripotent stem cell properties, and has been implicated as a marker of stemness. Higher magnification micrographs display regression of tubular and cristae-rich mitochondria (C) into spherical and cristae-poor remnants (D) during derivation of iPS cells. Original micrographs were obtained on a JEOL 1200 EXII electron microscope from ultramicrotome sections of glutaraldehyde and formaldehyde fixed cells.
iPS: Induced pluripotent stem.
The immature mitochondrial morphology of iPS cells may have a significant direct impact on mitochondrial function. Assessment of mitochondrial membrane potential indicated a hyperpolarization in iPS cells compared with parental fibroblasts [20,27,37]. It is uncertain why pluripotent cells, including ESCs, maintain a high mitochondrial membrane potential, but it may be due to low demand for oxidatively derived ATP, which would allow these cells to stay in an energetically primed state, so they can rapidly respond to an increase in energetic demands, such as that imposed during differentiation. Measurement of mitochondrial oxygen utilization at baseline and in response to electron transport chain uncoupling tracks changes in mitochondrial function. Compared with fibroblasts, mouse iPS cells have reduced basal oxygen consumption and an inability to increase oxygen consumption in response to electron transport chain uncoupling, suggesting that these bio-engineered pluripotent cells are already respiring at the maximal rate similar to their natural ESC counterparts [20]. Human iPS cells are also unable to respond to electron transport chain uncoupling, indicating a low reserve capacity [26]. Low functional oxidative capacity would generate significantly lower amounts of mitochondrial ROS. iPS cells have reduced generation of superoxide anions, which results in less oxidatively modified proteins, lipids and DNA, even after exposure to hydrogen peroxide, suggesting a low level of oxidative stress and greater antioxidant defense mechanisms [24,27]. This may enable iPS cells to rescue telomere-dependent cellular aging and senescence [47]. In fact, somatic cell telomere shortening is reversed during nuclear reprogramming, and adopts embryonic telomere features including increased abundance of telomere transcripts and decreased density of telomere region histone heterochromatic marks [48].
Anaerobicizing energy metabolism during nuclear reprogramming
Adult somatic cells have high bioenergetic demands in order to maintain cellular homeostasis. This demand is met by the complete oxidation of metabolic substrates including carbohydrates, fatty acids and amino acids in the mitochondrial tricarboxylic acid cycle to supply reduced cofactors (reduced nicotinamide adenine dinucleotide [NADH] and reduced flavin adenine dinucleotide [FADH2]) for the maximum generation of ATP by oxidative phosphorylation. However, nuclear reprogramming results in reduced basal and maximal oxygen consumption due to the observed structural and functional mitochondrial remodeling. Although iPS cells have lower cellular ATP levels and ADP:ATP ratio (an index of cellular energy turnover) than their parental sources [20,24–26], the reduced oxidative reserve capacity would necessitate metabolic reprogramming to alternative ATP-generating pathways to meet the new bioenergetic demands of iPS cells. To address the specifics of this remodeling we recently utilized a nuclear magnetic resonance (NMR) metabolomic profiling approach to quantify extracellular (‘footprinting’) and intracellular (‘fingerprinting’) metabolites [20]. Derived iPS cells had a metabolic profile that was significantly different from parental fibroblasts and resembled the pattern of ESCs. Similar to their natural pluripotent counterparts, iPS cells had greater utilization of extracellular glucose and accumulation of lactate and acetate compared with fibroblasts [20,21]. This profile is indicative of accelerated anaerobic glycolysis without the aerobic disposal of pyruvate, which requires the conversion to and accumulation of the glycolytic byproducts lactate (via lactate dehydrogenase) and acetate (via ATP-citrate lyase or acetyl-CoA synthase) to regenerate a sufficient nicotinamide adenine dinucleotide (NAD+) pool and maintain the high rates of glycolysis. Upregulation of glycolysis is a common trait, as observed by greater extracellular lactate accumulation [24,26], and intracellular accumulation of glucose-6-phosphate and decreased dihydroacetone phosphate, which is indicative of increased flux to pyruvate [28]. Despite the potential of nuclear reprogramming to induce mtDNA point mutations and changes in mitochondrial hetero-plasmy [28], iPS cells display a metabolic switch from somatic oxidative energy metabolism to glycolysis, irrespective of these mutations. Anaerobicizing somatic oxidative metabolism into a pluripotent glycolytic metabotype (Figure 2) is consistent across multiple species and iPS cell lines, and occurs independent of transduction of the oncogene c-Myc [20,21,24,26,28].
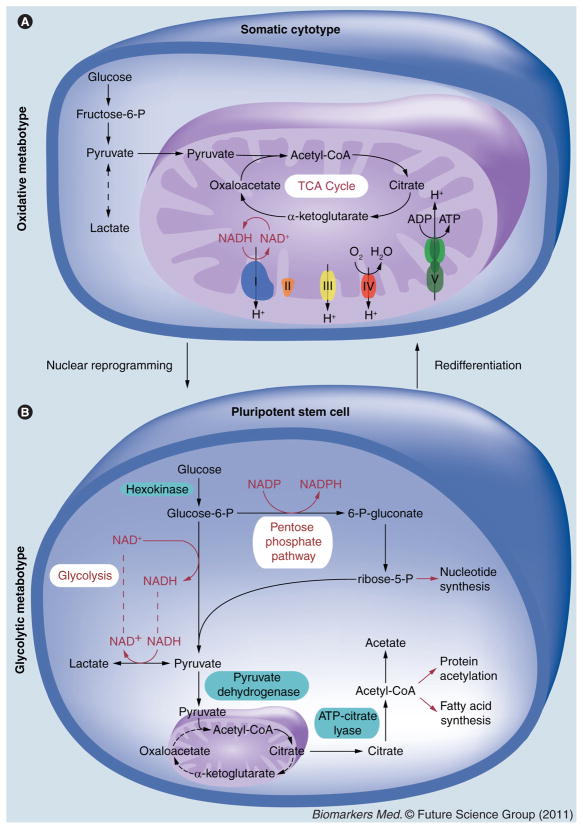
Figure 2. Mitochondrial and energy metabolism define cell fate.
