Abstract
BACKGROUND & AIMS
5-hydroxytryptamine receptor (5-HT4R) agonists promote gastrointestinal motility and attenuate visceral pain, but concerns about adverse reactions have restricted their availability. We tested the hypotheses that 5-HT4 receptors are expressed in the colonic epithelium and that 5-HT4R agonists can act intraluminally to increase motility and reduce visceral hypersensitivity.
METHODS
Mucosal expression of the 5-HT4R was evaluated by reverse-transcriptase polymerase chain reaction and immunohistochemical analysis of tissues from 5-HT4R(BAC)-enhanced green fluorescent protein mice. Amperometry, histology, and short-circuit current measurements were used to study 5-HT, mucus, and Cl− secretion, respectively. Propulsive motility was measured in guinea pig distal colon, and visceromotor responses were recorded in a rat model of colonic hypersensitivity. 5-HT4R compounds included cisapride, tegaserod, naronapride, SB204070, and GR113808.
RESULTS
Mucosal 5-HT4 receptors were present in the small and large intestines. In the distal colon, 5-HT4 receptors were expressed by most epithelial cells, including enterochromaffin and goblet cells. Stimulation of 5-HT4Rs evoked mucosal 5-HT release, goblet cell degranulation, and Cl− secretion. Luminal administration of 5-HT4R agonists accelerated propulsive motility; a 5-HT4R antagonist blocked this effect. Bath application of 5-HT4R agonists did not affect motility. Oral or intracolonic administration of 5-HT4R agonists attenuated visceral hypersensitivity. Intracolonic administration was more potent than oral administration, and was inhibited by a 5-HT4R antagonist.
CONCLUSIONS
Mucosal 5-HT4 receptor activation can mediate the prokinetic and antinociceptive actions of 5-HT4R agonists. Colon-targeted, intraluminal delivery of 5-HT4R agonists might be used to promote motility and alleviate visceral pain, while restricting systemic bioavailability and resulting adverse side effects.
Keywords: Constipation, ATI-7505, Peristaltic Reflex, Cavitation
5-hydroxytryptamine (5-HT, serotonin) is an important gastrointestinal (GI) signaling molecule involved in motor, secretory, and sensory functions.1,2 These actions are mediated by a large family of serotonin receptors located within the neural circuitry and on a variety of other cell types in the gut.3 Of the 5-HT receptors expressed in the intestines, the 5-HT4 receptor (5-HT4R) has been one of the most widely studied in regards to GI function, and 5-HT4R agonists have been developed for the treatment of constipation and visceral pain.4 Despite clinical effectiveness, the 5-HT4R agonists tegaserod and cisapride were removed from the market because of concerns related to the possibility of adverse cardiovascular effects.4
The 5-HT4R is a G-protein–coupled receptor that promotes activation of the adenylate cyclase/cyclic adenosine monophosphate (cAMP)/protein kinase A pathway, and can affect various cellular functions including facilitation of neurotransmitter release.3 Stimulation of presynaptic 5-HT4Rs on myenteric cholinergic nerve terminals enhances fast excitatory synaptic inputs to neurons and increases neurogenic muscle contractions in the intestines.5,6 As a result, presynaptic facilitation within the peristaltic reflex circuitry is thought to be responsible for the prokinetic actions of 5-HT4R agonists. It also is possible that 5-HT4R agonists act via a mucosal site of action. Luminal application of 5-HT4R agonists promotes propulsive motility and enhances the ascending contractile and descending relaxatory limbs of the peristaltic reflex.7,8 However, the existence and distribution of 5-HT4Rs in the mucosal layer of the intestines have not been investigated directly.
The aim of this investigation was to explore the hypothesis that 5-HT4Rs are expressed in the colonic mucosa and their activation promotes motility and/or alleviates visceral pain. 5-HT4Rs have been identified in the GI tracts of a number of species, including human beings. In these studies, we used assays that have been validated previously in mouse, rat, and guinea pig to evaluate functional responses to 5-HT4R activation. The expression pattern of 5-HT4Rs in the GI epithelium was determined by quantitative reverse-transcription polymerase chain reaction (RT-PCR) and by evaluation of green fluorescent protein (GFP) immunoreactivity in 5-HT4R(BAC)-enhanced GFP (eGFP) transgenic mice. Epithelial responses were evaluated by measuring 5-HT4R agonist–induced 5-HT release, mucus secretion, and ion transport. Colonic propulsive motility was measured in response to luminal vs serosal administration of 5-HT4R agonists. Finally, we evaluated the effects of luminal administration of 5-HT4R agonists on the visceromotor response (VMR) to colorectal distension (CRD) in a colonic hypersensitivity model. Our findings indicate that 5-HT4Rs are expressed in the epithelial layer of the colon, and suggest that targeted activation of these receptors has prokinetic and antinociceptive effects.
Materials and Methods
See Supplementary Materials and Methods section for details of immunohistochemistry protocols, strains and sources of animals used, and physiological solution recipes and reagents.
Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committees of the University of Vermont, Oklahoma City VA Medical Center and University of Oklahoma Health Sciences Center, and the University of Calgary Animal Care Committee. In all cases, animals were euthanized by isoflurane and exsanguination or cervical dislocation.
Human Biopsies
Human tissue biopsy specimens were obtained from patients of the Division of Gastroenterology and Hepatology using protocols approved by the University of Vermont Institutional Review Board. Individuals provided informed consent before their scheduled screening procedures. Mucosal samples were obtained using standard biopsy forceps. Samples were immediately placed in RNA stabilization solution (RNAlater; Ambion, Austin, TX).
RT-PCR
RNA was extracted from human biopsies and murine full-thickness preparations or mucosal scrapings using the RNeasy Mini Kit (Qiagen, Valencia, CA) and complementary DNA (cDNA) was generated by reverse-transcriptase reaction (Promega, Madison, WI). An Applied Biosystems 7500 Fast Realtime PCR System was used with Fast Universal PCR Master Mix and validated TaqMan Gene Expression Assays for human 5-HT4R (Hs00410577_m1), mouse 5-HT4R (Mm00434129_m1), human HPRT1 (Hs99999909_m1), and mouse HPRT1 (Mm00446968_m1) (Applied Biosystems, Foster City, CA). Resulting data were calculated using the standard curve method and the level of 5-HT4R expression was normalized to HPRT1. HPRT1 expression was consistent across the regions studied. To ensure that mucosal samples did not contain neuronal cell bodies, a subset of human samples were immunostained for anti-human neuronal protein HuC/D and neuron-specific enolase, and no neurons were observed. Furthermore, HuC/D transcript was not detected in cDNA from mouse mucosal samples.
