Abstract
Puberty is an important transition that enables reproduction of mammalian species. Precocious puberty, specifically early thelarche (the appearance of breast “buds”), in girls of multiple ethnic backgrounds is a major health problem in the United States and other countries. The cause for a continued decrease in the age of breast development in girls is unknown, but environmental factors likely play a major role. Laboratory and epidemiological studies have identified several individual environmental factors that affect breast development, but further progress is needed. Current research needs include increased attention to and recording of prenatal and neonatal environmental exposures, testing of marketed chemicals for effects on the mammary gland, and understanding of the mammary gland–specific mechanisms that are altered by chemicals. Such research is required to halt the increasing trend toward puberty at earlier ages.
Keywords: puberty, mammary gland, developmental basis of adult disease, environmental factors, diet, breast cancer
INTRODUCTION
Puberty is the period of transition from childhood to adolescence. It is marked by maturation of the hypothalamic-pituitary-gonadal (HPG) axis, development of secondary sexual characteristics, accelerated growth, behavioral changes, and ultimately attainment of reproductive ability. Puberty is influenced by hormones, particularly estrogens in females. However, the exact neuroendocrine mechanisms involved in its onset are complex and not completely understood (1). The earliest physical sign of puberty in girls is often the development of breast “buds” (thelarche), followed by development of pubic hair (pubarche). Initiation of menses (menarche) is usually a later pubertal endpoint. Timing and patterning of these milestones of puberty can yield information on the overall health status of an individual, adequacy of nutrition and growth, and past environmental/nutritional or pharmaceutical exposures; they may also predict future health outcomes (2, 3).
Recent studies have reported a progressive decrease in age of onset of puberty in girls, as determined by early breast development, especially among minority populations in the United States (4–8). Similar trends have been reported in Europe and among girls adopted from developing countries by Western parents (9, 10). Although the age of onset of breast development has decreased over the past several decades, age at menarche has not significantly changed (9, 11). The exact reason for early onset of breast development is not understood, but it is generally believed to be the outcome of a complex interaction among genetic, endocrine, and environmental factors (5, 8, 12, 13). Because precocious thelarche and puberty have been linked to adverse health outcomes in adults (Table 1), concern about this shift in timing of these developmental processes is warranted. The purpose of this review is to discuss (a) the regulation of puberty; (b) critical windows of perinatal development that may influence timing of mammary growth; (c) breast development comparisons in humans and rodents; (d ) factors that can influence mammary development timing in rodents or girls including body weight, diet, and exposures to environmental factors such as endocrine-disrupting chemicals (EDCs); (e) the usefulness of rodent models to study environmental effects on mammary development and maturation; and ( f ) future research needed in this area.
Table 1.
Risk factors in adults associated with early puberty in females
| Health risk | Reference(s) |
|---|---|
| Accelerated skeletal maturation and short adult height | 117–119 |
| Early sexual interests; risk-taking behaviors such as smoking, using alcohol and drugs, engaging in unprotected sex | 120–122 |
| Psychosocial and behavioral difficulties | 3, 117 |
| Obesity and eating disorders | 122–126 |
| Type 2 diabetes and insulin resistance | 118, 127–130 |
| Cardiovascular disease and hypertension | 118, 125, 127, 131 |
| Increased breast and reproductive cancers | 3, 118, 127 |
| Asthma | 127 |
| Polycystic ovarian syndrome | 128, 129, 132, 133 |
| Depression | 121, 122 |
REGULATION OF PUBERTY ONSET AND PROGRESSION
Humans
The onset of puberty occurs as a consequence of activation of the HPG axis. First activated in mid-fetal life, the HPG axis continues to function after birth into infancy but becomes quiescent during childhood. Hypothalamic changes initiate the reactivation of the HPG network, which then triggers the onset of puberty. Although the factors involved in the reactivation of the HPG axis have not been completely identified, kisspeptin, a hypothalamic neurotransmitter, directly stimulates gonadotropin-releasing hormone (GnRH) (14). GnRH, the main hormone of puberty, is responsible for increasing the frequency and amplitude of the increase in kisspeptin. Increases in GnRH also enhance the pulsatile secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary, resulting in stimulation of the gonads (ovaries or testes) to secrete their respective hormones (15, 16). An elevated concentration of gonadal hormones in morning urine samples is a marker of puberty (17). In females, the maturation of the neuroendocrine/reproductive axis leads to the onset of menses, followed by the establishment of regular menstrual cycles when appropriate LH and FSH signals cause the secretion of gonadal hormones. Ovarian theca cells secrete androgens and granulosa cells secrete estradiol before ovulation, whereas the corpus luteum (formed at the follicle following an ovulation) secretes progesterone and estradiol (6, 13, 18). In males, maturation of the neuroendocrine/reproductive axis differs from that of females; that is, LH initiates the secretion of androgens (testosterone and androstenedione) from testicular Leydig cells. Although activity in the HPG axis increases at puberty, it is functional throughout a child’s life, thus explaining why there are low levels of LH, FSH, and estradiol in boys and girls even prior to puberty (19, 20).
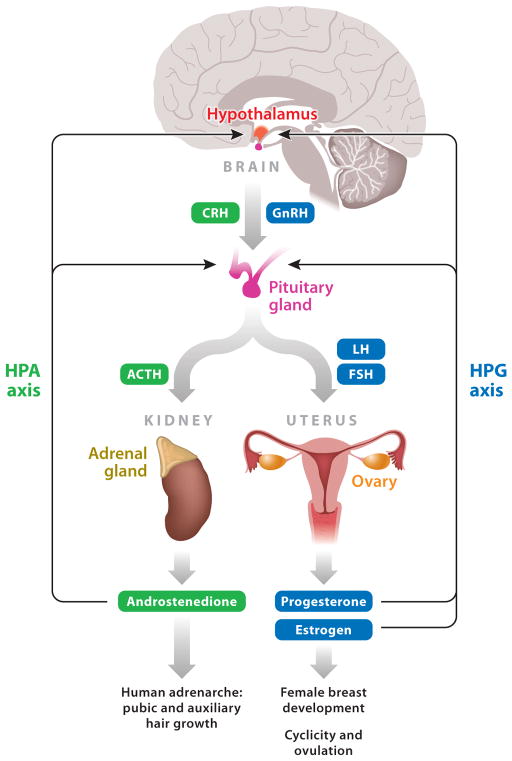
In addition to reactivation of the HPG axis, the secretion of adrenal androgens starts in early puberty and is under the control of the hypothalamic-pituitary-adrenal (HPA) axis. Androgens cause pubic and auxiliary hair growth, and they sensitize the androgen receptors of the hypothalamus and pituitary that play a role in attainment of puberty and reproductive maturation. Disruption of the interacting signals in the HPG and HPA pathways can result in altered timing of puberty (Figure 1).
Figure 1.
Hormonal control of female puberty. Pubertal timing in humans is controlled by both the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axes. Breast development (thelarche) and pubic and auxiliary hair development (adrenarche) are regulated by these hormonal circuits. Other abbreviations: ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
External physical markers that accompany biochemical signals at puberty include (a) thelarche, which may be unilateral; (b) pubarche; (c) menarche; and (d ) attainment of regular menstrual cycles, all of which signify pubertal onset and/or progression. Physical markers of breast development and pubic hair growth have been classified and staged using the Tanner Staging criteria defined by Marshall & Tanner (21, 22). These physical markers of puberty may be regulated by different triggers and may not necessarily be interrelated. For example, thelarche follows the secretion of ovarian estrogen, whereas pubarche, one of the processes that occurs within the context of adrenarche, is caused by androgen secretion from the adrenal glands (adrenarche) and ovaries. Adrenarche, or the initiation of pubic and auxiliary hair growth (in the armpits and on the legs and face), appears to be a phenomenon limited to primates and humans (23). Because estrogen secretion and androgen secretion are controlled separately, adrenarche and thelarche can occur either in a coordinated manner (which is usual) or independently (unusual). Menses are initiated in response to FSH and LH, usually late in puberty and sometimes several years after breast budding. The onset of menarche is often used to define puberty, but this provides a limited perspective relative to other markers of puberty and its progression.
Estrogens play essential roles at puberty, not only in breast development but also in bone and brain maturation in girls and boys. Estrogens control the timing of the “growth spurt,” a marker of puberty. The fact that girls start their growth spurt before boys adds support to the idea that estrogens are involved in bone growth at puberty. Furthermore, patients with estrogen receptor (ER) mutations demonstrate stunted epiphyseal maturation, altered skeletal proportions, and bone mineralization (16).
Rodents
Rodents have been extensively used to study the mechanisms involved in initiating mammalian puberty. Pubertal onset in female rodents has traditionally been assessed through the study of endpoints such as vaginal opening (VO) and age of first estrus (similar to menarche in humans) because VO occurs the day after the first preovulatory surge of gonadotropins (24). Few studies in animals have assessed the hallmarks of mammary gland development or differentiation as an indicator of pubertal progression (25–29), although thelarche is usually the initial and most sensitive marker in humans. VO is commonly used as a pubertal sign in rodents but not in humans. Adrenarche, on the other hand, is commonly used as a pubertal marker in girls but not in rodents. However, mammary gland development and progression are similar in rodents and humans.