Somatic cytotypes have mature mitochondria that support the efficient generation of ATP via the complete oxidation of substrates by the TCA cycle and the electron transport chain (A). Nuclear reprogramming induces mitochondria regression and acceleration of glycolysis as indicated by elevated glucose utilization and production of lactate and acetate (B). This metabotype is consistent among highly proliferative cells, including bioengineered and natural stem cells, and uniquely provides both a source of ATP and anabolic precursors in the form of NADPH, ribose-5-P and acetyl-CoA. Regeneration of the cytosolic NAD+ pool is coupled with lactate production and is required to maintain high glycolytic rates. Differentiation of pluripotent cells reactivates mitochondrial biogenesis and maturation, to once again support efficient oxidative metabolism.
NAD+: Nicotinamide adenine dinucleotide; NADH: Reduced nicotinamide adenine dinucleotide;
NADP: Nicotinamide adenine dinucleotide phosphate; P: Phosphate; TCA: Tricarboxylic acid.
Metabolic gene & protein remodeling during nuclear reprogramming
Global transcriptional analysis has demonstrated that iPS cell transcriptomes are distinct from their parental source and similar but not identical to ESCs [24,49]. Pathway analysis did not prioritize mitochondrial related pathways, indicating that transcription of nuclear-encoded mitochondrial genes persists during nuclear reprogramming [24,50]. However, nuclear mitochondria biogenesis factors are upregulated in iPS cells compared with parental fibroblasts [24,50]. Microarray and Glucose RT2 Profiler PCR™ array data demonstrated significant reconfiguration of glucose metabolism at the transcriptional level [26,28]. Genes regulating the initial steps of glycolysis, glucose uptake (SLC2A3 encoding GLUT3) and glucose phosphorylation (HK3 and GCK), and the final steps of glycolysis (PGAM2, ENO, PKLR and LDH) were significantly upregulated in iPS cells compared with fibroblasts, while gene expression of glycolytic enzymes downstream of glucose-6-phosphate (GPI, PFK and ALDO) were down-regulated [28]. Pluripotent cells also have greater expression of genes involved in the nonoxidative branch of the pentose phosphate pathway (TKT and RPIA) [26]. This transcriptional profile and the accumulation of glucose-6-phosphate suggest that glycolytic intermediates are diverted into the pentose phosphate pathway to provide anabolic precursors for nucleotide synthesis, as well as to maintain cellular antioxidant defenses [26,28]. The dynamics of these transcriptional changes were examined during nuclear reprogramming by selecting an emergent population of cells residing in small compact clusters with high mitochondrial membrane potential, as assessed by tetramethylrhodamine methyl ester. These cells were demonstrated to have significantly elevated glycolytic gene expression that preceded the induction of pluripotent genes, which only occurred following 2 weeks of reprogramming in the high-tetramethylrhodamine methyl ester cells [20]. This indicates that observed bioenergetic changes are not simply a consequence of the acquisition of pluripotentcy, but rather, are required as an energetic switch replacing oxidative metabolism in favor of anaerobic glycolysis in order to undergo nuclear reprogramming [21].
Remodeling of the metabolic infrastructure supports the bioenergetic transition during pluripotent induction. Examination of the metabolic enzyme proteome demonstrated that iPS cells clustered closer to ESCs than to parental fibroblasts, based upon a predominant down-regulation of electron transport chain complex I subunits and broad upregulation of glycolytic enzymes [20]. Decreased expression of complex I would impair coupling between oxidative metabolism and ATP generation, resulting in reduced oxygen consumption. By contrast, the subunits of complex III and V are predominantly upregulated in iPS cells, which may be a compensatory change to allow for increased ATP production in response to stimuli [20,26]. Nuclear reprogramming accelerates glycolytic flux by regulating expression of specific targets, including an isoform switch from hexokinase I to II [20]. Western blotting demonstrated that hexokinase II, which has previously been demonstrated to be responsible for the high glycolytic rates of hepatoma cells [51], was preferentially localized to the mitochondria in pluripotent cells [26]. Hexokinase catalyzes the first step of glycolysis by phosphorylating and trapping glucose within the cell, thus playing an important regulatory role. The importance of the hexokinase II isoform is the twin catalytic domains that gives hexokinase II the capacity to double the glycolytic rate, in comparison to isoforms I and III where the N-terminal no longer has catalytic activity and rather plays a regulatory function [51]. In addition, mitochondrial localization of hexokinase II can enhance glycolytic capacity as it has preferential access to mitochondrial ATP from the adenine nucleotide transporter for its catalytic activity and can escape product inhibition by glucose-6-phosphate [51–54]. Treatment during nuclear reprogramming with the hexo-kinase II inhibitor, 3-bromopyruvic acid, which may also inhibit additional glycolytic enzymes, reduced induction of glycolysis resulting in vigorous oxidative metabolism and compromised pluripotent transition [20,55,56].
An additional key regulatory step in controlling the balance between glycolysis and oxidative energy metabolism is pyruvate dehydrogenase (PDH), which catalyzes the decarboxylation of pyruvate to acetyl-CoA, for further oxidation in the tricarboxylic acid cycle. This complex is highly regulated by both allosteric mechanisms as well as phosphorylation/dephosphorylation by PDH kinase and phosphatase [57]. Proteomic analysis demonstrated significantly elevated levels of PDH α- and β-subunits as well as the upstream kinase PDHK1, which results in phosphorylation and inactivation of the PDH complex [20,26]. This configuration would shunt pyruvate away from oxidative metabolism and stimulate lactate production to maintain a sufficient NAD+ pool for the high rates of glycolytic flux. Inhibition of PDHK1 with dichloroacetate during nuclear reprogramming relieves the inhibition of PDH, thus elevating pyruvate flux into the tricarboxylic acid cycle and reducing lactate production, resulting in a decreased efficiency of pluripotency induction. Specific remodeling of the transcriptome and proteome thus underlies the metabolic infrastructure supporting nuclear reprogramming.