Immunohistochemistry/BAC Transgenic Mice
Tissue samples from 5-HT4(BAC)-eGFP mice with a Swiss Webster (SW) strain genetic background (kindly provided by Eric Schmidt and Nathaniel Heintz, Rockefeller University) were fixed with 4% paraformaldehyde, paraffin-embedded, and sectioned at 10 μm. The eGFP signal was amplified by GFP immunostaining, which yielded similar, but more intense, fluorescence than emitted by eGFP alone. Sections were examined on an Olympus AX70 fluorescence microscope (Olympus America, Inc, Melville, NY), and some sections were double-stained for 5-HT or mucin 2. Images of microscopic fields were captured with an Optronics MagnaFire digital camera (Optronics, Goleta, CA) using identical exposure settings.
Amperometry
Boron-doped diamond microelectrodes were used for continuous amperometric recordings.9,10 The holding potential for the electrode was set at 700–750 mV with an Axoclamp-2B amplifier (Axon Instruments, Union City, CA). This potential was determined previously to oxidize 5-HT at a mass transfer limited rate. Electrical signals were acquired using a MiniDigi 1A (Axon Instruments) interfaced with pClamp software (Molecular Devices, Sunnyvale, CA) on an iMac computer (Apple, Cupertino, CA). Experiments on guinea pigs were performed with the colon bathed in oxygenated (95% O2, 5% CO2) Krebs solution at room temperature to minimize muscle contractions (flow rate 2 mL min-1). Mouse experiments were conducted at 37°C. After a 30-minute or longer equilibration period and confirmation of basal 5-HT release, recordings were obtained with the electrode placed 50 μm above the mucosal surface.
Mucus Release
Full-thickness colonic segments were equilibrated for 30 minutes in oxygenated, 37°C Krebs solution, followed by 30 minutes of an experimental condition. Tissues were rinsed and fixed in 10% formalin overnight at 4°C. Preparations were paraffin-embedded and sectioned, and stained with periodic acid– Schiff and Alcian Blue. The percentage of goblet cells that were cavitated was evaluated blindly by counting cavitated and non-cavitated goblet cells at 400× magnification.
Measurement of Ion Transport
Full-thickness preparations were mounted in Ussing chambers bathed in oxygenated Krebs solution warmed to 37°C.11,12 The tissue was voltage-clamped at 0 mV. Short circuit current (ISC, μA/cm2) responses were measured as the maximum increase occurring within 10 minutes of drug application. At the end of each experiment, forskolin (10 μmol/L) was added to confirm tissue viability.
Motility Analysis
A Gastrointestinal Motility Monitor (Catamount Research and Development, Inc, St. Albans, VT) was used to record and analyze the rate of propulsive motility in guinea pig colonic segments.13 A segment of distal colon was pinned onto a Sylgard-lined (Dow Corning Co, Midland MI) organ bath perfused with recirculating warmed (37°C) and oxygenated Krebs solution at a flow rate of 10 mL min−1. Intraluminally delivered compounds were delivered at a flow rate of 0.25 mL min−1. An epoxy-coated pellet was inserted into the oral end of an isolated segment, and the motility pattern of the pellet was tracked with a digital camera coupled to the Gastrointestinal Motility Monitor computer analysis software. After a 30-minute equilibration period, at least 3 trials were recorded, with a 5-minute recovery between each trial.
Assessment of Colonic Sensitivity
Acclimatized rats were anesthetized with isoflurane (5% induction; 1%–2% maintenance in O2) and a strain gauge force transducer was sutured to the right external oblique muscle. Twenty minutes after administration of compounds via oral gavage or an intracolonic catheter, rats were infused intracolonically (i.c.) with 1.5 mL 0.6% acetic acid, and a 5-cm balloon catheter was inserted 8 cm into the distal colon. Sixty minutes later, the colorectal balloon was attached to a barostat (Distender Series IIR; G & J Electronics Inc, Toronto, Ontario, Canada) and chart recorder, and VMR was measured as the number of abdominal contractions 10 min−1 to randomized isobaric distension pressures (0, 15, 30, 45, and 60 mm Hg). Contractions were scored by a deflection in the chart recording accompanied by observation of abdominal flexion.
Data Analysis
The data presented are means ± standard error of the mean for n animals or subjects. Statistical analyses were performed using GraphPad Prism software (v. 5.0a; GraphPad Software, San Diego, CA). Differences were determined by unpaired Student t test, 1-way analysis of variance, or a 2-way analysis of variance with Bonferroni post-test. A P value less than .05 was considered statistically significant.
Results
5-HT4Rs Are Expressed in the Intestinal Epithelium
Murine mucosal 5-HT4R expression
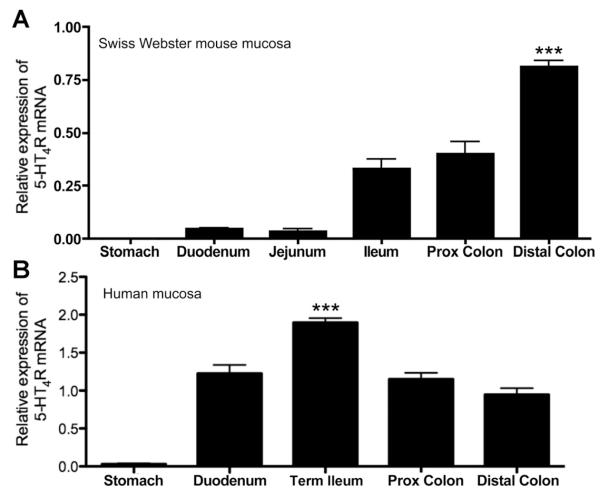
To assess the presence and relative levels of 5-HT4R transcript throughout the GI tract, real-time quantitative RT-PCR was performed in samples from the gastric corpus, duodenum, jejunum, ileum, and proximal and distal colons of SW and BALB/cJ mice. In full-thickness preparations, 5-HT4R transcript was detected from all regions, and when normalized to the endogenous control Hprt1, significantly higher levels were found in the distal and proximal colon as compared with more proximal regions of the gut (data not shown). 5-HT4R transcript was not detected in gastric mucosal samples. In intestinal mucosa samples, a clear gradient was observed, with low levels of expression in the small intestine, and highest in the mucosa of the distal colon (SW, Figure 1A; Balb/cJ, Supplementary Figure 1). Mucosal 5-HT4 transcript expression also was confirmed in mucosal scrapings from rat and CD1 mouse colon (data not shown). The normalized expression level in the distal colon was higher in mucosal samples than full-thickness samples (P < .05), suggesting that the density of expression is highest in the mucosa.