Although humans and rodents have similar biochemical signals and regulators of the HPG and HPA axes, there are differences in the controlling mechanisms (6). In humans, control of GnRH activation is centralized in the hypothalamus and is independent of ovarian input, whereas in rodents, ovarian secretions control GnRH regulation. Thus, the same pathways that activate GnRH at puberty are operational in both rodents and humans. The discovery of new regulatory genes has revealed additional similarities and differences in brain development and neuroendocrine signaling between humans and rodents (6).
Precocious Puberty
Precocious puberty, i.e., the development of secondary sexual characteristics before age 8 in girls (4), can have either central (gonadotropin-dependent) or peripheral (gonadotropin-independent) origins. Premature thelarche is considered an indicator of precocious puberty; the full range of pubertal changes is not observed, but specific changes in breast development occur. As reported in 1997 in an assessment of 17,000 U.S. girls (4), ~8% of white girls and 25% of black girls exhibited evidence of early breast buds. Such data led to guidelines published in 1999 (30) that recommended puberty be considered precocious only when breast development or pubic hair appear before ages 6 and 7 in black and white girls, respectively.
A recent study suggests that the incidence of precocious puberty continues to rise (31). This study of nearly 1,200 girls from three areas of the U.S. showed that of girls aged 7 and 8, respectively, puberty had begun in 10% and 18% of white non-Hispanic girls, 23% and 43% of black girls, and 15% and 31% of Hispanic girls. White non-Hispanic girls showed the largest decrease in age of puberty onset between the 1997 (4) and the 2010 reports (31); the girls studied in the 2010 report were nearly twice as likely to have begun puberty by ages 7 and 8 than were girls of those ages in the 1997 study.
Usually premature thelarche progresses slowly; sometimes it is rate limiting and stops, or it can develop into central precocious puberty. Either scenario extends the period leading up to mature breast development. At the opposite end of the spectrum, delayed puberty can be defined as the absence of breast development by age 13 and can result from primary hypothalamic, pituitary, and/or ovarian dysfunction (6). Both precocious and delayed puberty, as determined by the stage of breast development, can have long-term health consequences. Although precocious or delayed puberty in girls can have genetic and/or environmental causes, this review focuses on potential environmental influences.
In summary, puberty is a complex, multifaceted process that occurs over a period of years and that is characterized by numerous events including breast growth. Because puberty is influenced by hormones, alterations in their balance during development can have adverse consequences on the timing of puberty and its progression.
WINDOWS OF SUSCEPTIBILITY IN PERINATAL DEVELOPMENT
The term window of susceptibility refers to a time in development when environmental factors can significantly alter the developing organism and result in structural, functional, and/or cellular changes. Alterations occurring during these windows may not be identifiable early in life but become apparent only later; thus, changes during early prenatal or neonatal development may alter pubertal timing later in life.
Fetal and neonatal developmental periods are particularly sensitive times when external influences can disrupt active organogenesis, tissue remodeling, and signaling. The developing organism lacks much of the protective machinery available to adults such as DNA repair mechanisms, a fully competent immune system, an intact blood-brain barrier, detoxifying enzymes, and mature liver metabolism (32–34). Furthermore, although cell differentiation for neuroendocrine circuitry and breast development starts early in prenatal life, tissue differentiation continues in infants and adolescents. Moreover, signaling pathways are established during perinatal development for proper functioning of the HPG and HPA axes at puberty.
Developmental exposure to environmental factors, including body weight, diet, drugs, and chemicals, can interfere with maturation of endocrine signaling pathways and cause adverse consequences later in life (35–37). In humans, prenatal exposure to diethylstilbestrol (DES) is the best example of an environmental estrogen that causes delayed or long-term effects (34). Effects of prenatally administered DES include vaginal carcinoma and structural alterations of the reproductive tract during puberty; later effects include reproductive problems, such as an increased incidence of uterine fibroids, and breast cancer (38). Similar effects occur in animal models following perinatal exposure to DES (39). Other important windows of growth and differentiation also exist. For the mammary gland, three such windows of susceptibility to exogenous factors have been identified: (a) fetal (mammary bud development), (b) puberty (exponential mammary growth), and (c) pregnancy (differentiating breast) (12).
BREAST/MAMMARY GLAND DEVELOPMENT
Rodents are often used to study human breast development because the biological processes involved in mammary growth and differentiation are conserved across species. In embryonic/fetal development, developmental processes that occur prenatally in humans may happen both prenatally and neonatally in rodents; see the following Web site for an overview: http://www.endocrinedisruption.com/prenatal.criticalwindows.overview.php. For this comparison, the term breast tissue is used when referring to humans and the term mammary gland is used when referring to rodents and other animals.
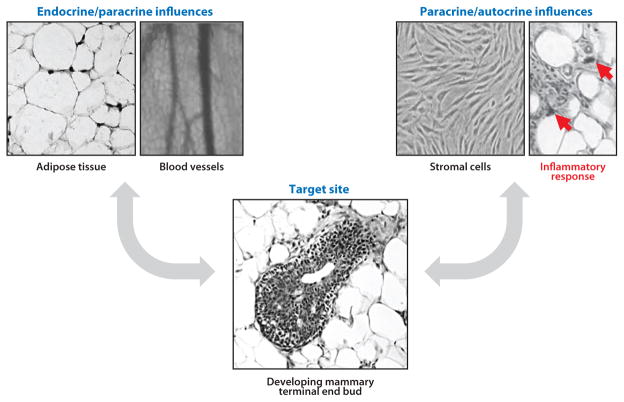
Breast/mammary gland development is complex. Branching epithelial cells proliferate and differentiate to fill the fat pad of adipocytes, and the stromal and myoepithelial cells (often referred to as mesenchyme) encompass and support much of the epithelium; all of these cells are in close communication with immune, stem, and endothelial cells. This development is controlled by a carefully orchestrated series of events characterized by extensive proliferation followed by differentiation of cells and structures. Critical events in breast development begin during fetal life with epithelial bud sprouting, whereas extensive branching morphogenesis continues into postnatal life. This is followed by exponential epithelial outgrowth during puberty; the fat pad rapidly fills with epithelia to produce the adult breast. Mature breast tissue subsequently undergoes only minor changes, largely dependent on the stage of the menstrual (humans) or estrous (rodent) cycle. Breast tissue undergoes prominent differentiation during late pregnancy, ultimately becoming competent for functional lactation (12, 40). Early breast development is regulated by endogenous hormones—mainly estrogen, which promotes growth of the ducts, and progesterone, which promotes lobuloalveolar development—whereas prolactin, insulin, and corticosteroids play a primary role in lactation (41). The mammary gland is also an autocrine/paracrine tissue, producing and responding to hormones produced by its own cells, as shown in Figure 2. At the cellular level, these hormones control cell proliferation by regulating the expression of critical growth factors, and they also control synthesis and secretion of cell type–specific proteins in the breast. These effects are primarily mediated by the interaction of these hormones with their receptors in breast tissue (for estrogens, the interaction is mainly with ER-α and ER-β). The various hormone receptors can also interact with exogenous compounds such as DES, which binds to ERs and interacts with developing breast tissue, causing altered proliferation and differentiation. Other chemicals can interact with developing rodent breast tissue and later impair growth and lactation; such chemicals include Ziracin™, an antibacterial compound that demonstrates androgen-like effects (42); dioxin, a pollutant that binds the aryl hydrocarbon receptor (43); atrazine (27); and perfluorooctanoic acid (PFOA) (44). The mechanisms by which most chemicals alter breast development are not known.
Figure 2.
The multiple influences on mammary development. Environmental exposures and endogenous signals may affect endocrine organs as well as tissues near the mammary gland (i.e., fat), and those nearby tissues can send altered messages through the vascular system, culminating in perturbed mammary development. Mammary tissue may also be a direct target of environmental exposures; epithelia, fibroblasts, fat cells, and inflammatory cells express unique and shared receptors that are targets for environmental chemicals. The tight cellular junctions signal across cell types, affecting neighboring cells as well as the target cells. These various endocrine/paracrine/ autocrine signaling mechanisms may affect the status of mammary epithelia over the lifetime of the individual.
Humans
In humans, the breast is present early in prenatal life; its development starts as a thickening of the milk lines on the ventral surface of the fetus as early as 4–5 weeks postconception. Epithelial budding and branching begins between 6–20 weeks of gestation; the breast buds extend, producing cords of epithelia that grow through the underlying mesenchyme. The mesenchyme develops supporting stroma between weeks 20 and 32. The epithelial cords develop a lumen during the last 2 months of gestation when ductal branching occurs, yielding a primitive gland composed of ducts ending in ductules at birth. Close to birth, the nipple is formed by invagination of the breast surface (45). ERs and progesterone receptors have been localized in breast epithelial cells as early as gestational weeks 30 and 41, respectively (46), suggesting that this tissue has the cellular machinery to respond to hormones or hormonal mimics early in prenatal differentiation. During childhood and adolescence, growth of the breast keeps pace with overall body growth but greatly accelerates at puberty.
Rodents
Similar development patterns occur in the rodent and human mammary glands. In the mouse, mammary gland development begins approximately at embryonic day 10.5. By embryonic day 11.5, five lens-shaped ectodermal structures appear along two mammary lines; these structures invaginate into the dermis by embryonic day 13.5. The mesenchyme adjacent to the mammary epithelium becomes denser than the surrounding mesenchyme and develops several concentric layers of fibroblasts that align around the epithelial structure (47). At fetal day 15.5, in response to mesenchymal signals, the epithelial bud starts to elongate to become a cord. At fetal day 16, proliferation of the primary cord increases as the cord pushes through the surrounding mammary mesenchyme and grows through the fetal fat pad found deep in dermal tissue. By fetal day 18, just before birth, branching of the structure is apparent and a lumen that will differentiate into a mammary duct starts to form (48).