Glycolytic requirement for pluripotency induction
Distinct rationales have been proposed to explain the glycolytic metabotype of highly proliferative cells, in most cases referring to cancer cells, but which could also be applied to pluripotent cells [6,58]. Although glycolysis produces ATP less efficiently than oxidative phosphorylation as less ATP is produced per molecule of glucose, if glycolytic rates are sufficiently high, the percentage of ATP produced from glycolysis can exceed that of oxidative phosphorylation [59,60]. Therefore, the inefficient nature of glycolysis may only represent a hurdle if resources become limiting [6]. Typical cell culture conditions maintain an adequate supply of metabolic substrates (e.g., glucose, glutamine and pyruvate) to maintain cellular homeostasis. Even if glycolytic cells are stimulated to divide at an accelerated rate they still maintain high ratios of NADH/NAD+ and ATP/ADP, suggesting that resources do not become scarce [58,61]. These cells may benefit from the greater rate of ATP generation from glycolysis compared with oxidative phosphorylation [62]. If the ability of proliferating cells to produce glycolytic ATP is impaired, they could undergo cell cycle arrest and reactivate oxidative catabolism [63,64].
The reliance on glycolysis may also be due to the anabolic requirements of proliferating cells [6]. Not all of the cellular constituents required for cellular replication can be obtained from the environment and, hence, cells must synthesize the basic building blocks of amino acids, nucleotides and fatty acids. Although biosynthetic pathways require ATP, they require greater equivalents of carbon and reducing cofactors (nicotinamide adenine dinucleotide phosphate [NADPH]). As the major catabolic sources of these precursors are glucose and glutamine in the majority of mammalian cell culture, the complete oxidation of glucose would not meet the requirements of these proliferating cells [6,58]. By contrast, glycolytic metabolism provides a balance between the catabolic generation of ATP and the production of biosynthetic substrates to meet the anabolic requirements. Glycolytic intermediates can also be shunted into the pentose phosphate pathway to generate NADPH. In extreme cases, glioblastoma cells can metabolize up to 60% of glutamine and 90% of glucose to alanine and lactate to produce high quantities of NADPH [65]. Carbon precursors can also be produced from the glycolytic end product, pyruvate, which is first converted to acetyl-CoA and incorporated into citrate in the mitochondrial matrix. Under the high ATP/ADP conditions of proliferating cells, citrate is exported from the mitochondria and cytosolic acetyl-CoA, an anabolic precursor, can be produced by the action of ATP citrate lyase [66,67]. Acetyl-CoA is also a precursor for the acetylation of a number of proteins involved in diverse cellular responses ranging from regulation of energy metabolism, to nuclear import and regulation of gene expression [68]. Inhibition of histone deacetylases increases the efficiency of nuclear reprogramming by promoting histone acetylation and activation of a transcriptional state similar to that of ESCs [69–72]. In yeast and proliferating cells, high rates of glycolysis have been associated with generation of nucleocytosolic acetyl-CoA and a resultant increase in histone acetylation [67,73,74]. Thus, the high glycoytic capacity of iPS cells may link energy metabolism with nuclear reprogramming-induced epigenetic resetting, which ultimately primes the cell for pluripotent induction.
Metabolic modulation influences the efficiency of nuclear reprogramming
The balance between glycolysis and oxidative metabolism contributes to the mechanism of nuclear reprogramming and can be manipulated to increase its efficiency. Increased expression of glycolytic enzymes, particularly phosphoglycerate mutase and glucose-6-phosphate isomerase, and increased rates of glycolysis are sufficient to increase cellular life span and are required for the immortalization of mouse fibroblasts [75]. Stimulation of glycolytic metabolism by hypoxia significantly increases the efficiency of nuclear reprogramming [76] and has been demonstrated to improve the maintenance of the pluripotent ground state [77–80]. In addition, inhibition of the p53 pathway, which would in part stimulate glycolysis [81], also improves nuclear reprogramming [82–86]. Direct metabolic modulation has also been successfully used to manipulate reprogramming efficiency, with agents that inhibit glycolysis and stimulate oxidative metabolism, such as 2-deoxyglucose, 3-bromopyruvic acid, 6-aminonicotinamide, oxalate or dichloroacetate suppressing the efficiency of nuclear reprogramming. More importantly, reprogramming efficiency can be augmented by stimulating glycolysis in favor of oxidative metabolism by increased glucose concentrations, or using agents such as PS48 (a PDHK1 activator), dinitro-phenol, N-oxaloylglycine and quercetin [20,87]. Stimulation of glycolysis with PS48 in combination with a histone deacetylase inhibitor (sodium butyrate), TGF-β receptor inhibitor (A-83–01) and MAPK/ERK inhibitor (PD0325901) was sufficient to reduce the number of reprogramming factors required to generate pluripotent colonies from four to one (Oct4), indicating the importance of optimizing energy metabolism to the reprogramming process [87].
Mitochondria & energy metabolism underlies redifferentiation of pluripotent cells
Nuclear reprogramming-induced dedifferentiation requires specific mitochondrial and metabolic remodeling to obtain the pluripotent state. Redifferentiation of these cells also requires remodeling of bioenergetic systems, implicating mitochondrial dynamics and energy metabolism in controlling cell fate. Upon downregulation of pluripotent genes and spontaneous differentiation, mtDNA replication increases, resulting in an elevated mtDNA copy number and mitochondrial maturation into elongated tubular structures with complex cristae and dense matrices [24,25,36,37,40,41,43]. These mature mitochondria migrate into more dispersed cytoplasmic areas to form complex mitochondrial networks to allow energetic transfers to cytosolic organelles [24,25,37,40,41,44,88–90]. However, it remains unknown if there is a specific mitochondrial density and functional capacity that must be met to specify defined lineages and whether there is a specific time during the differentiation process that these requirements must be met. In comparison to low passage number primate stromal cells, high passage number cells have greater spontaneous differentiation associated with fewer perinuclear localized mitochondria [91]. If ESCs are sorted based on their resting mitochondrial membrane potential into low and high populations, they are indistinguishable based upon expression of pluripotency markers and morphology [92]. However, the population with lower potential has increased propensity for in vitro mesodermal differentiation, but fails to efficiently form teratomas in vivo, with the opposite results observed in the high potential group [92]. It has also been demonstrated that disruption of mitochondrial respiratory chain function compromises formation of the mitochondrial network and impairs the ability of ESCs to differentiate into cardiomyocytes [37], although this may be specific to inhibition of complex III of the electron transport chain [93].