Figure 1.
(A) SW mouse mucosal 5-HT4R transcript levels detected in the distal colon were significantly greater than levels detected in all other regions. The transcript levels in the proximal colon were significantly greater than levels in the duodenum or jejunum (P < .001). Data were normalized to HPRT1. (B) 5-HT4R transcript was detected in all regions of the human intestinal mucosa, with the highest level in the terminal ileum. 5-HT4R transcript was undetectable in murine, and detected at low levels in human gastric mucosal samples. ***P < .001 as compared with other regions (n = 3–5 for mouse samples, and 7–12 for human samples).
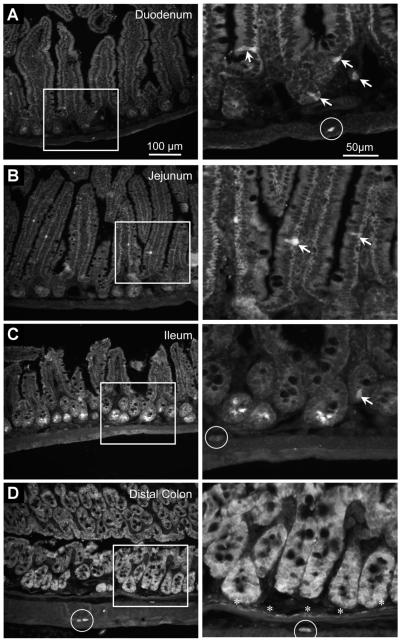
As an additional approach to evaluate the distribution of 5-HT4Rs and to identify the cell types expressing the receptor, we examined tissue from mice expressing eGFP under the regulatory elements for the 5-HT4 promoter (5-HT4[BAC]-eGFP; available: www.gensat.org) and observed a pattern similar to that detected with PCR. In the duodenum and jejunum, GFP-immunoreactive neurons were observed in submucosal and myenteric ganglia, and a small number of cells that appeared to be enteroendocrine cells were detected in the epithelium (Figure 2A and B). In the ileum, intense GFP immunofluorescence was observed in cells at the base of the crypts, enteroendocrine-like cells, and neurons (Figure 2C). In the distal colon, essentially all cells in the epithelium were GFP-immunoreactive (Figure 2D). GFP-immunoreactive cells also were observed in the muscularis mucosa, and in enteric ganglia (Figure 2D).
Figure 2.
Photomicrographs of sections from a 5-HT4R(BAC)-eGFP mouse showing GFP immunoreactivity in the (A) duodenum, (B) jejunum, (C) ileum, and (D) distal colon. White boxes indicate regions shown at higher magnification. In the duodenum and jejunum, GFP immunoreactivity was detected in enteric neurons (circle) and enteroendocrine cells (arrows). In the ileum, GFP immunoreactivity was detected in epithelial cells at the base of crypt glands, in enteroendocrine cells, and in neurons. In the distal colon, GFP immunoreactivity was observed throughout the epithelial layer, in a monolayer of cells along the muscularis mucosa (asterisks), and in neurons (circles).
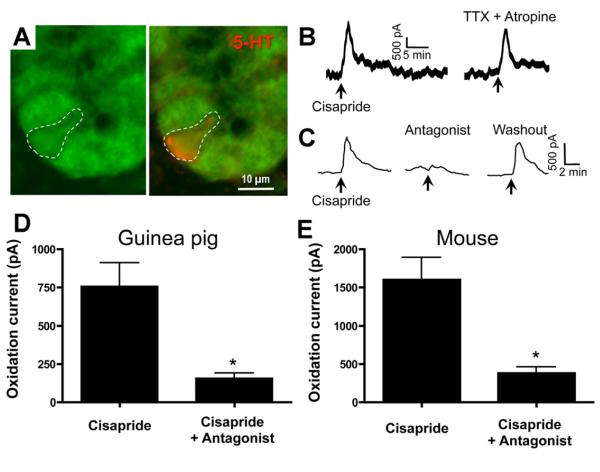
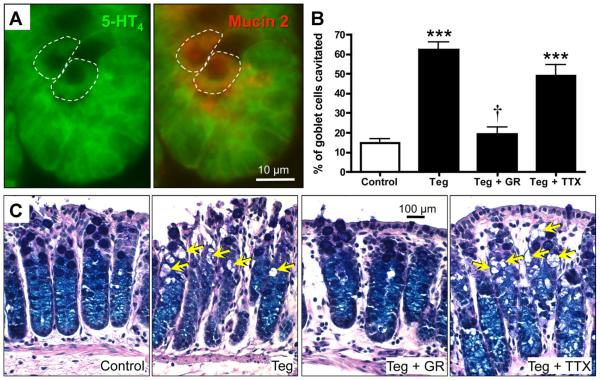
To identify the subtypes of epithelial cells that express 5-HT4R, double-labeling was performed in sections from 5-HT4(BAC)-eGFP mouse intestines. Immunostaining antibodies directed against 5-HT and mucin 2 were used to identify 5-HT–containing enterochromaffin (EC) cells (Figure 3A), and goblet cells (Figure 4A), respectively. In the colon, 5-HT– and mucin 2–immunoreactive cells were also GFP-immunoreactive, indicating that EC cells and goblet cells express the 5-HT4R.
Figure 3.
(A) Photomicrographs from a 5-HT4R(BAC)-eGFP mouse section showing that 5-HT–immunoreactive EC cells are GFP-immunoreactive. (B) In guinea pig distal colon, the 5-HT4R agonist, cisapride (1 μmol/L), increased the oxidation current for 5-HT in a TTX-(0.3 μmol/L) and atropine-(10 μmol/L) insensitive manner. (C and D) In guinea pig, the cisapride response was blocked by the 5-HT4R antagonist, SB204070 (0.1 μmol/L) (n = 5–8). (E) In SW mice, the cisapride response was blocked by the 5-HT4R antagonist, GR113808 (1 μmol/L) (n = 5). *P < .02 vs cisapride alone.