Mammary bud development in the rat begins around embryonic day 12 and follows the same developmental and temporal pattern as in the mouse, culminating on gestation day 21, just before birth, in six sets of glands with a slightly more branched structure than that found in the mouse. Growth and branching of the mammary structure in rodents continue after birth. Rat mammary duct ends are more complex than those of the mouse, with extensive alveolar budding and lobule formation following puberty.
Interference in branching and differentiation of mammary tissue could lead to altered timing of maturation at puberty, abnormal morphology, and permanent effects on glandular structures (e.g., altered number of primary ducts, blind ducts, or unusual presence of nipples/areola) (12). Furthermore, mammary glands in rodents, like those in humans, undergo exponential growth in the peripubertal period. During peripuberty (several weeks in rodents and several years in girls), structures termed terminal end buds (TEBs) are at the tips of the epithelial ducts of the growing mammary gland and are composed of actively proliferating cells that facilitate the growth of the mammary epithelial ducts to form a tree-like pattern (28). The ends of the tear-shaped TEBs are multiple cell layers thick and are sites of further branching. TEBs disappear from the mature gland as it differentiates. Susceptibility of these developing mammary gland structures to carcinogens has been described in more than two decades of work (49–51). Thus, any environmental factor, drug, or chemical that prolongs the period of TEBs or slows maturation of the developing mammary gland may have the potential to affect the sensitivity of the gland to the activity of carcinogens.
Similarities and Differences in Mammary Gland Development Between Humans and Rodents
Slight timing differences exist in mammary gland development between humans and rodents, although ductal branching originates in prenatal life for both species. Furthermore, minor differences in the extent of ductal branching can be seen at birth across species, and alveolar buds are prominent in young rat and human mammary glands but not in immature mice. The growing mammary ducts end in TEBs in rodents and in morphologically analogous terminal ductal lobular units in humans. Both structure types contain actively proliferating cells and potentially stem cells, likely accounting for their sensitivity to carcinogens. Precocious onset of breast development at puberty may represent a direct effect on the mammary tissue itself (i.e., exposures during bud and branching development), an indirect effect (i.e., an effect acting on the neuroendocrine pathway of puberty), or a combination of both. Altered prenatal imprinting or altered stem cell populations, whether direct or indirect, is a promising area for additional research.
To summarize, although developmental milestones are similar between humans and rodents, the timing of the events may differ slightly. Because molecular and cellular pathways of mammalian differentiation are conserved across species, the rodent is an excellent model by which to study the role of environmental factors that disturb normal maturation of the mammary gland. The mammary gland in humans and rodents undergoes dramatic changes during pregnancy in preparation for lactation, its primary function. Interruption of these differentiation processes can lead to malnutrition or death of the offspring; this is particularly important in animals that rely solely on maternal nourishment for survival. However, for humans, a potential effect of an environmental exposure on lactation can be missed if formula feeding is used.
ENVIRONMENTAL FACTORS INVOLVED IN PRECOCIOUS BREAST DEVELOPMENT
Body Weight and Diet
Childhood obesity, measured as body mass index, has been associated with early pubertal development (52). The pioneering work of Herman-Giddens et al. (4) raised the possibility of a link between increasing rates of obesity and the trend toward early puberty. Various mechanisms have been proposed to explain such an association; these include increased levels of circulating estrogens in obese girls and/or increased aromatase activity in breast fat that results in increased conversion of local or systemic androgens to estrogens, thereby overexposing breast tissues to estrogen during prepubertal years (53). Another possibility is that leptin produced by body fat or breast cells triggers the onset of breast development. Studies in humans suggest that leptin has a permissive role in puberty onset (54), but it is not a crucial modulator, and increased leptin may be a consequence of pubertal development (55). Other molecular regulators may influence pubertal timing (54): (a) GPR54, a G protein–coupled receptor gene that regulates GnRH; (b) the kisspeptin family of neuropeptides; and (c) EAP1, which is known as the puberty gene. All of these are considered essential for normal puberty and may be altered in obese children or altered by components in their diets. Studies in rodents offer insight into the role of estrogen agonist or antagonist activity of dietary components, such as genistein and other soy constituents, in altering pubertal mammary development. A variety of mammary-specific effects depend on the timing, route and dose of exposure, the species evaluated, and diet (56). Dietary components may also be important in children, especially in those fed soy-based formulas.
Most important, the relationship of puberty and body weight/diet may be related to changes during perinatal life rather than changes in adolescence or puberty. The Developmental Origins of Health and Disease paradigm (57, p. 356) describes the association of proper nutritional support (i.e., the rate of weight increase during infancy and early childhood) as a crucial determinant in body mass index and in the timing of puberty (37). Therefore, the prenatal and neonatal periods are critical times during which diet and nutritional status can “program” or permanently change subsequent growth and development, including the timing of puberty and risk of obesity.
Hormones and Endocrine-Disrupting Chemicals
Both endogenous and exogenous environmental influences can cause precocious TEB differentiation and breast development. Endogenous hormones, such as steroids, influence mammary tissue development, but over the past 15 years, it has become evident that exogenous drugs/chemicals may alter the effects of these hormones (35). EDCs are exogenous compounds that mimic, block, or alter the activity of endogenous hormones synthesized by the endocrine system (58). Estrogens have been studied most, although chemicals with antiestrogenic, androgenic, antiandrogenic, progesterone-like, and thyroid-like activity can be endocrine disruptors and may play a role in precocious puberty and breast development, as discussed below and summarized in Table 2.
Table 2.
Chemicals known to induce effects in rodents on breast [mammary gland (MG)] development and in adulthood
| Compound | Rodent model tested | Accelerated MG development | Delayed MG development | Related putative long-term consequences | Reference(s) |
|---|---|---|---|---|---|
| Atrazine | Rat | ✓ |
|
27, 29, 102, 134, 135 | |
| Bisphenol A | Rat and mouse | ✓ | ✓ |
|
25, 48, 72, 76, 77, 79, 80, 81 |
| Cadmium | Rat | ✓ |
|
94–96 | |
| Dieldrin | Rat | ✓ |
|
136, 137 | |
| Dioxin or TCDD | Rat | ✓ |
|
26, 43, 56, 104, 105, 138 | |
| DES | Rat and mouse | ✓ |
|
38, 63, 68, 139–141 | |
| Genistein, zearalenone, resveratrol | Rat | ✓ | ✓ |
|
28, 86–93 |
| Nonylphenol | Rat | ✓ |
|
82–84, 134 | |
| Organochlorines | Rat | ✓ |
|
138, 142 | |
| Phthalates | Rat | ✓ |
|
85, 143 | |
| PCBs | Rat | ✓ | ✓ |
|
144 |
| PhIP | Rat | ✓ |
|
145, 146 | |
| PFOA | Mouse | ✓ |
|
44, 112, 113, 147 | |
| High-fat diet (PUFA) | Rat and mouse | ✓ | ✓ |
|
97–99, 148 |
| PBDE | Rat | ✓ |
|
110, 111 | |
| Ziracin™ (antibacterial) | Rat | ✓ |
|
42 |
Abbreviations: DES, diethylstilbestrol; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFOA, perfluorooctanoic acid; PUFA, polyunsaturated fatty acid; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine.
Sex hormones
The endogenous hormones of puberty have roles in mammary gland development, and altered exposure to hormones can shift the delicate balance needed for normal maturation. Female rats exposed prenatally to testosterone have altered reproduction later in life and no nipple development (i.e., masculinized mammary morphology), suggesting that gestational exposure to testosterone severely inhibits normal development of females, rendering them unable to nurse their offspring (59). However, early postnatal treatment of mice with testosterone stimulates ductal branching in the mammary gland around the time of puberty (60). These divergent outcomes point out the importance of timing in the exposure to EDCs and potentially other environmental factors.
Perinatal treatment of mice with estradiol eventually leads to accelerated mammary gland development. Estradiol exposure on postnatal days 1–5 dose-dependently increases the number of duct junctions and the mammary growth area at 5 weeks; an increased estradiol dose increases ductal branching, while the ratio between branching and total mammary area remains constant (61). That effect is either not evident or attenuated prior to puberty. Similar effects have been noted in mice administered estradiol on neonatal days 1–5: inhibition of ductal branching in the first 6 days of life with tripling of the number of duct branches postpuberty, surpassing growth in age-matched untreated mice (60). This accelerated development after estradiol exposure correlated with a greater number of undifferentiated TEBs. TEBs were evident earlier in the estradiol-treated mice than they were in the controls, but the density of the epithelial ducts was comparable around the time of puberty. In early adulthood, TEBs in control animals differentiated, whereas TEBs in estradiol-treated mice remained unchanged, making the animal more susceptible to neoplastic lesions later in life (49–51, 62).
Chemicals with hormone-like activity
Numerous environmental compounds have demonstrated hormone-like activity in rodent studies and humans, with estrogenic effects most often noted. The compounds exhibiting hormone-like activity are pharmaceuticals, dietary factors, and environmental chemicals.