Differentiation-induced increases in mitochondrial mass and function is associated with a significant increase in ROS generation, which results in increased oxidative damage in differentiated cells despite a concomitant increase in antioxidant defenses [24,27,40]. As mitochondria mature in differentiating ESCs, oxygen consumption and cellular respiration are accelerated such that ATP production increases and reliance on anaerobic glycolysis is reduced [37,40,75,94]. Differentiation into specific lineages including the cardiomyocyte, results in increased expression of electron transport chain subunits and tri-carboxylic acid enzymes, and downregulation of glycolytic enzymes [37,40,43,44]. In vivo teratoma differentiation of ESCs and iPS cells also resulted in the upregulation of selected mitochondrial biogenesis and electron transport genes; however, these genes were predominantly downregulated during spontaneous in vitro differentation [50]. Differentiation also engages a maturation of the metabolic signaling and phosphotransfer infrastructure, including increased expression of AMP-activated protein kinase, creatine kinase and adenlyate kinase [88,95]. This remodeling may support an alternative function of the glycolytic pathway to facilitate substrate supply and transfer high-energy phosphoryls from the mitochondria to sites of ATP utilization [44]. Blockade of mitochondrial function or inhibition of adenylate kinase resulted in maintenance of the pluripotent state and inhibition of differentiation [37,95,96]. Mesenchymal stem cell lineage specification is also characterized by a reduction in glycolytic enzyme expression and lactate production, and an increase in oxygen consumption [46]. Yet, the importance of this transition in energy metabolism away from glycolysis to more efficient oxidative metabolism in controlling the differentiation process is unknown. Inhibition of mTOR reduced oxygen consumption, mitochondrial membrane potential and lactate production, and was associated with increased in vitro mesodermal differentiation in ESCs [92]. These observations suggest that the glycolytic phenotype of pluripotent stem cells is a critical component required to maintain these cells in the pluripotent state, while mitochondrial and metabolic infrastructure maturation may be integral to the differentiation process. Bioenergetics thus contributes to the balance between the maintenance of stem cell pluripotency and self-renewal and their redifferentiation and specification into defined lineages.
Metabolomics
Beyond the traditional scientific approaches that focus on individual genes, molecules or pathways, advances in decoding the biology of dedifferentiation and redifferentiation relies on the multiplexing of high-throughput data sets and systems biology, which offers an opportunity for the integration of multilevel connectivity with biological complexity [97,98]. Recent advances allow for the simultaneous profiling of a significant number of low-molecular-weight organic and inorganic compounds (metabolites) in a biological system. This allows for the unbiased examination of metabolite dynamics and fluxes in stressed, diseased or abnormal physiological states. As the concentrations of metabolites cannot be deduced from the genomic information, it has necessitated the development of specialized techniques that spawned the field of metabonomics or metabolomics [99–101]. Although the metabolome is relatively small and tightly conserved compared with the genome, transcriptome and proteome, with an order of several thousand primary metabolites, these small molecules have diverse chemical properties ranging from polar to nonpolar with diverse molecular weights (~50–1400 g/mol). As these small molecules have no common chemical backbone, such as nucleotides or amino acids, there is no single current analytical technique that allows for the measurement of this diverse group of compounds. The predominate methods currently used to interrogate the metabolome are extensions of the long-existing techniques of NMR spectroscopy and mass spectrometry (MS) coupled with compound separation by liquid or gas chromatography. These techniques can measure a wide range of chemical compounds with high analytical precision and can provide structural information for identification purposes. These analytical techniques and their specific usage in metabolomics have been extensively reviewed [102–105]. Due to the lack of annotation in metabolite databases, many studies now use a more targeted approach to identify biomarkers by first identifying mass spectral features that differ among groups, and then concentrate on elucidating the chemical identities of these key features instead of the complete profile [106]. Metabolomics can interrogate energy metabolism within cells in culture by examining both the intracellular metabolites (fingerprinting), as well as by monitoring the metabolites consumed in or secreted into the growth media (footprinting) [100,107–109]. This provides a global energetic snapshot of the cell, and is readily amenable to surrogate metabolic biomarker discovery (Figure 3).
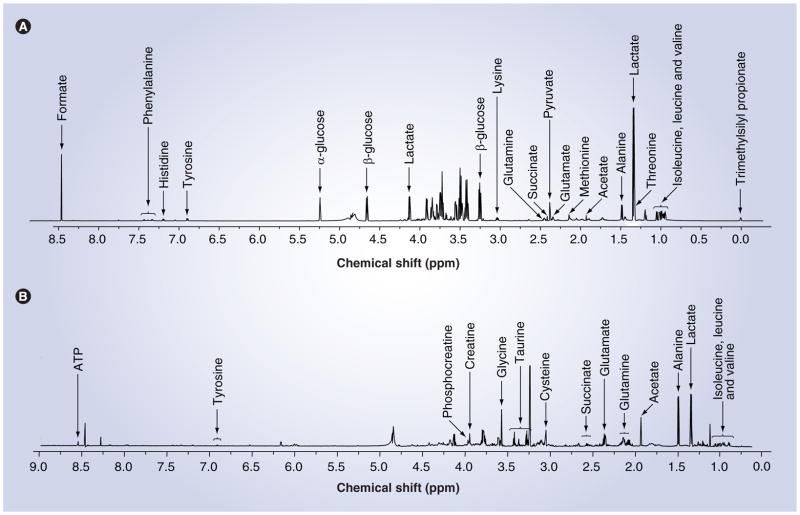
Figure 3. Metabolomic profiling using nuclear magnetic resonance spectroscopy.
Representative nuclear magnetic resonance spectra from (A) mouse induced pluripotent stem cells indicating extracellular metabolites identifiable within the cell culture media (metabolomic footprint) and (B) intracellular metabolites found within the cell (metabolomic fingerprint). Original nuclear magnetic resonance spectra were obtained on a Bruker Ultrashield 700 MHz spectrometer with a zgpr water suppression presaturation pulse.