Figure 4.
(A) Photomicrographs from a 5-HT4R(BAC)-eGFP mouse section showing that mucin 2–immunoreactive goblet cells are GFP-immunoreactive. (B) Percentage of goblet cells that were cavitated under conditions tested. (C) Application of the 5-HT4R agonist, tegaserod (Teg, 1 μmol/L), to the mucosal surface of the SW mouse distal colon elicited an increase in goblet cell cavitation (yellow arrows) in periodic acid–Schiff and Alcian Blue (PAS/AB)–stained sections. The tegaserod response was blocked by the 5-HT4R antagonist, GR113808 (1 μmol/L), but not by TTX (0.3 μmol/L). ***P < .001 vs vehicle; †P < .001 vs tegaserod, and >0.05 vs vehicle (n = 5 per group).
Human mucosal 5-HT4R expression
To assess 5-HT4R transcript in the human GI tract, real-time quantitative RT-PCR was performed in mucosal biopsy specimens from the gastric corpus, duodenum, terminal ileum, proximal colon, and distal colon. Very low levels of 5-HT4R transcript were detected in gastric biopsies (Figure 1B). In intestinal samples, 5-HT4R transcript was present in all regions tested, with the highest transcript level in the terminal ileum (Figure 1B). In separate studies, 5-HT4R transcript was detected in rectal biopsies (data not shown).
Effects of 5-HT4R Activation on Colonic Epithelial Cells
Mucosal 5-HT4R activation elicits 5-HT release
To test whether activation of 5-HT4Rs on EC cells affects 5-HT release, we used in vitro amperometry with diamond-coated microelectrodes calibrated to detect 5-HT as an oxidation current. Cisapride was used for these studies because tegaserod oxidizes at the same voltage as 5-HT; therefore, tegaserod-mediated oxidation currents cannot be distinguished from serotonergic currents. Upon application of cisapride (1.0 μmol/L), transient increases in the oxidation current were detected in the distal colon of guinea pigs (Figure 3B–D) and SW mice (Figure 3E). The cisapride-induced response was inhibited by the 5-HT4R antagonist administration (Figure 3C–E), but persisted in the presence of tetrodotoxin (TTX) (0.3 μmol/L). These findings indicate that cisapride elicits 5-HT release from the mucosa by directly activating 5-HT4Rs on EC cells, rather than via a neural mechanism.
Mucosal 5-HT4R Activation Elicits Mucus Secretion
Data from 5-HT4(BAC)-eGFP mouse sections immunostained for mucin 2 suggest that goblet cells express 5-HT4Rs. Previous work has shown that 5-HT causes mucus secretion by activation of goblet cells, which can be visualized as large vacuoles in the epithelial layer, referred to as cavitations, in periodic acid–Schiff and Alcian Blue– stained sections.14 To determine whether 5-HT4R activation leads to mucus secretion, preparations from SW mice (Figure 4B and C) and guinea pig (Supplementary Figure 2) distal colon were exposed to vehicle, 1 μmol/L tegaserod, tegaserod plus TTX (0.3 μmol/L), or tegaserod plus the 5-HT4R antagonist, GR113808 (1 μmol/L). The proportion of goblet cells that were cavitated was increased in preparations treated with tegaserod or tegaserod and TTX (P < .001), and this effect was inhibited by GR113808.
Mucosal 5-HT4R Activation Elicits Cl− Secretion
The effects of mucosal vs serosal administration of a 5-HT4R agonist on ISC were evaluated in full-thickness segments of CD1 mouse duodenum and distal colon mounted in Ussing chambers. In the colon, 1 μmol/L tegaserod increased ISC when applied to the mucosal side of the chamber (Figure 5A; P < .001), but had no effect when applied serosally (P = .19; Figure 5B). The peak response to mucosally applied tegaserod was 25 ± 8 μA/cm2 (n = 6). This effect was reduced by GR113808 (1 μmol/L; P < .001, n = 6; Figure 5A). Similar results were obtained in the distal colons of SW mice and guinea pigs (Supplementary Figure 3). The tegaserod-mediated increase in I involves Cl− SC secretion because tegaserod did not alter the I when Cl− SC was excluded from the Krebs solution. Furthermore, the response to mucosal application of tegaserod was blocked by TTX, indicating that this response was mediated via a neural mechanism. Mucosal application of tegaserod in the murine duodenum did not alter ISC (P = .54; Figure 5C).
Figure 5.
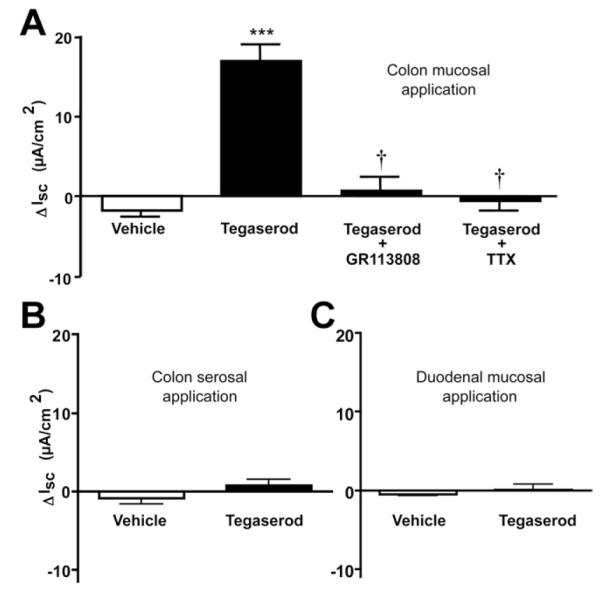
(A) Tegaserod (1 μmol/L) caused a significant increase in ISC that was blocked by the 5-HT4R antagonist, GR113808 (1 μmol/L), and by neural blockade with TTX (0.3 μmol/L). (B) Serosal application of tegaserod did not alter the ISC. (C) No response was detected by mucosal application of tegaserod in the duodenum. ***P < .001 vs vehicle; †P < .001 vs tegaserod (n = 6).