Diethylstilbestrol
DES, a potent synthetic estrogen, was prescribed to millions of pregnant women from the 1940s to the 1970s with the mistaken belief that it could prevent miscarriages. It is now well documented that prenatal DES treatment of men and women results in a low but significant increase in neoplastic lesions in the reproductive tract and a high incidence of benign reproductive lesions (38). Administration of DES to pregnant mice causes similar reproductive abnormalities and dysfunction in their offspring (63). An increased incidence of breast cancer has also been noted in prenatally DES-exposed women (64–66) and their mothers (38, 67). Women >40 years of age and exposed in utero to DES have an estimated 1.9 times increased risk of developing breast cancer compared with unexposed women of the same age. Additionally, the highest risks were correlated with the highest cumulative doses of DES during gestation (65).
Many studies in rodents have demonstrated alterations in mammary gland development following perinatal exposure to DES. Prenatal DES exposure caused excessive nipple development in both male and female offspring of pregnant rats. Furthermore, females are unable to nurse their offspring, most likely because of the absence of the nipple sheath, an indication that the connection between the mammary ducts and the nipple failed to form (68). Gestational or lactational exposure of rats to DES produces extensive lobuloalveolar proliferation, decreased tumor latency, and greater multiplicity of spontaneous tumors (69). Prenatal or neonatal exposure to DES also increases mammary tumorigenesis in Syrian golden hamsters and rats treated with a known mammary gland carcinogen, dimethylbenz(a)anthracene. DES increased the number of mammary tumors, tumor multiplicity, and the grade of tumor malignancy, suggesting that in addition to being carcinogenic, it also increases the sensitivity of the mammary gland to other carcinogens (38).
Mice exposed to DES just after birth demonstrate increased outgrowth of the mammary gland ducts around the time of puberty, dilated mammary ducts noted at 12 weeks of age, and precocious lactation. Additionally, following DES exposure during fetal life, mice exhibit increased mammary tumor incidence as adults (70).
Bisphenol A
Bisphenol A (BPA), a component of polycarbonate plastics and epoxy resins, is produced at high volumes (>800 million kg annually in the U.S.), and humans have widespread exposure. BPA can leach into consumables from the linings of food cans, baby bottles, and beverage containers and is found in dental sealants and composites. Measurable BPA levels have been found in human urine, serum, breast milk, maternal and fetal plasma, amniotic fluid, and placental tissues (71, 72). BPA can be brominated or chlorinated to produce flame retardants; tetrabromo-bisphenol A is the most commonly used, with >60,000 tons produced annually (72). Serum levels of brominated flame retardants are increasing in adults, but, more important, levels in infants and children are markedly higher than in adults (73).
BPA is described as a weak estrogen, but numerous studies find that it may have other biological activities at low, environmentally relevant exposure levels. In addition to binding to the nuclear ER-α and ER-β, BPA interacts with other cellular targets, including the nonclassical membrane-bound form of the ER (ncmER); a recently identified orphan nuclear receptor termed estrogen-related receptor gamma (ERR-γ); a seven-transmembrane estrogen receptor (GPR30); and the aryl hydrocarbon receptor. Interactions with ncmER and ERR-γ are especially noteworthy because BPA binds to these receptors with high affinity (IC50 value of 13.1 nM); 4-nonylphenol and DES bind with a 5–50-fold lower affinity than that exhibited by BPA (74). BPA can also act as an androgen receptor antagonist and can interact with thyroid hormone receptors (75).
Studies in animals have shown that low doses of BPA (i.e., in the range of human exposures) can exert adverse effects if given during development. Numerous low-dose studies have suggested that perinatal BPA exposure is associated with abnormalities in male and female reproductive tissues (72, 76). BPA alters mammary gland cell proliferation, ductal elongation, and ductal differentiation during the peripubertal and early adult life stages. Low-dose BPA treatment (25 μg kg−1) of mice from mid-pregnancy until birth causes greater mammary duct elongation than in controls, whereas high-dose treatment (250 μg kg−1) causes less ductal elongation. Additionally, increases occur in the number of alveolar and ductal structures of 6-month-old mice treated with both low and high doses of BPA (25). In another study, environmentally relevant doses (25 and 250 ng kg−1 per day) of BPA administered to mice during pregnancy and lactation resulted in female offspring with more TEBs relative to the ductal area and fewer apoptotic TEB cells around the time of puberty compared with the controls; low-dose-exposed mice had a greater number of lateral branches and ducts (77). Exposure to 250 ng kg−1 BPA from mid-pregnancy until parturition altered the histologic architecture and tissue organization of the fetal mammary gland to produce increased ductal growth, smaller epithelial cells, and accelerated fat pad maturation, the latter of which resulted in a less dense fat pad, increased ductal collagen density, and decreased collagen density in the stroma (48). Changes in stromal composition and fat pad density may be involved in determining lifetime risk for tumor development (78).
BPA also heightens the susceptibility to carcinogens with increasing age in rats and initiates the development of “beaded” ducts in 9-month-old mice (79, 80). Ductal beading represents the merging of actively proliferating luminal epithelial cells (intraductal hyperplasia). This hyperplasia is considered a precursor to ductal carcinoma, suggesting that BPA induces both an elevated susceptibility to carcinogens (81) and spontaneous neoplasia—changes that are associated with early-life changes in development around the time of puberty.
Nonylphenol
Nonylphenol is used as a surfactant, emulsifier, and detergent, and it has estrogenic properties. It is found in the lining of food containers and wraps, in cleaning compounds, and in spermicides. Administration of nonylphenol to rats produces a dose-dependent, 200%–400% increase in mammary cell proliferation, with increased S phase relative to G0 and G1 phases of the mammary epithelial cell cycle (82). Nonylphenol also produces DNA mutations and chromosomal abnormalities (83). These changes can cause genetic instability and can increase the risk for developing neoplastic lesions and mammary tumorigenesis.
When rats are given nonylphenol during pregnancy, their female offspring have increased proliferative mammary epithelial branching and budding just after birth. By puberty, such rats had more alveolar buds and increased TEB differentiation compared with controls, thus indicating precocious mammary epithelial development (84).
Phthalates
Di-n-butyl phthalate is used to soften plastics (such as those used for medical tubing and children’s toys) and to aid in the dispersal of health and beauty aids (e.g., perfume). Environmental contamination of phthalates is widespread; they are detectable in human urine samples. Following perinatal di-n-butyl phthalate exposure from late pregnancy until weaning, adult female offspring exhibit poor mammary alveolar branching and hypoplasia, adolescent male offspring demonstrate retained nipples (normally absent) at high doses, and adult males exhibit dilation of mammary alveolar buds and ducts (85).
Phytoestrogens
Phytoestrogens are naturally occurring chemicals found in plants. Efforts to implement healthier lifestyles by decreasing fat intake have resulted in increased consumption of vegetables, especially soy products, and supplements that are high in phytoestrogens (28). Effects of developmental exposure to genistein, one of the most abundant phytoestrogens in soy, have been studied, in part because some infants rely solely on soy formula for sustenance during their first year of life (28, 86). Genistein has varying effects on development in rodents depending on time, dose, and method of exposure (86); notable outcomes include alteration of mammary gland development and ovarian dysfunction following exposure during early life. Mice given genistein just after birth show dose-dependent changes in TEB differentiation and ductal branching around the time of puberty. The highest dose tested caused a decrease in the number of TEBs and decreased branching, suggesting delayed mammary gland development, but mice exposed to a low dose of genistein displayed increased branching and ductal elongation. The morphological differences (reduced lobuloalveolar development and dilated ducts) appeared to be permanent because they were also observed at 9 months of age (28). Prepubertal exposure to genistein in rats decreases the number of TEBs and increases the number of terminal ducts in 50-day-old animals. These results suggest an enhanced rate of differentiation of TEBs into terminal ducts after genistein treatment, thus signifying precocious development (87).
The effects of genistein exposure during development and cancer susceptibility seem to depend on the timing of exposure. In two studies that included 5 days of postnatal exposure, TEBs appeared earlier in treated than in control animals (precocious mammary development), and these TEBs differentiated into mature structures earlier than did those of the controls; both studies showed a decreased risk of developing cancer (88, 89). One of those studies observed a decrease in multiplicity of tumors in rats treated with genistein (88), and the other found a significant increase in the density of lobuloalveolar structures, which correlated with a decreased susceptibility to environmental carcinogens (89). Conversely, another study showed altered growth and developmental programming of ERs in mammary tissue exposed to genistein during neonatal life, a setting that typically puts one at increased tumor risk (28). Other studies in rodents have found results consistent with an increased risk of mammary tumorigenesis following prenatal genistein exposure: precocious development of TEBs and decreased differentiation with age. These findings indicate a longer period for potential TEB exposure to environmental toxins and provide evidence that treatment of rats with genistein increases the incidence of tumors if animals also receive a mammary gland carcinogen (90, 91).
In a multigenerational study conducted by the National Toxicology Program, rats received genistein in their diet starting during prenatal development. Alterations were seen in mammary glands of both males and females, and changes in peripubertal males were most apparent (92, 93). As genistein-treated rats aged, they had an increased incidence of mammary carcinomas.
Metals
Naturally occurring metals can be classified as EDCs because they mimic or perturb the normal hormonal milieu. Prenatal exposure to low levels of cadmium, for example, can alter mammary development in mice and rats, mimicking estrogen effects (94). Treatment of a human breast cancer cell line (MCF-7 cells) with cadmium decreases ER protein and mRNA, stimulates the estrogen response element, and induces cell growth (95). These results suggest that cadmium can modulate and promote growth of breast cancer cells (95).