Metabolomic profiling of cell fate
Stem cells require specific metabolic pathways to meet the energetic demands of the functional and structural remodeling that occurs during differentiation into defined lineages and nuclear reprogramming-induced dedifferentiation back to the pluripotent ground state. Metabolomic profiling allows for the detection of metabolite profiles specific for distinct states, and the identification of their metabolic biomarkers. These markers would have immediate research and clinical implications as a novel method to monitor cell fate. To date, relatively few studies have applied metabolomic techniques to the study of stem cell biology. Initial profiling only identified a few NMR-detectable intracellular metabolites of ESCs [110]. These cells were characterized by high phosphocholine content and nondetectable glycerophosphocholine, a precursor of phospholipid synthesis, compared with derivative neural stem cells, suggesting a specific choline metabolism for cell growth [110]. Metabolomic footprinting of the extracellular metabolites of hepatic stem cells identified a glycolytic profile, which included high glucose consumption and high levels of lactate and alanine accumulation, consistent with other stem cell types [111]. Comparison of liquid chromatography–MS/MS-derived metabolite profiles of ESCs and leukemia inhibitory factor-deprived differentiated cells within embryoid bodies identified a class of metabolites that were present at a higher abundance in ESCs [112]. The greatest changes in abundance were observed in metabolites involved in purine biosynthesis (folic acid, methyl-tetrahydrofolate, 5-aminoimidazole carboxamide ribonucleotide, guanosine, adenosine and inosine) [112]. Consistent with the single carbon requirement of this pathway, ESCs have 1000-fold higher threonine dehydrogenase expression and low levels of threonine compared with differentiated cells [112]. Withdrawal of threonine from cell culture media resulted in impaired growth and loss of stem cell markers, indicating that threonine metabolism is required to maintain stem cell pluripotency [112]. A comprehensive study examining over 150 metabolites in ESCs during maintenance of pluripotency and differentiation into neuronal and cardiac lineages identified that the ESC metabolome has a high degree of unsaturation (extensive multiple carbon–carbon bonds) compared with differentiated cells [113]. As a significant change in redox status occurs during differentiation, the acceleration of oxidative metabolism may drive cell differentiation [113]. Indeed, inhibition of the eicosanoid pathway maintained a high level of unsaturated fatty acids and promoted maintenance of pluripotency, while treatment with eicosanoids or oxidative substrates (saturated fatty acids and acyl-carnitines) supported cardiac and neuronal differentiation [113]. These studies indicate that metabolomic profiling of stem cells and their differentiated progeny can lead to identification of novel pathways that control cell fate and can lead to the identification of metabolites as markers of specific lineages.
Metabolomic profiling has only recently been applied to the nuclear reprogramming process, with a combined NMR footprinting and finger-printing approach demonstrating a glycolytic signature of greater glucose utilization and accumulation of lactate and acetate in the iPS cells [20]. Selective profiling of glycolytic intermediates with liquid chromatography–MS/MS indicated increased flux towards pyruvate generation due to elevated levels of glucose-6-phosphate and reduction of dihydroacetone phosphate [28].
Stem cell energy metabolism: implications for biomarker discovery
Stem cells have a specific metabolic signature in order to meet their anabolic and catabolic demands, which differs dramatically from their differentiated progeny. Biomarkers of this signature provide a valuable resource not only to follow fate choices during nuclear reprogramming and cellular differentiation, but as additional criteria for the establishment and maintenance of the pluripotent state. The perinuclear localization of sparse and immature mitochondria characteristic of iPS cells [20,23–27] and other stem cell populations [37–43,45,46,91] is consistent with the localization of mitochondria to the pronuclei in fertilized oocytes and nuclei of embryos [33,114], and has been suggested as a biomarker of stem cell competence [34,91]. Recent evidence suggests that elevated mitochondrial membrane potential, as assessed by fluorescent mitochondrial probes, is a marker of the pluripotent state [20]. Recently, the fluorescent probe CDy1 has been identified as a marker of ESCs and iPS cells, and may be dependent on mitochondrial polarization, as it has similar staining patterns to mitochondrial-specific dyes [115,116]. Metabolites have already been utilized as clinical biomarkers of disease and would provide information beyond mitochondrial structure and function. A number of metabolites could be utilized as surrogate markers of elevated glycolytic flux, including increased glucose uptake and the accumulation of lactate, acetate and alanine [6,7,20]. Similar to cancer cells, it may be possible to track stem cells in vivo using fluoro-2-deoxyglucose as a measure of glucose uptake [7]. Loss of the pluripotent state would be consistent with the reduction of glycolytic makers and the rise of markers of oxidative metabolism, including an increase in oxygen consumption, ROS generation and subsequent oxidatively modified proteins and lipids. Therefore, metabolic biomarkers may provide a useful tool for following cell fate specification. However, further studies are required to identify and validate markers of specific states.
Applications of metabolomics & metabolic biomarkers in stem cell biology
Although stem cell energy metabolism is a recent field of investigation, studies have applied metabolic biomarkers for discovery science. A proof-of-concept study demonstrated that MS metabolomic profiling was able to identify significant differences in the extracellular metabolites involved in kynurenine and glutamate metabolism when ESCs were treated with valproate [102]. This study introduces metabolomic profiling of ESCs and derivatives as a means to perform efficacy and toxicity assessments of pharmaceuticals and for the identification of predictive biomarkers of toxicity [102,117,118]. Several studies have taken advantages of metabolomic footprinting analysis to monitor stem cell maintenance in response to different culture conditions. Serial metabolite profiling has examined the effect of plating conditions (cell culture plastic versus hyaluronan matrix hydrogels) on energy metabolism, demonstrating that changes in matrix composition resulted in changes in the cellular growth and differentiation properties, which is associated with a specific metabolomic profile [111]. This NMR footprinting technique has also been used to define the metabolite composition of conditioned media that is utilized to maintain human ESCs [119]. This provides a valuable tool to monitor batch-to-batch variation due to both the conditioning process and subsequent storage.
Future perspective
The field of stem cell energy metabolism is in its infancy, with current knowledge focused on the understanding of the bioenergetic requirements for maintenance of pluripotency, differentiation into defined lineages and dedifferentiation back to the pluripotent ground state. As metabolomic profiling techniques continue to evolve, they will provide a sharper snapshot of these discrete states and will define the metabolic dynamics required for transition among states. Defining these metabolic biomarkers will ultimately allow for real-time tracking of nuclear reprogramming and subsequent differentiation, and would enable a platform for rapid testing of small molecules and protocols to improve these processes. Indeed, the glycolytic metabotype appears to be consistent across stem cell lines, and may represent an additional criterion for pluripotency. A thorough understanding of the underlying bioenergetics will allow for the optimization of energy metabolism using small molecules or defined media to manipulate cellular fate. This has important implications in the derivation of iPS cells by nuclear reprogramming, maintenance of self-renewal and pluripotency, and differentiation or transdifferentiation into defined lineages. Therefore, remodeling of energy metabolism is now considered an active participant in defining cell fate.