Effects of 5-HT4R Agonists on Propulsive Motility and Visceral Hypersensitivity in the Colon
The findings described earlier show that 5-HT4Rs are widely expressed in the colonic epithelium and that stimulation of these receptors elicits fluid, mucus, and 5-HT secretion. 5-HT4R agonists promote colonic motility and attenuate visceral hypersensitivity in animal models and human beings.15,16 Therefore, we investigated whether luminal application of 5-HT4R agonists could elicit these effects. For these studies, we had access to naronapride (ATI-7505), which is a more selective 5-HT4R agonist than tegaserod or cisapride.4,17 Unlike cisapride and tegaserod, naronapride does not interact with other 5-HT4Rs.4,17
Mucosal Administration of a 5-HT4R Agonist Promotes Colonic Propulsive Motility
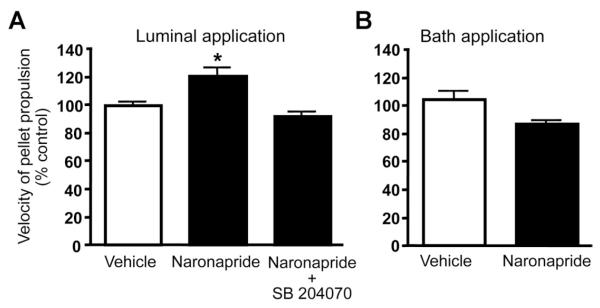
Previous studies have shown that luminal 5-HT4R agonist application accelerates propulsive motility in isolated segments of guinea pig distal colon.18 To evaluate the effects of mucosal 5-HT4R activation, as compared with stimulation of myenteric receptors, we tested the effects of luminal vs bath application of naronapride using this model. Luminally applied naronapride (0.1 μmol L−1) increased the rate of propulsive motility (Figure 6A), and this effect was blocked by the 5-HT4R antagonist, SB204070 (10 nmol L−1). The addition of naronapride to the bathing solution did not alter the rate of propulsive motility (Figure 6B). Comparable data were obtained with luminal vs bath application of tegaserod (data not shown).
Figure 6.
(A) Intraluminal administration of naronapride (100 nmol/L) increased the rate of propulsive motility, and this effect was blocked by the 5-HT4R antagonist SB204070 (10 nmol/L) (*P < .05 vs vehicle; n = 5). (B) No change in pellet propulsion was detected when naronapride was added to the bathing solution (n = 6–19).
Visceral Hypersensitivity is Attenuated by Intraluminal Administration of a 5-HT4R Agonist
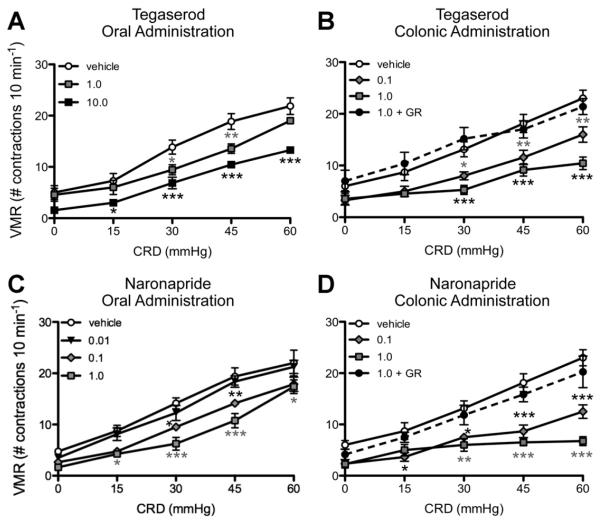
Previous studies have shown that colonic infusion of acetic acid enhances VMRs to CRD in awake, freely moving rats,19 and that intraperitoneal administration of tegaserod suppresses this colonic hypersensitivity in rats.16 In the current study, we compared the effects of oral vs intracolonic administration of 5-HT4R agonists. Oral administration of tegaserod attenuated the VMR at both 1- and 10-mg kg−1 doses (Figure 7A). Intraluminal administration of tegaserod also decreased the VMR at doses of 1 and 0.1 mg kg−1, and this response was blocked by luminal pretreatment with GR113808 (1 mg kg−1) (Figure 7B).
Figure 7.
(A) Oral doses of 10 mg kg−1 and 1.0 mg kg−1 tegaserod significantly reduced the VMR at distension pressures of 30 mm Hg and greater (*P < .05 vs control; **P < .01 vs control; ***P < .001 vs vehicle; n = 6–7). (C) Oral naronapride decreased the VMR at doses of 0.1 mg kg−1 and higher, with a maximal effect at 1 mg kg−1. Intracolonic administration of (B) tegaserod and (D) naronapride decreased the VMR to CRD at doses of 0.1 and 1.0 mg kg−1 (n = 5–6). This effect was inhibited by the 5-HT4R antagonist, GR113808 (1.0 mg kg−1), administered intracolonically (dashed lines; n = 5–6).
To further establish that the antinociceptive effects of tegaserod on colonic hypersensitivity involves mucosal 5-HT4Rs, the actions of naronapride were tested. Oral administration of naronapride attenuated visceral hypersensitivity at doses of 0.1–30 mg kg−1, with the maximal decrease in VMR observed at 1.0 mg kg−1 (Figure 7C). Similar to the effects observed with tegaserod, intracolonic administration of naronapride (0.1 and 1 mg kg−1) decreased VMR to CRD at pressures of 15 mmHg and greater (Figure 7D). As observed with tegaserod, GR113808 (1 mg kg−1 i.c.) blocked the antinociceptive action of naronapride (1 mg kg−1 i.c.) (Figure 7D).
Discussion
This study was performed to test the hypotheses that 5-HT4Rs are expressed in the colonic mucosa, and, when activated, promote propulsive motility and attenuate visceral hypersensitivity. Our findings provide novel molecular, morphologic, and physiological evidence for 5-HT4R expression in the colonic epithelium of mouse, rat, guinea pig, and human beings. Expression of this receptor was found on serotonin-containing EC cells and mucin 2–immunoreactive goblet cells, and activation of mucosal 5-HT4Rs elicited 5-HT release, mucus release, and increased short-circuit current. Furthermore, luminal administration of 5-HT4R agonists increased the velocity of propulsive motility and decreased colonic hypersensitivity. Collectively, these studies contribute new knowledge regarding the expression and function of the 5-HT4R in the colonic mucosa, and support the concept that 5-HT4R agonists formulated to target the colonic mucosa could provide an effective and safer method of delivery.