Mice exposed postnatally to cadmium also have more fat globules and degenerating alveolar epithelial cells than do controls, along with a negative dose-response relationship between cadmium and milk-protein expression. At the highest dose, litter weights were reduced compared with control offspring, suggesting that alterations of the lactating mammary gland caused by postnatal exposure to cadmium may perturb its function and impair development of the offspring (96).
Other Factors
Lipids and adipose-derived hormones affect pubertal development. Maternal diet and health can have major effects on fetal development, thus assessing the effect of dietary changes on developing mammary glands is an important endpoint.
n-6 polyunsaturated fatty acid
Maternal rodent diets high in n-6 polyunsaturated fatty acid (PUFA) correlate with larger mammary fat pad areas in female offspring and increased epithelial cell density at puberty; increased density of TEBs occurs concurrently with reduced differentiation into alveolar buds (51). The latter findings are consistent with precocious mammary gland development, yet delayed and prolonged differentiation at puberty was evident, and increased risk of tumorigenesis occurred later in life (97).
n-3 polyunsaturated fatty acid
In contrast, a diet high in n-3 PUFA suppresses mammary gland development and ductal growth in mice. These suppressive effects were found only during stages of intense proliferation, e.g., in early development and puberty, suggesting that pubertal mammary gland development is a sensitive period for effects of dietary fatty acids (98). Upon exposure to a mammary carcinogen, mice on a diet high in n-3 PUFA had a decreased incidence of mammary tumors and increased tumor latency, whereas a diet high in n-6 PUFA enhanced tumor development and decreased tumor latency (99). This may be related to increased numbers of TEBs in n-6 PUFA female offspring and reduced numbers of TEBs in the n-3 PUFA group.
Leptin
The adipose-derived endogenous hormone leptin is best known for its role in energy balance through its actions on leptin receptors in the hypothalamus, but it also plays a role in mammary gland development and function. Leptin-deficient mice are unable to support pups after birth because of undeveloped mammary glands (100). Leptin may also promote mammary tumor development. Breast epithelial cells (HBL-100 and T-47D) treated with leptin exhibit greater proliferation than do the same cells in controls (101). Also, transgenic mice overexpressing leptin and fed a high-fat diet (thus having high serum levels of leptin) developed mammary tumors earlier than did lean mice (101).
ENVIRONMENTAL FACTORS CAUSING DELAYED BREAST DEVELOPMENT
Although less studied, some environmental chemicals delay mammary development. This delay can have detrimental effects on mammary function and morphology and may also be associated with future problems. We note a few examples here and others in Table 2.
Atrazine
Atrazine, an herbicide commonly applied to food and grain crops in the U.S., has undergone risk assessment since the late 1990s owing to its toxicity in rodents and its ability to permeate waterways, soil, and drinking water supplies. Its use is banned in the European Union. Several paradigms of exposure were tested to assess effects of atrazine on mammary gland development and VO; these approaches included a cross-fostering design (102) and limited gestational exposures (27). Atrazine-exposed rats, especially if exposed both in utero and through nursing, exhibit abnormal mammary gland development (decreased branching, delayed migration into the fat pad, and fewer TEBs) just after birth and extending past the time of VO. By age 6 weeks, most TEBs in control animals were differentiated, whereas atrazine-exposed animals retained large numbers of TEBs, suggesting an extended time for mammary tissue development. In timed-exposure studies (27), rats exposed to atrazine for 3 days in late pregnancy had the most persistent delays in mammary development. Although such delayed development was observed in these animals after they reached sexual maturity, VO timing was disturbed only with extended periods of exposure (5 or 7 days). Rats exposed 5 days prenatally to chlorotriazine metabolite mixtures at concentrations 10–1,000 times lower than that of atrazine itself also exhibited abnormal mammary development, with no change in timing of VO (29). These low atrazine exposure levels did not affect VO, suggesting that mammary development and VO timing are regulated by different mechanisms. These results suggest that atrazine metabolites are biologically active, and because atrazine normally occurs in nature in a mixture form, the effects of the mixture may be much more potent than the effects of atrazine alone. Thus, further studies are needed to evaluate the effect of mixtures of compounds on pubertal endpoints.
Second-generation pups born to mothers exposed in utero to atrazine have lower body weights than those born to control dams, suggesting a decreased lactational capacity in rats with abnormal mammary gland development when they become pregnant (27). Moreover, lifetime administration of atrazine to rats in the diet caused early onset and increased incidence of mammary tumors compared with controls. The latter results support the hypothesis that exposure to atrazine causes acceleration of endocrine-changing effects that result in an earlier onset of tumorigenesis, although the mechanism of action for mammary changes is unknown (103).
2,3,7,8-Tetrachlorodibenzo-p-dioxin
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), an incineration- and chemical reaction–dependent pollutant, is an EDC that binds the aryl hydrocarbon receptor to induce adverse effects in development and reproduction, including the mammary glands. The carcinogenic potential of TCDD is well documented (56).
The effect of TCDD exposure on mammary development of the rat has been studied extensively. The female offspring of three different rat strains, each of which was exposed to a single dose of this lipophilic compound a week before birth, exhibited severe and persistent delays in mammary gland differentiation from birth into adulthood. Adverse effects included decreased ductal branching, delayed epithelial migration into the fat pad, and fewer differentiated TEBs. However, exposure near the time of birth or during the nursing period did not alter offspring mammary gland development (26, 104).
Prenatal TCDD treatment of rats, followed by a carcinogen challenge in early adulthood, doubled the incidence of mammary tumors and decreased tumor latency compared with controls. These results suggest that TCDD permanently changes the mammary glands during gestation, resulting in a heightened risk for tumors later in life (105). TCDD also impairs normal lactation in mice exposed during the rapid mammary development of pregnancy (43), leading to malnutrition and death in offspring.
An epidemiological study conducted in Seveso, Italy, correlated TCDD exposure following an industrial accident with an increased risk of breast cancer (106). The hazard ratio for breast cancer associated with a 10-fold increase in serum TCDD levels was 2.1 (95% confidence interval, 1.0–4.6). Because women in the study who had the greatest TCDD exposure are not yet between the ages of 40 and 55, the age of highest breast cancer risk (107), it will be important to follow these women as they age and to continue observing the incidence of breast cancer. Two studies evaluating early-life exposures to dioxins or polychlorinated biphenyls found delayed breast development in adolescents with the highest circulating levels (Seveso, Italy) (108) or the highest prenatal/lactational levels (The Netherlands) of dioxin (109), but later-life adverse effects cannot yet be determined.
Polybrominated Diphenyl Ether
Brominated chemicals are widely used for commercial and industrial products (e.g., flame retardants in textiles, construction materials, and polymers used in electronics). Mammary gland development was delayed in the female offspring of rat dams treated from early pregnancy through weaning with a polybrominated diphenyl ether (PBDE) mixture (DE-71); this treatment provided both gestational and lactational exposure. Mammary glands of the offspring had decreased epithelial growth, limited TEB development, and decreased lateral branches just prior to puberty (110), consistent with other altered reproductive endpoints in rodents exposed to PBDEs (111).
Perfluorooctanoic Acid
PFOA, which is used in fire-fighting foams, electronics, and products designed to be grease-and water-proof, is the final degradation product of other >8-carbon perfluorinated materials. Persistent traces have been found in humans and wildlife, making it an important target for developmental toxicity studies (112). Studies in mice have revealed stunted mammary development after gestational PFOA exposure, although mechanisms for these effects are undetermined (113).
The lowest doses tested produced blood levels in mice that overlap with those reported in humans living in PFOA-contaminated communities in Ohio and West Virginia (112). An expert panel found that delayed puberty in girls in this cohort was associated with the highest levels of serum PFOA (114). However, in reports from a British cohort, prenatal exposure to PFOA and other related compounds was not associated with age at menarche (115). These two studies assessed pubertal outcomes but did not evaluate timing of breast development.
RESEARCH NEEDS AND DATA GAPS
There are known links between breast cancer risk and pubertal timing, breast density, and endocrine disruption. Breast cancer is a leading cause of morbidity in women worldwide; therefore, there is an important need to understand the environmental basis of the disease. Childhood obesity and precocious puberty, both of which may influence breast cancer risk, are at epidemic levels in many countries (8, 9, 31, 52, 57). An understanding of the connection between environmental exposures and breast cancer risk factors may be the missing link that will lead to eventual prevention of the disease. Young women begin breast development earlier than their mothers or grandmothers did, and they require a longer period to attain full breast size, thus lengthening the time of susceptibility to EDCs and other environmental influences. More studies that expose rodents to specific EDCs or mixtures of EDCs during critical windows of mammary tissue development and that include long-term adult health follow-up should help define the environmental factors that influence breast cancer risk in women.