Executive summary.
Energy metabolism
Cells have significant metabolic flexibility in order to match their anabolism and catabolism to their state-specific bioenergetic requirements.
Monitoring cellular energy metabolism is informative of cellular status and can be utilized for the identification of metabolic biomarkers.
Nuclear reprogramming to pluripotency
Nuclear reprogramming induces regression of mitochondrial morphology from complex networks of tubular and cristae-rich mitochondria to few perinuclear localized cristae-poor remnants.
Pluripotent cells have low rates of oxygen consumption and respire at their maximal capacity, with no reserve capacity.
Nuclear reprogramming anaerobicizes somatic oxidative bioenergetics into glycolytic flux of pluripotent stem cells.
Transcriptome and proteome remodeling supports the metabolic infrastructure transition underlying nuclear reprogramming.
Mitochondrial potential and glycolytic genes are induced prior to pluripotent gene expression during nuclear reprogramming, suggesting that metabolic remodeling is not simply a consequence of pluripotency acquisition, but plays a causative role.
The mitochondrial and metabolic signature of induced pluripotent stem cells is consistent with that of natural pluripotent stem cells.
Glycolysis uniquely provides a balance between the generation of ATP and anabolic precursors to support the requirements of highly proliferative cells.
Modulation of energy metabolism can influence the efficiency of pluripotency induction, with the stimulation of glycolysis promoting and suppression inhibiting nuclear reprogramming.
Mitochondria & energy metabolism contribute to redifferentiation of induced pluripotent stem cells
Differentiation of pluripotent cells results in re-establishment of an extensive network of mature mitochondria.
Differentiated cells utilize efficient mitochondrial oxidative metabolism to produce ATP to maintain cell homeostasis.
Metabolomics
High-throughput metabolomics using nuclear magnetic resonance and mass spectrometry allows for the examination of the metabolic dynamics and fluxes in different physiological and pathological states.
Metabolite profiling has resolved novel metabolic pathways that control cell fate and is useful for the identification of novel biomarkers.
Stem cell energy metabolism: implications for biomarker discovery
Mitochondrial morphology/localization and hyperpolarization are potential markers of stem cell competence.
Surrogate markers of glycolysis, such as increased glucose uptake and accumulation of lactate and acetate, are biomarkers of pluripotency.
Loss of pluripotency is consistent with a reduction in glycolytic biomarkers and the rise of markers of oxidative metabolism.
Conclusion & future perspective
Remodeling of energy metabolism is an active participant in defining cell fate, rather than a simple consequence of these decisions.
Definition and validation of metabolic biomarkers will enable the real-time tracking of nuclear reprogramming and subsequent differentiation of pluripotent stem cells.
Thorough understanding of the underlying bioenergetics of nuclear reprogramming and differentiation will allow for the optimization of energy metabolism to manipulate cellular fate.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ of considerable interest
- 1.Terzic A, Folmes CD, Martinez-Fernandez A, Behfar A. Regenerative medicine. on the vanguard of health care. Mayo Clin Proc. 2011;86(7):600–602. doi: 10.4065/mcp.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson TJ, Behfar A, Yamada S, Martinez-Fernandez A, Terzic A. Stem cell platforms for regenerative medicine. Clin Transl Sci. 2009;2(3):222–227. doi: 10.1111/j.1752-8062.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪▪.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. Describes the reprogramming of somatic fibroblasts back to the pluripotent ground state. [DOI] [PubMed] [Google Scholar]
- 4.Nelson TJ, Terzic A. Induced pluripotent stem cells: an emerging theranostics platform. Clin Pharmacol Ther. 2011;89(5):648–650. doi: 10.1038/clpt.2010.304. [DOI] [PubMed] [Google Scholar]
- 5.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206(Pt 12):2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 6▪.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect. The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. Details the benefits of utilizing glycolysis for highly proliferative cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez F, Boue S, Izpisua Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet. 2011;12(4):231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 11.Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen TS, Hanna J, Zhang X, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454(7200):49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25(10):1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 14.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 15.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Fernandez A, Nelson TJ, Yamada S, et al. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ Res. 2009;105(7):648–656. doi: 10.1161/CIRCRESAHA.109.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120(5):408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105(15):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson TJ, Martinez-Fernandez A, Yamada S, Mael AA, Terzic A, Ikeda Y. Induced pluripotent reprogramming from promiscuous human stemness-related factors. Clin Transl Sci. 2009;2(2):118–126. doi: 10.1111/j.1752-8062.2009.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Folmes CD, Nelson TJ, Martinez-Fernandez A, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14(2):264–271. doi: 10.1016/j.cmet.2011.06.011. Demonstrates metabolic reprogramming from somatic oxidative bioenergetics to glycolysis during pluripotency induction. Also indicates that metabolic remodeling occurs prior to induction of pluripotent genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panopoulos AD, Izpisua Belmonte JC. Anaerobicizing into pluripotency. Cell Metab. 2011;14(2):143–144. doi: 10.1016/j.cmet.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 23.Zeuschner D, Mildner K, Zaehres H, Scholer HR. Induced pluripotent stem cells at nanoscale. Stem Cells Dev. 2010;19(5):615–620. doi: 10.1089/scd.2009.0159. [DOI] [PubMed] [Google Scholar]
- 24▪.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28(4):721–733. doi: 10.1002/stem.404. Demonstrates a regression of mitochondrial infrastructure during nuclear reprogramming and an increase in lactate production. [DOI] [PubMed] [Google Scholar]
- 25.Suhr ST, Chang EA, Tjong J, et al. Mitochondrial rejuvenation after induced pluripotency. PLoS One. 2010;5(11):e14095. doi: 10.1371/journal.pone.0014095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Varum S, Rodrigues AS, Moura Mb, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6(6):e20914. doi: 10.1371/journal.pone.0020914. Describes the metabolic phenotype of human induced pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong L, Tilgner K, Saretzki G, et al. Human induced pluripotent stem cell lines show similar stress defense mechanisms and mitochondrial regulation to human embryonic stem cells. Stem Cells. 2010;28(4):661–673. doi: 10.1002/stem.307. [DOI] [PubMed] [Google Scholar]