It generally is accepted that 5-HT4R agonists have prokinetic actions and can be used to improve symptoms related to constipation. Emerging data involving more selective 5-HT4R agonists, including naronapride (ATI-7505), prucalopride, and velusetrag (TD-5108) support this receptor as an effective therapeutic target for promoting gut motility. However, the mechanism of action of these compounds has not been clearly resolved. One possibility is that 5-HT4R agonists promote motility by stimulating receptors on enteric nerve terminals and increasing neurotransmitter release. It is clear from a number of investigations that 5-HT4Rs are located on enteric nerve terminals, and 5-HT4R agonists facilitate synaptic transmission through a presynaptic mechanism.5,6,20–22 Furthermore, morphologic and molecular studies have shown that 5-HT4Rs are expressed by enteric neurons.22–24 Consistent with this model, bath application of cisapride increases peristalsis in the ileum.6 However, in the current investigation, when 5-HT4R agonists were applied to the bathing solution, where they would have access to the myenteric plexus, propulsive motility in the distal colon was not detectably altered.
Another possible mechanism for the prokinetic action of 5-HT4R agonists involves a mucosal site of action. In the current and previous8,18 studies of 5-HT4R responses in the colon, agonists increased the rate of propulsive motility when administered intraluminally, and these responses were inhibited by 5-HT4R antagonists. Furthermore, Grider et al7 reported that application of tegaserod to the colonic mucosa activates ascending excitatory and descending relaxant peristaltic reflex responses. Yet, until now, a lack of direct evidence for epithelial 5-HT4Rs has limited acceptance of a mucosal site of action for 5-HT4R agonists.
The findings reported here indicate that 5-HT4Rs are expressed in the intestinal mucosa, where they are distributed differentially and expressed by a number of epithelial cell types. These data show that there is a gradient of mucosal 5-HT4R expression in the murine GI tract, with highest expression in the distal colon where all or most cells appear to express this receptor. 5-HT4R messenger RNA also was present in human and rat colonic mucosal samples. It is possible that activation of 5-HT4Rs on colonic epithelial cells could mediate the prokinetic actions of 5-HT4R agonists. In the current investigation, we show 5-HT4R expression by EC cells, goblet cells, and enterocytes, and it is conceivable that stimulation of secretion by any or all of these cell types could promote colonic transit. For example, 5-HT release could activate peristaltic reflex activity, mucus release could decrease friction along the epithelial lining, and fluid secretion could soften the stool and facilitate propulsion.
In the current study, 5-HT–immunoreactive EC cells also expressed GFP immunoreactivity in sections from 5-HT4(BAC)-eGFP transgenic mice, indicating that EC cells in the distal colon express 5-HT4Rs. The effects of 5-HT4R activation on 5-HT release have been conducted in the small intestine, where 5-HT4R agonists are reported to decrease basal 5-HT release.25,26 However, in this study, using continuous electrochemical recordings with electrodes calibrated for measurement of 5-HT, application of a 5-HT4R agonist to the colonic mucosa evoked an increase in oxidation current that was TTX-insensitive, and was blocked by a 5-HT4R antagonist. The concept that 5-HT4R activation enhances 5-HT release is consistent with the fact that this receptor signals through the cAMP pathway. Furthermore, freshly isolated mammalian ECcells,27 as well as the EC cell models, BON cells28 and KRJ-1 cells,29 promote 5-HT release via cAMP signaling.
Previous studies have shown that 5-HT causes TTX-insensitive mucus secretion and goblet cell cavitation in the rat colon,14 but the receptor(s) responsible for these actions were not identified. Evidence for mucosal 5-HT4Rs mediating mucus secretion includes the findings that goblet cells are GFP-immunoreactive in sections from 5-HT4(BAC)-eGFP transgenic mice, and 5-HT4R activation increases cavitation in goblet cells. The concept that 5-HT4R stimulation elicits mucus secretion is supported by previous findings that stimulation of the cAMP/PKA pathway causes mucus secretion in the T84 human colonic adenocarcinoma cell line,30 which includes goblet-like cells with mucin-containing secretory granules.
Serotonin stimulates chloride secretion in the intestine, and 5-HT4Rs may contribute to this response.31,32 In the current study, luminal administration of a 5-HT4R agonist to the distal colon elicited an increase in Cl− secretion, whereas serosal application had no effect. Furthermore, in the mouse duodenum, where 5-HT4R expression was barely detectable, administration of a 5-HT4R agonist to the mucosa had no effect on ISC. The ISC response in the colon was eliminated in the presence of TTX, indicating that this response was mediated neurally. This was some-what surprising because data from the 5-HT4(BAC)-eGFP transgenic mice suggest that enterocytes express the 5-HT4R, and as indicated earlier, this receptor is linked to the cAMP pathway. It is possible that tegaserod elicits Cl− secretion by activating 5-HT release because EC cell activation leads to a neurally mediated secretory response.33 Regardless of the mechanism, these findings are consistent with a prokinetic effect of 5-HT4R activation, and may contribute to the relief of constipation.
Previous human15 and animal16 studies have shown that tegaserod alleviates abdominal pain and discomfort, with the compound administered orally in human beings and intraperitoneally in rats. We report here that intracolonic infusion of tegaserod or naronapride reduced the VMR in a dose-dependent manner when infused into the colon, and the agonists were more potent when administered intracolonically.
There has been considerable debate as to whether the antinociceptive actions of tegaserod are mediated via activation of 5-HT4Rs and/or antagonism of 5-HT2BRs.34,35 5-HT2BR antagonists suppress VMR responses in Wistar Kyoto rats35 and in a model of trinitrobenzene sulfonic acid (TNBS)–induced colonic hypersensitivity.36 Also, tegaserod is an antagonist at the 5-HT2BR, in addition to its more potent action as a 5-HT4R agonist.37 However, previous studies of rats with acetic acid–induced colonic hypersensitivity showed that the tegaserod-induced attenuation of VMR was partially inhibited by 5-HT4R antagonism, but no additional inhibition of the antinociceptive response was observed after co-administration of a 5-HT2BR antagonist.34 Here, we report that intracolonically administered naronapride, a compound with 1000-fold greater affinity for the 5-HT4R than other 5-HTRs,17 caused a decrease in the VMR to CRD in the sensitized colon. Furthermore, the antinociceptive responses to tegaserod and naronapride were blocked by a 5-HT4R antagonist. Collectively, these findings support the concept that exposure of the colonic mucosa to 5-HT4R agonists alleviates visceral hypersensitivity, but the precise mechanisms of action are unknown.