Although research in the past 20 years has made great strides toward understanding the effects of a limited subset of environmental EDCs and how they affect mammary gland development, more information is required. Table 3 defines some of the research needed to make progress in defining the sources for precocious puberty, specifically as it pertains to early breast development. This review identifies many known environmental EDCs that alter mammary gland development, but only a minute fraction of the 85,000+ marketed chemicals have been tested for effects on timing and other aspects of puberty, including mammary development. Chemicals with effects in girls and rodents have been reviewed recently (12, 116). Few of the studies in rodents report biological endpoints in addition to mammary effects, or a dose range for mammary and other effects, so assessing the sensitivity of mammary tissue compared with other tissues is often difficult or impossible. As knowledge of the toxicity of environmental EDCs becomes more widespread, applying it to human-relevant endpoints will be crucial. This can be achieved by cooperative efforts between toxicological and epidemiological studies that include human-relevant data and endpoints in animal studies, such as those provided by internal dosimetry. In this review, we note a few studies that have used doses relevant to humans, but it not clear whether they achieved serum levels similar to those of humans, or if animals received doses comparable with those to which humans are exposed. Identification of biologically relevant mixtures of chemicals that affect mammary tissue is another important research gap.
Table 3.
Research topics that need to be addressed for a better understanding of environmental effects on mammary tissue
| Research needs | Rodent | Human |
|---|---|---|
| Understanding regulation of fetal and neonatal mammary development (which receptor populations are present and when; involvement of other regulatory factors and genes) | ✓ | ✓ |
| Evaluating mammary gland development as a required endpoint in toxicology screening and testing designs | ✓ | |
| Establishing whether early-life (prenatal and perinatal) environmental exposures are associated with changes in timing of puberty | ✓ | ✓ |
| Evaluating mammary development in tandem with other pubertal endpoints so the sensitivity of the tissue can be better assessed | ✓ | ✓ |
| Defining the dose-response relationships between mammary effect and internal dosimetry or body burden of compounds in the environment | ✓ | |
| Examining an association between altered pubertal timing and additional adult adverse health consequence | ✓ | ✓ |
| Determining if mammary-specific effects are multigenerational | ✓ | ✓ |
Many toxicological studies using rodents address pubertal endpoints such as first estrus, estrous cyclicity, and VO. It is not clear whether these endpoints provide information relevant to human health, as different mechanisms may control these and somewhat related human endpoints. Although these are important markers in determining rodent puberty, they do not assess effects on mammary differentiation and development, which are the hallmark endpoints of puberty in humans. Therefore, evaluation of mammary gland development in toxicological studies is vital, especially in the pursuit of using animals as surrogates for humans. Even more important is the need for research that identifies the mechanisms by which EDCs alter mammary gland development, sometimes permanently in female and male offspring (as noted above). These mechanisms may prove crucial in preventing further increases in precocious breast development and breast cancer.
SUMMARY POINTS.
The HPG axis has a primary role in regulating the timing of puberty, ovulation, and cyclicity in rodents and humans, and the HPA axis has proven roles in humans. VO in rodents has no corresponding endpoint in humans, and adrenarche in humans has no corresponding endpoint in rodents.
Mammary gland development is similar across species in the developmental windows of susceptibility, the relative order and pace at which developmental milestones occur, and the regulation by hormones and growth factors.
Precocious breast development is an ongoing problem. Recent reports indicate that the percentage of 7-year-old girls with Tanner Stage II breast development is still on the rise. Environmental factors are thought to contribute to this trend.
Environmental chemicals dysregulate the timing of breast development in rodent models; estrogenic compounds (e.g., DES, genistein, BPA) tend to decrease the time needed for gland differentiation, whereas other chemicals (e.g., atrazine, dioxin, PFOA) delay gland development and prolong maturation. Acceleration or delay in mammary development has been associated with risk for later tumorigenesis.
Although there is information on the effects of EDCs on mammary gland development and related adverse adult health outcomes in rodent models, limited data are available regarding environmental factors associated with precocious thelarche.
Glossary
- HPG
hypothalamic-pituitary-gonadal
- Thelarche
initiation of breast development
- Menarche
initiation of menses or menstruation
- Endocrine-disrupting chemicals (EDCs)
exogenous compounds that mimic, block, or alter the activity of endogenous hormones synthesized by the endocrine system
- GnRH
gonadotropin-releasing hormone
- HPA
hypothalamic-pituitary-adrenal
- Adrenarche
initiation of pubic and auxiliary hair growth, controlled by adrenal androgens
- ER
estrogen receptor
- VO
vaginal opening
- Window of susceptibility
a time in development when environmental factors can cause alterations in the developing organism and result in structural, functional, and/or cellular changes
- DES
diethylstilbestrol
- TEB
terminal end bud
Footnotes
This is a work of the U.S. Government and is not subject to copyright protection in the United States.
DISCLOSURE STATEMENT
The information in this document has been reviewed by the National Institute for Environmental Health Sciences and approved for publication. Approval does not necessarily reflect the views of the Institute.
Contributor Information
Suzanne E. Fenton, Email: fentonse@niehs.nih.gov.
Retha R. Newbold, Email: newbold1@niehs.nih.gov.
LITERATURE CITED
- 1.Ojeda SR, Roth C, Mungenast A, Heger S, Mastronardi C, et al. Neuroendocrine mechanisms controlling female puberty: new approaches, new concepts. Int J Androl. 2006;29:256–63. doi: 10.1111/j.1365-2605.2005.00619.x. discussion 286–90. [DOI] [PubMed] [Google Scholar]
- 2.Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: The National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–43. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- 3.Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–30. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- 4.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 5.Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008;121(Suppl 3):S167–71. doi: 10.1542/peds.2007-1813C. [DOI] [PubMed] [Google Scholar]
- 6.Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(Suppl 3):S192–207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- 7.Mouritsen A, Aksglaede L, Sørensen K, Mogensen SS, Leffers H, et al. Hypothesis: Exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int J Androl. 2010;33:346–59. doi: 10.1111/j.1365-2605.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 8.Toppari J, Juul A. Trends in puberty timing in humans and environmental modifiers. Mol Cell Endocrinol. 2010;324:39–44. doi: 10.1016/j.mce.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 10.Parent AS, Rasier G, Gerard A, Heger S, Roth C, et al. Early onset of puberty: tracking genetic and environmental factors. Horm Res. 2005;64(Suppl 2):41–47. doi: 10.1159/000087753. [DOI] [PubMed] [Google Scholar]
- 11.Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123:e932–39. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 12.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 13.Rasier G, Toppari J, Parent AS, Bourguignon JP. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. 2006;254–255:187–201. doi: 10.1016/j.mce.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Tena-Sempere M. Kisspeptin signaling in the brain: recent developments and future challenges. Mol Cell Endocrinol. 2010;314:164–69. doi: 10.1016/j.mce.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–51. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 16.Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson AM. The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Hum Reprod Update. 2006;12:341–49. doi: 10.1093/humupd/dml018. [DOI] [PubMed] [Google Scholar]
- 17.Wu FC, Brown DC, Butler GE, Stirling HF, Kelnar CJ. Early morning plasma testosterone is an accurate predictor of imminent pubertal development in prepubertal boys. J Clin Endocrinol Metab. 1993;76:26–31. doi: 10.1210/jcem.76.1.8421096. [DOI] [PubMed] [Google Scholar]
- 18.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, et al. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–74. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 19.Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K. Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab. 1992;74:890–97. doi: 10.1210/jcem.74.4.1548356. [DOI] [PubMed] [Google Scholar]
- 20.Klein D, Wan YJ, Kamyab S, Okuda H, Sokol RZ. Effects of toxic levels of lead on gene regulation in the male axis: increase in messenger ribonucleic acids and intracellular stores of gonadotrophs within the central nervous system. Biol Reprod. 1994;50:802–11. doi: 10.1095/biolreprod50.4.802. [DOI] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species. Endocrinology. 2002;143:4665–72. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- 24.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3 Vol. 1. San Diego, CA: Academic/Elsevier; 2006. pp. 2061–126. [Google Scholar]
- 25.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–23. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 26.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci. 2005;87:255–66. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- 28.Padilla-Banks E, Jefferson WN, Newbold RR. Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology. 2006;147:4871–82. doi: 10.1210/en.2006-0389. [DOI] [PubMed] [Google Scholar]
- 29.Enoch RR, Stanko JP, Greiner SN, Youngblood GL, Rayner JL, Fenton SE. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environ Health Perspect. 2007;115:541–47. doi: 10.1289/ehp.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Pediatrics. 1999;104:936–41. doi: 10.1542/peds.104.4.936. [DOI] [PubMed] [Google Scholar]