- 28.Prigione A, Lichtner B, Kuhl H, et al. Human iPSCs harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining hESC-like metabolic reprogramming. Stem Cells. 2011;29(9):1338–1348. doi: 10.1002/stem.683. [DOI] [PubMed] [Google Scholar]
- 29.Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 30.Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gore A, Li Z, Fung Hl, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mummery C. Induced pluripotent stem cells – a cautionary note. N Engl J Med. 2011;364(22):2160–2162. doi: 10.1056/NEJMcibr1103052. [DOI] [PubMed] [Google Scholar]
- 33.Batten BE, Albertini DF, Ducibella T. Patterns of organelle distribution in mouse embryos during preimplantation development. Am J Anat. 1987;178(2):204–213. doi: 10.1002/aja.1001780212. [DOI] [PubMed] [Google Scholar]
- 34.Lonergan T, Bavister B, Brenner C. Mitochondria in stem cells. Mitochondrion. 2007;7(5):289–296. doi: 10.1016/j.mito.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berlin) 2010;88(10):981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Facucho-Oliveira JM, St John JC. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev Rep. 2009;5(2):140–158. doi: 10.1007/s12015-009-9058-0. [DOI] [PubMed] [Google Scholar]
- 37.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baharvand H, Matthaei KI. The ultrastructure of mouse embryonic stem cells. Reprod Biomed Online. 2003;7(3):330–335. doi: 10.1016/s1472-6483(10)61873-1. [DOI] [PubMed] [Google Scholar]
- 39.Baharvand H, Piryaei A, Rohani R, Taei A, Heidari MH, Hosseini A. Ultrastructural comparison of developing mouse embryonic stem cell and in vivo derived cardiomyocytes. Cell Biol Int. 2006;30(10):800–807. doi: 10.1016/j.cellbi.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Cho YM, Kwon S, Pak YK, et al. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348(4):1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 41.St John JC, Ramalho-Santos J, Gray HL, et al. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7(3):141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- 42.Oh SK, Kim HS, Ahn HJ, et al. Derivation and characterization of new human embryonic stem cell lines. SNUhES1, SNUhES2, and SNUhES3 Stem Cells. 2005;23(2):211–219. doi: 10.1634/stemcells.2004-0122. [DOI] [PubMed] [Google Scholar]
- 43.Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci. 2007;120(Pt. 22):4025–4034. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- 44.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48(4):725–734. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccoli C, Ria R, Scrima R, et al. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P) H oxidase activity. J Biol Chem. 2005;280(28):26467–26476. doi: 10.1074/jbc.M500047200. [DOI] [PubMed] [Google Scholar]
- 46.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 47.Passos JF, Saretzki G, Ahmed S, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5(5):e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marion RM, Strati K, Li H, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4(2):141–154. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Chin MH, Mason MJ, Xie W, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5(1):111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prigione A, Adjaye J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol. 2010;54(11–12):1729–1741. doi: 10.1387/ijdb.103198ap. [DOI] [PubMed] [Google Scholar]
- 51.Mathupala SP, Ko YH, Pedersen PL. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim Biophys Acta. 2010;1797(6–7):1225–1230. doi: 10.1016/j.bbabio.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988;263(33):17422–17428. [PubMed] [Google Scholar]
- 53.Bustamante E, Morris HP, Pedersen PL. Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J Biol Chem. 1981;256(16):8699–8704. [PubMed] [Google Scholar]
- 54.Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture. Role of mitochondrial hexokinase. Proc Natl Acad Sci USA. 1977;74(9):3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer. Characterization and targeting hexokinase. Cancer Lett. 2001;173(1):83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- 56.Pereira DA, Silva AP, El-Bacha T, Kyaw N, et al. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J. 2009;417(3):717–726. doi: 10.1042/BJ20080805. [DOI] [PubMed] [Google Scholar]
- 57.Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112(3):139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 58.Deberardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer. metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 60.Guppy M, Greiner E, Brand K. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur J Biochem. 1993;212(1):95–99. doi: 10.1111/j.1432-1033.1993.tb17637.x. [DOI] [PubMed] [Google Scholar]
- 61.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 62.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 63.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lum JJ, Bauer DE, Kong M, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(2):237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 65.Deberardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatzivassiliou G, Zhao F, Bauer De, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 69.Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 71.Liang G, Taranova O, Xia K, Zhang Y. Butyrate promotes induced pluripotent stem cell generation. J Biol Chem. 2010;285(33):25516–25521. doi: 10.1074/jbc.M110.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mali P, Chou BK, Yen J, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28(4):713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friis RM, Wu BP, Reinke SN, Hockman DJ, Sykes BD, Schultz MC. A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA. Nucleic Acids Res. 2009;37(12):3969–3980. doi: 10.1093/nar/gkp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell. 2011;42(4):426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kondoh H, Lleonart ME, Gil J, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65(1):177–185. [PubMed] [Google Scholar]
- 76.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5(3):237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102(13):4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Powers DE, Millman JR, Huang RB, Colton CK. Effects of oxygen on mouse embryonic stem cell growth, phenotype retention, and cellular energetics. Biotechnol Bioeng. 2008;101(2):241–254. doi: 10.1002/bit.21986. [DOI] [PubMed] [Google Scholar]