Interestingly, although various classes of 5-HT4R agonists are useful for the treatment of functional gastrointestinal disorders, their efficacies for alleviating upper vs lower GI symptoms appear to vary. For example, cisapride is well known for its effects on gastric emptying and gastroparesis, whereas tegaserod and prucalopride are more recognized for improving colonic transit. Tegaserod is poorly absorbed,38 and its selectivity for the colon may involve a direct action on mucosal 5-HT4Rs.39 Formulation of 5-HT4R agonists to prevent systemic absorption and deliver the drug effectively to the colonic mucosa may enhance their clinical effects while avoiding systemic bioavailability and potential side effects.
The findings presented here provide evidence for mucosal expression of 5-HT4Rs in the guinea pig, rat, and human colons, and 3 strains of mice (Supplementary Table 1). Support for 5-HT4R–mediated 5-HT release and goblet cell degranulation are provided from both SW mice and guinea pigs. Furthermore, mucosal application of a 5-HT4R agonist elicited an increase in ISC in CD1 and SW mice, and guinea pigs. Collectively, these findings indicate that these epithelial responses are not species-specific effects. By using assays previously used to study 5-HT4R functions in the gut, we present data showing that luminal administration of 5-HT4R agonists promotes propulsive motility and suppresses visceral hypersensitivity in guinea pigs and rats, respectively. A limitation of the current study was that the 5-HT4R was localized in mouse colonic mucosa, but the motility and visceral sensitivity assays were conducted in guinea pigs and rats, respectively. This was performed to maintain consistency with previous studies of 5-HT4R agonists on gut function and sensation. Additional studies will be required to confirm the mucosal distributions of 5-HT4Rs and determine whether the mucosal actions of 5-HT4R agonists are comparable across species, including human beings.
In conclusion, these findings show that 5-HT4Rs are expressed on a variety of epithelial cells of the colon, and activation of these mucosal 5-HT4Rs leads to mucus, serotonin, and fluid secretion. Furthermore, activation of mucosal 5-HT4Rs promotes propulsive motility and attenuates visceral hypersensitivity, but the precise mechanisms remain to be resolved. Moreover, these data support the novel concept that the colonic mucosa should be explored as an effective target for 5-HT4R agonists in the treatment of constipation and abdominal pain.
Supplementary Material
Supplementary Figure 1. As was detected in Swiss Webster mice (Figure 1A), mucosal 5-HT4R transcript levels detected in the distal colon of Balb/cJ mice were significantly greater than levels detected in all other regions. The transcript levels in the proximal colon were significantly greater than levels in the duodenum or jejunum (P < .01). ***P < .001 as compared with other regions; n = 5–6.
Supplementary Figure 2. Application of the 5-HT4R agonist, tegaserod (Teg; 1 μmol/L), to the mucosal surface of the guinea pig distal colon elicited an increase in goblet cell cavitation (yellow arrows) in periodic acid–Schiff and Alcian Blue (PAS/AB)–stained sections. The tegaserod response was blocked by the 5-HT4R antagonist, GR113808 (1 μmol/L), but not by TTX (0.3 μmol/L). n = 5 per group.
Supplementary Figure 3. Mucosal application of the 5-HT4R agonist, tegaserod (1 μmol/L), elicited an increase in ISC in distal colon preparations of (A) Swiss Webster mice and (B) guinea pigs. In both species, the response to mucosally applied tegaserod was inhibited by the 5-HT4R antagonist, GR113808 (1 μmol/L). Serosal application of tegaserod failed to induce a change in ISC. n = 4 per group. ***P < .001.
Supplementary Table 1. Composite of Data Collected From the Different Species Studied Regarding Mucosal 5-HT4 Receptors in the Colon
Acknowledgments
The authors would like to thank Dr Brigitte Lavoie for consultation during the project, and Marion France for supplying electrodes for the mouse amperometry studies. The authors also would like to thank Drs Nathaniel Heintz and Eric Schmidt of Rockefeller University for 5-HT4R(BAC)-eGFP mouse tissue samples, and Drs John McRorie and Russell Spruell of Proctor and Gamble for 5-HT4R agonists.
Funding This work was supported by National Institutes of Health grants DK62267 (G.M.M.) and R21HD056197 (B.G.V.M.), and P20 RR16435 from the Centers of Biomedical Research Excellence (COBRE) Program of the National Center for Research Resources, as well as a grant from the Canadian Institutes of Health Research (K.A.S.); Sarah MacEachern is supported by the Dr. T. Chen Fong Doctoral Scholarship in Neuroscience through the Hotchkiss Brain Institute; and Keith Sharkey is an Alberta Heritage Foundation for Medical Research Medical Scientist and the Crohn’s and Colitis Foundation of Canada Chair in IBD Research at the University of Calgary.