- 31.Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126:e583–90. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landrigan P, Garg A, Droller DB. Assessing the effects of endocrine disruptors in the National Children’s Study. Environ Health Perspect. 2003;111:1678–82. doi: 10.1289/ehp.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol. 2008;102:125–33. doi: 10.1111/j.1742-7843.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newbold RR, Heindel JJ. Developmental exposures and implications for early and latent disease. In: Woodruff TJ, Janssen S, Guillette LJ Jr, Giudice LC, editors. Environmental Impacts on Reproductive Health and Fertility. New York: Cambridge Univ. Press; 2010. pp. 92–102. [Google Scholar]
- 35.Colborn T, Dumanoski D, Myers JP. Our Stolen Future. New York: Penguin; 1996. [Google Scholar]
- 36.Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–39. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 37.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–41. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natl. Inst. Health (NIH) NIH Publ 00-4722. U.S. Dep. Health Hum. Serv; Washington, D.C: 1999. DES Research Update 1999: current knowledge, future directions. [Google Scholar]
- 39.Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertil Steril. 2008;89:e55–56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 40.Russo J, Russo IH. Development of the human breast. Maturitas. 2004;49:2–15. doi: 10.1016/j.maturitas.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- 42.Poulet FM, Veneziale R, Vancutsem PM, Losco P, Treinen K, Morrissey RE. Ziracin-induced congenital urogenital malformations in female rats. Toxicol Pathol. 2005;33:320–28. doi: 10.1080/01926230590925061. [DOI] [PubMed] [Google Scholar]
- 43.Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: Exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci. 2004;78:248–57. doi: 10.1093/toxsci/kfh062. [DOI] [PubMed] [Google Scholar]
- 44.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96:133–44. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 45.Rosen P. Anatomy and physiologic morphology. In: Rosen PR, editor. Rosen’s Breast Pathology. 3 New York: Lippincott, Williams & Wilkins; 2008. pp. 1–22. [Google Scholar]
- 46.Friedrichs N, Steiner S, Buettner R, Knoepfle G. Immunohistochemical expression patterns of AP2α and AP2γ in the developing fetal human breast. Histopathology. 2007;51:814–23. doi: 10.1111/j.1365-2559.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 47.Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4:9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- 48.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–27. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Investig. 1990;62:244–78. [PubMed] [Google Scholar]
- 50.Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;2000(27):17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 51.Hilakivi-Clarke L, Cho E, Raygada M, Kenney N. Alterations in mammary gland development following neonatal exposure to estradiol, transforming growth factor α, and estrogen receptor antagonist ICI 182,780. J Cell Physiol. 1997;170:279–89. doi: 10.1002/(SICI)1097-4652(199703)170:3<279::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 52.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(Suppl 3):S208–17. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 53.Aksglaede L, Juul A, Olsen LW, Sørensen TIA. Age at puberty and the emerging obesity epidemic. PLoS ONE. 2009;4(12):e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009;20:237–42. doi: 10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Carlsson B, Ankarberg C, Rosberg S, Norjavaara E, Albertsson-Wikland K, Carlsson LMS. Serum leptin concentrations in relation to pubertal development. Arch Dis Child. 1997;77:396–400. doi: 10.1136/adc.77.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–94. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lustig RH, editor. Obesity Before Birth: Maternal and Prenatal Influences on the Offspring. New York: Springer; 2010. p. 412. [Google Scholar]
- 58.Rice C, Birnbaum LS, Cogliano J, Mahaffey K, Needham L, et al. Exposure assessment for endocrine disruptors: some considerations in the design of studies. Environ Health Perspect. 2003;111:1683–90. doi: 10.1289/ehp.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman AS, Shapiro B, Neumann F. Role of testosterone and its metabolites in the differentiation of the mammary gland in rats. Endocrinology. 1976;99:1490–95. doi: 10.1210/endo-99-6-1490. [DOI] [PubMed] [Google Scholar]
- 60.Tomooka Y, Bern HA. Growth of mouse mammary glands after neonatal sex hormone treatment. J Natl Cancer Inst. 1982;69:1347–52. [PubMed] [Google Scholar]
- 61.Warner MR. Effect of various doses of estrogen to BALB/cCrgl neonatal female mice on mammary growth and branching at 5 weeks of age. Cell Tissue Kinet. 1976;9:429–38. doi: 10.1111/j.1365-2184.1976.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 62.Hilakivi-Clarke L, Shajahan A, Yu B, de Assis S. Differentiation of mammary gland as a mechanism to reduce breast cancer risk. J Nutr. 2006;136:2697S–99S. doi: 10.1093/jn/136.10.2697S. [DOI] [PubMed] [Google Scholar]
- 63.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–50. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 64.Palmer JR, Hatch EE, Rosenberg CL, Hartge P, Kaufman RH, et al. Risk of breast cancer in women exposed to diethylstilbestrol in utero: preliminary results (United States) Cancer Causes Control. 2002;13:753–58. doi: 10.1023/a:1020254711222. [DOI] [PubMed] [Google Scholar]
- 65.Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, et al. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomark Prev. 2006;15:1509–14. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- 66.Troisi R, Hatch EE, Titus-Ernstoff L, Hyer M, Palmer JR, et al. Cancer risk in women prenatally exposed to diethylstilbestrol. Int J Cancer. 2007;121:356–60. doi: 10.1002/ijc.22631. [DOI] [PubMed] [Google Scholar]
- 67.Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Wise LA, et al. Mortality in women given diethylstilbestrol during pregnancy. Br J Cancer. 2006;95:107–11. doi: 10.1038/sj.bjc.6603221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boylan ES. Morphological and functional consequences of prenatal exposure to diethylstilbestrol in the rat. Biol Reprod. 1978;19:854–63. doi: 10.1095/biolreprod19.4.854. [DOI] [PubMed] [Google Scholar]
- 69.Rothschild TC, Boylan ES, Calhoon RE, Vonderhaar BK. Transplacental effects of diethylstilbestrol on mammary development and tumorigenesis in female ACI rats. Cancer Res. 1987;47:4508–16. [PubMed] [Google Scholar]
- 70.Newbold RR, Jefferson WN, Padilla-Banks E, Haseman J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol. 2004;18:399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Zoeller RT. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol. 2005;242:10–15. doi: 10.1016/j.mce.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Shelby MD. NIH Publ 08-5994. Natl. Toxicol. Program; Research Triangle Park, N.C: 2008. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. [PubMed] [Google Scholar]
- 73.Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ Sci Technol. 2002;36:1414–18. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- 74.Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi H. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett. 2006;167:95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muñoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–47. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, et al. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103:1179–89. doi: 10.1093/jnci/djr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–19. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–90. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colerangle JB, Roy D. Exposure of environmental estrogenic compound nonylphenol to noble rats alters cell-cycle kinetics in the mammary gland. Endocrine. 1996;4:115–22. doi: 10.1007/BF02782756. [DOI] [PubMed] [Google Scholar]
- 83.Roy D, Colerangle JB, Singh KP. Is exposure to environmental or industrial endocrine disrupting estrogen-like chemicals able to cause genomic instability? Front Biosci. 1998;3:d913–21. doi: 10.2741/a332. [DOI] [PubMed] [Google Scholar]
- 84.Moon HJ, Han SY, Shin JH, Kang IH, Kim TS, et al. Gestational exposure to nonylphenol causes precocious mammary gland development in female rat offspring. J Reprod Dev. 2007;53:333–44. doi: 10.1262/jrd.18055. [DOI] [PubMed] [Google Scholar]
- 85.Lee KY, Shibutani M, Takagi H, Kato N, Takigami S, et al. Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology. 2004;203:221–38. doi: 10.1016/j.tox.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008;98:1485–93. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–57. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- 88.Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Skaar T, et al. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999;80:1682–88. doi: 10.1038/sj.bjc.6690584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabanes A, Wang M, Olivo S, DeAssis S, Gustafsson JA, et al. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–48. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 90.Hilakivi-Clarke L, Cho E, Clarke R. Maternal genistein exposure mimics the effects of estrogen on mammary gland development in female mouse offspring. Oncol Rep. 1998;5:609–16. doi: 10.3892/or.5.3.609. [DOI] [PubMed] [Google Scholar]
- 91.Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R. Maternal exposure to genistein during pregnancy increases carcinogen-induced mammary tumorigenesis in female rat offspring. Oncol Rep. 1999;6:1089–95. doi: 10.3892/or.6.5.1089. [DOI] [PubMed] [Google Scholar]
- 92.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, et al. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol. 2009;28:342–53. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Delclos KB, Bucci TJ, Lomax LG, Latendresse JR, Warbritton A, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague-Dawley) rats. Reprod Toxicol. 2001;15:647–63. doi: 10.1016/s0890-6238(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 94.Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9:1081–84. doi: 10.1038/nm902. [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Morales P, Saceda M, Kenney N, Kim N, Salomon DS, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem. 1994;269:16896–901. [PubMed] [Google Scholar]
- 96.Ohrvik H, Yoshioka M, Oskarsson A, Tallkvist J. Cadmium-induced disturbances in lactating mammary glands of mice. Toxicol Lett. 2006;164:207–13. doi: 10.1016/j.toxlet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 97.Hilakivi-Clarke L, Cho E, Cabanes A, DeAssis S, Olivo S, et al. Dietary modulation of pregnancy estrogen levels and breast cancer risk among female rat offspring. Clin Cancer Res. 2002;8:3601–10. [PubMed] [Google Scholar]
- 98.Welsch CW, O’Connor DH. Influence of the type of dietary fat on developmental growth of the mammary gland in immature and mature female BALB/c mice. Cancer Res. 1989;49:5999–6007. [PubMed] [Google Scholar]