- 79.Westfall SD, Sachdev S, Das P, et al. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. 2008;17(5):869–881. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology. A critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 81.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 82.Banito A, Rashid ST, Acosta JC, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23(18):2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawamura T, Suzuki J, Wang YV, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H, Collado M, Villasante A, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marion RM, Strati K, Li H, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87▪.Zhu S, Li W, Zhou H, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7(6):651–655. doi: 10.1016/j.stem.2010.11.015. Demonstrates that agents which stimulate glycolysis can increase the efficiency of nuclear reprogramming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung S, Dzeja PP, Faustino RS, Terzic A. Developmental restructuring of the creatine kinase system integrates mitochondrial energetics with stem cell cardiogenesis. Ann NY Acad Sci. 2008;1147:254–263. doi: 10.1196/annals.1427.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Terzic C, Behfar A, Mery A, Van Deursen JM, Terzic A, Puceat M. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ Res. 2003;92(4):444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- 90.Perez-Terzic C, Faustino RS, Boorsma BJ, et al. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S68–S76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 91.Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208(1):149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 92.Schieke SM, Ma M, Cao L, et al. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283(42):28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spitkovsky D, Sasse P, Kolossov E, et al. Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. FASEB J. 2004;18(11):1300–1302. doi: 10.1096/fj.03-0520fje. [DOI] [PubMed] [Google Scholar]
- 94.Kondoh H, Lleonart ME, Nakashima Y, et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9(3):293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 95.Dzeja PP, Chung S, Faustino RS, Behfar A, Terzic A. Developmental enhancement of adenylate kinase–AMPK metabolic signaling axis supports stem cell cardiac differentiation. PLoS One. 2011;6(4):e19300. doi: 10.1371/journal.pone.0019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res. 2009;3(2–3):142–156. doi: 10.1016/j.scr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arrell DK, Terzic A. Network systems biology for drug discovery. Clin Pharmacol Ther. 2010;88(1):120–125. doi: 10.1038/clpt.2010.91. [DOI] [PubMed] [Google Scholar]
- 98.Faustino RS, Chiriac A, Niederlander NJ, et al. Decoded calreticulin-deficient embryonic stem cell transcriptome resolves latent cardiophenotype. Stem Cells. 2010;28(7):1281–1291. doi: 10.1002/stem.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’. Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 100.Fiehn O. Metabolomics: the link between genotypes and phenotypes. Plant Mol Biol. 2002;48(1–2):155–171. [PubMed] [Google Scholar]
- 101.Nicholson JK, Lindon JC. Systems biology. Metabonomics Nature. 2008;455(7216):1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 102.Cezar GG, Quam JA, Smith Am, et al. Identification of small molecules from human embryonic stem cells using metabolomics. Stem Cells Dev. 2007;16(6):869–882. doi: 10.1089/scd.2007.0022. [DOI] [PubMed] [Google Scholar]
- 103▪.Lindon JC, Nicholson JK. Spectroscopic and statistical techniques for information recovery in metabonomics and metabolomics. Ann Rev Anal Chem (Palo Alto Calif) 2008;1:45–69. doi: 10.1146/annurev.anchem.1.031207.113026. Compares the advantages and disadvantages of metabolomic profiling using nuclear magnetic resonance and mass spectrometry. [DOI] [PubMed] [Google Scholar]
- 104.Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem. 2011;286(29):25435–25442. doi: 10.1074/jbc.R111.238691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weckwerth W. Metabolomics. An integral technique in systems biology. Bioanalysis. 2010;2(4):829–836. doi: 10.4155/bio.09.192. [DOI] [PubMed] [Google Scholar]
- 106.Griffiths WJ, Koal T, Wang Y, Kohl M, Enot DP, Deigner HP. Targeted metabolomics for biomarker discovery. Angew Chem Int Ed Engl. 2010;49(32):5426–5445. doi: 10.1002/anie.200905579. [DOI] [PubMed] [Google Scholar]
- 107.Allen J, Davey HM, Broadhurst D, et al. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat Biotechnol. 2003;21(6):692–696. doi: 10.1038/nbt823. [DOI] [PubMed] [Google Scholar]
- 108.Ellis DI, Dunn WB, Griffin JL, Allwood JW, Goodacre R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics. 2007;8(9):1243–1266. doi: 10.2217/14622416.8.9.1243. [DOI] [PubMed] [Google Scholar]
- 109.Kell DB, Brown M, Davey HM, Dunn WB, Spasic I, Oliver SG. Metabolic footprinting and systems biology. The medium is the message. Nat Rev Microbiol. 2005;3(7):557–565. doi: 10.1038/nrmicro1177. [DOI] [PubMed] [Google Scholar]
- 110.Jansen JF, Shamblott MJ, Van Zijl PC, et al. Stem cell profiling by nuclear magnetic resonance spectroscopy. Magn Reson Med. 2006;56(3):666–670. doi: 10.1002/mrm.20968. [DOI] [PubMed] [Google Scholar]
- 111.Turner WS, Seagle C, Galanko JA, et al. Nuclear magnetic resonance metabolomic footprinting of human hepatic stem cells and hepatoblasts cultured in hyaluronan-matrix hydrogels. Stem Cells. 2008;26(6):1547–1555. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- 112.Wang J, Alexander P, Wu L, Hammer R, Cleaver O, Mcknight SL. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325(5939):435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113▪.Yanes O, Clark J, Wong DM, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6(6):411–417. doi: 10.1038/nchembio.364. Comprehensive metabolomic study examining the energy metabolism of embryonic stem cells and their differentiated progeny. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilding M, Dale B, Marino M, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16(5):909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 115.Im CN, Kang NY, Ha HH, et al. A fluorescent rosamine compound selectively stains pluripotent stem cells. Angew Chem Int Ed Engl. 2010;49(41):7497–7500. doi: 10.1002/anie.201002463. [DOI] [PubMed] [Google Scholar]
- 116.Kang NY, Yun SW, Ha HH, Park SJ, Chang YT. Embryonic and induced pluripotent stem cell staining and sorting with the live-cell fluorescence imaging probe CDy1. Nat Protoc. 2011;6(7):1044–1052. doi: 10.1038/nprot.2011.350. [DOI] [PubMed] [Google Scholar]
- 117.Cezar GG, Donley EL. Stemina biomarker discovery. Regen Med. 2008;3(5):665–669. doi: 10.2217/17460751.3.5.665. [DOI] [PubMed] [Google Scholar]
- 118.West PR, Weir AM, Smith AM, Donley EL, Cezar GG. Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicol Appl Pharmacol. 2010;247(1):18–27. doi: 10.1016/j.taap.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 119.Macintyre DA, Melguizo Sanchis D, Jimenez B, Moreno R, Stojkovic M, Pineda-Lucena A. Characterisation of human embryonic stem cells conditioning media by 1H-nuclear magnetic resonance spectroscopy. PLoS One. 2011;6(2):e16732. doi: 10.1371/journal.pone.0016732. [DOI] [PMC free article] [PubMed] [Google Scholar]