- 99.Jurkowski JJ, Cave WT., Jr Dietary effects of menhaden oil on the growth and membrane lipid composition of rat mammary tumors. J Natl Cancer Inst. 1985;74:1145–50. [PubMed] [Google Scholar]
- 100.Malik NM, Carter ND, Murray JF, Scaramuzzi RJ, Wilson CA, Stock MJ. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology. 2001;142:5198–202. doi: 10.1210/endo.142.12.8535. [DOI] [PubMed] [Google Scholar]
- 101.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–11. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 102.Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol Appl Pharmacol. 2004;195:23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 103.Wetzel LT, Luempert LG, III, Breckenridge CB, Tisdel MO, Stevens JT, et al. Chronic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. J Toxicol Environ Health. 1994;43:169–82. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- 104.Lewis BC, Hudgins S, Lewis A, Schorr K, Sommer R, et al. In utero and lactational treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs mammary gland differentiation but does not block the response to exogenous estrogen in the postpubertal female rat. Toxicol Sci. 2001;62:46–53. doi: 10.1093/toxsci/62.1.46. [DOI] [PubMed] [Google Scholar]
- 105.Brown NM, Manzolillo PA, Zhang JX, Wang J, Lamartiniere CA. Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis. 1998;19:1623–29. doi: 10.1093/carcin/19.9.1623. [DOI] [PubMed] [Google Scholar]
- 106.Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, et al. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2002;110:625–28. doi: 10.1289/ehp.02110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lipworth L. Epidemiology of breast cancer. Eur J Cancer Prev. 1995;4:7–30. doi: 10.1097/00008469-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 108.Den Hond E, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, et al. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ Health Perspect. 2002;110:771–76. doi: 10.1289/ehp.02110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leijs MM, Koppe JG, Olie K, van Aalderen WM, Voogt P, et al. Delayed initiation of breast development in girls with higher prenatal dioxin exposure; a longitudinal cohort study. Chemosphere. 2008;73:999–1004. doi: 10.1016/j.chemosphere.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 110.Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, et al. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- 111.Talsness CE, Kuriyama SN, Sterner-Kock A, Schnitker P, Grande SW, et al. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Perspect. 2008;116:308–14. doi: 10.1289/ehp.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127(1–2):16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, et al. Prenatal perfluorooctanoic acid exposure in CD-1 mice: low dose developmental effects and internal dosimetry. Toxicol Sci. 2011;122:134–45. doi: 10.1093/toxsci/kfr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, et al. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol. 2011;45(19):8160–66. doi: 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- 115.Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, et al. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int. 2011;37:129–35. doi: 10.1016/j.envint.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rudel RA, Fenton SE, Ackerman JM, Euling SY, Makris SL. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119:1053–61. doi: 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Massart F, Parrino R, Seppia P, Federico G, Saggese G. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006;58:247–54. [PubMed] [Google Scholar]
- 118.Wolff MS, Britton JA, Boguski L, Hochman S, Maloney N, et al. Environmental exposures and puberty in inner-city girls. Environ Res. 2008;107:393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lorentzon M, Norjavaara E, Kindblom JM. Pubertal timing predicts leg length and childhood body mass index predicts sitting height in young adult men. J Pediatr. 2011;158:452–57. doi: 10.1016/j.jpeds.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. 2007;36:263–74. doi: 10.1111/j.1552-6909.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 121.Kaltiala-Heino R, Kosunen E, Rimpelä M. Pubertal timing, sexual behaviour and self-reported depression in middle adolescence. J Adolesc. 2003;26:531–45. doi: 10.1016/s0140-1971(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 122.Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpelä M. Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med. 2003;57:1055–64. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 123.Garn SM, LaVelle M, Rosenberg KR, Hawthorne VM. Maturational timing as a factor in female fatness and obesity. Am J Clin Nutr. 1986;43:879–83. doi: 10.1093/ajcn/43.6.879. [DOI] [PubMed] [Google Scholar]
- 124.Kemper HC, Koppes LL, de Vente W, van Lenthe FJ, van Mechelen W, et al. Effects of health information in youth and young adulthood on risk factors for chronic diseases—20-year study results from the Amsterdam Growth and Health Longitudinal Study. Prev Med. 2002;35:533–39. doi: 10.1006/pmed.2002.1107. [DOI] [PubMed] [Google Scholar]
- 125.Hulanicka B, Lipowicz A, Koziel S, Kowalisko A. Relationship between early puberty and the risk of hypertension/overweight at age 50: evidence for a modified Barker hypothesis among Polish youth. Econ Hum Biol. 2007;5:48–60. doi: 10.1016/j.ehb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Kaltiala-Heino R, Rimpelä M, Rissanen A, Rantanen P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. J Adolesc Health. 2001;28:346–52. doi: 10.1016/s1054-139x(01)00195-1. [DOI] [PubMed] [Google Scholar]
- 127.Macsali F, Real FG, Plana E, Sunyer J, Anto J, et al. Early age at menarche, lung function, and adult asthma. Am J Respir Crit Care Med. 2011;183:8–14. doi: 10.1164/rccm.200912-1886OC. [DOI] [PubMed] [Google Scholar]
- 128.Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev. 2000;21:671–96. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- 129.Dimartino-Nardi J. Premature adrenarche: findings in prepubertal African-American and Caribbean-Hispanic girls. Acta Paediatr Suppl. 1999;88:67–72. doi: 10.1111/j.1651-2227.1999.tb14406.x. [DOI] [PubMed] [Google Scholar]
- 130.Denburg MR, Silfen ME, Manibo AM, Chin D, Levine LS, et al. Insulin sensitivity and the insulin-like growth factor system in prepubertal boys with premature adrenarche. J Clin Endocrinol Metab. 2002;87:5604–9. doi: 10.1210/jc.2002-020896. [DOI] [PubMed] [Google Scholar]
- 131.Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90:2718–24. doi: 10.1210/jc.2004-1991. [DOI] [PubMed] [Google Scholar]
- 132.Meas T, Chevenne D, Thibaud E, Léger J, Cabrol S, et al. Endocrine consequences of premature pubarche in post-pubertal Caucasian girls. Clin Endocrinol. 2002;57:101–6. doi: 10.1046/j.1365-2265.2002.01579.x. [DOI] [PubMed] [Google Scholar]
- 133.Franceschi R, Gaudino R, Marcolongo A, Gallo MC, Rossi L, et al. Prevalence of polycystic ovary syndrome in young women who had idiopathic central precocious puberty. Fertil Steril. 2010;93:1185–91. doi: 10.1016/j.fertnstert.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 134.Fukamachi K, Han BS, Kim CK, Takasuka N, Matsuoka Y, et al. Possible enhancing effects of atrazine and nonylphenol on 7,12-dimethylbenz[a]anthracene-induced mammary tumor development in human c-Ha-ras proto-oncogene transgenic rats. Cancer Sci. 2004;95:404–10. doi: 10.1111/j.1349-7006.2004.tb03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ueda M, Imai T, Takizawa T, Onodera H, Mitsumori K, et al. Possible enhancing effects of atrazine on growth of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in ovariectomized Sprague-Dawley rats. Cancer Sci. 2005;96:19–25. doi: 10.1111/j.1349-7006.2005.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cameron HL, Foster WG. Developmental and lactational exposure to dieldrin alters mammary tumorigenesis in Her2/neu transgenic mice. PLoS ONE. 2009;4:e4303. doi: 10.1371/journal.pone.0004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tarraf C, El-Sabban M, Bassam R, Beyrouthy M, Chamoun J, Talhouk R. Functional consequence of exposure to dieldrin on mammary development and function. Food Addit Contam. 2003;20:819–28. doi: 10.1080/0265203031000138231. [DOI] [PubMed] [Google Scholar]
- 138.Desaulniers D, Leingartner K, Russo J, Perkins G, Chittim BG, et al. Modulatory effects of neonatal exposure to TCDD, or a mixture of PCBs, p,p′-DDT, and p-p′-DDE, on methylnitrosourea-induced mammary tumor development in the rat. Environ Health Perspect. 2001;109:739–47. doi: 10.1289/ehp.01109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boylan ES, Calhoon RE. Prenatal exposure to diethylstilbestrol: ovarian-independent growth of mammary tumors induced by 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1981;66:649–52. [PubMed] [Google Scholar]
- 140.Boylan ES, Calhoon RE, Vonderhaar BK. Transplacental action of diethylstilbestrol on reproductive and endocrine organs, mammary glands, and serum hormone levels in two- and nine-month-old female rats. Cancer Res. 1983;43:4872–78. [PubMed] [Google Scholar]
- 141.Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog. 2007;46:783–96. doi: 10.1002/mc.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Desaulniers D, Leingartner K, Musicki B, Cole J, Li M, et al. Lack of effects of postnatal exposure to a mixture of aryl hydrocarbon-receptor agonists on the development of methylnitrosourea-induced mammary tumors in Sprague-Dawley rats. J Toxicol Environ Health A. 2004;67:1457–75. doi: 10.1080/15287390490483818. [DOI] [PubMed] [Google Scholar]
- 143.Moral R, Wang R, Russo IH, Mailo DA, Lamartiniere CA, Russo J. The plasticizer butyl benzyl phthalate induces genomic changes in rat mammary gland after neonatal/prepubertal exposure. BMC Genomics. 2007;8:453. doi: 10.1186/1471-2164-8-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Muto T, Wakui S, Imano N, Nakaaki K, Takahashi H, et al. Mammary gland differentiation in female rats after prenatal exposure to 3,3′,4,4′,5-pentachlorobiphenyl. Toxicology. 2002;177:197–205. doi: 10.1016/s0300-483x(02)00224-x. [DOI] [PubMed] [Google Scholar]
- 145.Snyderwine EG. Mammary gland carcinogenesis by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rats: possible mechanisms. Cancer Lett. 1999;143:211–15. doi: 10.1016/s0304-3835(99)00127-5. [DOI] [PubMed] [Google Scholar]
- 146.Snyderwine EG. Mammary gland carcinogenesis by food-derived heterocyclic amines: metabolism and additional factors influencing carcinogenesis by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Environ Mol Mutagen. 2002;39:165–70. doi: 10.1002/em.10053. [DOI] [PubMed] [Google Scholar]
- 147.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect. 2011;119:1070–76. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M. A maternal diet high in n – 6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci USA. 1997;94:9372–77. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]