Abbreviations used in this paper
- BAC
bacterial artificial chromosome
- CRD
colorectal distension
- EC
enterochromaffin
- eGFP
enhanced green fluorescent protein
- GFP
green fluorescent protein
- GI
gastrointestinal
- 5-HT
5-hydroxytryptamine or serotonin
- 5-HT4R
5-hydroxytryptamine receptor
- ISC
short-circuit current
- RT-PCR
reverse-transcription polymerase chain reaction
- SW
Swiss Webster
- TTX
tetrodotoxin
- VMR
visceromotor response
Footnotes
Supplementary Materials Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.12.041.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 4.De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi: 10.1111/j.1365-2982.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 5.Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol. 1994;266:G230–G238. doi: 10.1152/ajpgi.1994.266.2.G230. [DOI] [PubMed] [Google Scholar]
- 6.Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology. 1989;96:1257–1264. doi: 10.1016/s0016-5085(89)80012-5. [DOI] [PubMed] [Google Scholar]
- 7.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 8.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- 9.Park J, Quaiserova-Mocko V, Patel BA, et al. Diamond microelectrodes for in vitro electroanalytical measurements: current status and remaining challenges. Analyst. 2008;133:17–24. doi: 10.1039/b710236b. [DOI] [PubMed] [Google Scholar]
- 10.Patel BA, Bian X, Quaiserova-Mocko V, et al. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2007;132:41–47. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 11.Green CL, Ho W, Sharkey KA, et al. Dextran sodium sulfate-induced colitis reveals nicotinic modulation of ion transport via iNOS-derived NO. Am J Physiol Gastrointest Liver Physiol. 2004;287:G706–G714. doi: 10.1152/ajpgi.00076.2004. [DOI] [PubMed] [Google Scholar]
- 12.Maceachern S, Patel B, McKay D, et al. Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J Physiol. 2011;589:3333–3348. doi: 10.1113/jphysiol.2011.207902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal Motility Monitor (GIMM) J Vis Exp. 2010;46 doi: 10.3791/2435. doi: 10.3791/2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plaisancie P, Barcelo A, Moro F, et al. Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Physiol. 1998;275:G1073–G1084. doi: 10.1152/ajpgi.1998.275.5.G1073. [DOI] [PubMed] [Google Scholar]
- 15.Chey WD, Pare P, Viegas A, et al. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217–1225. doi: 10.1111/j.1572-0241.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood-Van Meerveld B, Venkova K, Hicks G, et al. Activation of peripheral 5-HT receptors attenuates colonic sensitivity to intraluminal distension. Neurogastroenterol Motil. 2006;18:76–86. doi: 10.1111/j.1365-2982.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 17.Bowersox SS, Lightning L, Rao S, et al. Metabolism and pharma-cokinetics of naronapride, a serotonin 5-HT4 receptor agonist for gastrointestinal motility disorders. Drug Metab Dispos. 2011;39:1170–1180. doi: 10.1124/dmd.110.037564. [DOI] [PubMed] [Google Scholar]
- 18.Jin JG, Foxx-Orenstein AE, Grider JR. Stimulation of colonic propulsion by 5-HT4 receptor agonists: synergism by delta opioid receptor antagonists. Gastroenterology. 1997;112:A754. doi: 10.1152/ajpgi.1998.275.5.G979. [DOI] [PubMed] [Google Scholar]
- 19.Langlois A, Pascaud X, Junien JL, et al. Response heterogeneity of 5-HT3 receptor antagonists in a rat visceral hypersensitivity model. Eur J Pharmacol. 1996;318:141–144. doi: 10.1016/s0014-2999(96)00857-6. [DOI] [PubMed] [Google Scholar]
- 20.Galligan JJ, Pan H, Messori E. Signalling mechanism coupled to 5-hydroxytryptamine4 receptor-mediated facilitation of fast synaptic transmission in the guinea-pig ileum myenteric plexus. Neurogastroenterol Motil. 2003;15:523–529. doi: 10.1046/j.1365-2982.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 21.Ren J, Zhou X, Galligan JJ. 5-HT4 receptor activation facilitates recovery from synaptic rundown and increases transmitter release from single varicosities of myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1376–G1383. doi: 10.1152/ajpgi.00078.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Geddis MS, Wen Y, et al. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–G1163. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 23.Fiorica-Howells E, Liu MT, Ponimaskin EG, et al. Distribution of 5-HT4 receptors in wild-type mice and analysis of intestinal motility in 5-HT4 knockout mice. Gastroenterology. 2003;124:A-342. [Google Scholar]
- 24.Poole DP, Xu B, Koh SL, et al. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res. 2006;325:413–422. doi: 10.1007/s00441-006-0181-9. [DOI] [PubMed] [Google Scholar]
- 25.Gebauer A, Merger M, Kilbinger H. Modulation by 5-HT3 and 5-HT4 receptors of the release of 5-hydroxytryptamine from the guineapig small intestine. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:137–140. doi: 10.1007/BF00169258. [DOI] [PubMed] [Google Scholar]
- 26.Schworer H, Ramadori G. Autoreceptors can modulate 5-hydroxy-tryptamine release from porcine and human small intestine in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:548–552. doi: 10.1007/pl00005206. [DOI] [PubMed] [Google Scholar]
- 27.Kidd M, Modlin IM, Eick GN, et al. Isolation, functional characterization, and transcriptome of Mastomys ileal enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G778–G791. doi: 10.1152/ajpgi.00552.2005. [DOI] [PubMed] [Google Scholar]
- 28.von Mentzer B, Murata Y, Ahlstedt I, et al. Functional CRF receptors in BON cells stimulate serotonin release. Biochem Pharmacol. 2007;73:805–813. doi: 10.1016/j.bcp.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Kidd M, Eick GN, Modlin IM, et al. Further delineation of the continuous human neoplastic enterochromaffin cell line, KRJ-I, and the inhibitory effects of lanreotide and rapamycin. J Mol Endocrinol. 2007;38:181–192. doi: 10.1677/jme.1.02037. [DOI] [PubMed] [Google Scholar]
- 30.Bradbury NA. Protein kinase–A-mediated secretion of mucin from human colonic epithelial cells. J Cell Physiol. 2000;185:408–415. doi: 10.1002/1097-4652(200012)185:3<408::AID-JCP11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Budhoo MR, Harris RP, Kellum JM. 5-Hydroxytryptamine-induced Cltransport is mediated by 5-HT3 and 5-HT4 receptors in the rat distal colon. Eur J Pharmacol. 1996;298:137–144. doi: 10.1016/0014-2999(95)00752-0. [DOI] [PubMed] [Google Scholar]
- 32.Ning Y, Zhu JX, Chan HC. Regulation of ion transport by 5-hydroxytryptamine in rat colon. Clin Exp Pharmacol Physiol. 2004;31:424–428. doi: 10.1111/j.1440-1681.2004.04015.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooke HJ. “Enteric tears”: chloride secretion and its neural regulation. News Physiol Sci. 1998;13:269–274. [PubMed] [Google Scholar]
- 34.MeGreenwood-Van erveld B, Campbell-Dittmeyer K, Johnson AC, et al. 5-HT2B receptors do not modulate sensitivity to colonic distension in rats with acute colorectal hypersensitivity. Neurogastroenterol Motil. 2006;18:343–345. doi: 10.1111/j.1365-2982.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Mahony SM, Bulmer DC, Coelho AM, et al. 5-HT(2B) receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil. 2010;22:573–578. e124. doi: 10.1111/j.1365-2982.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- 36.Ohashi-Doi K, Himaki D, Nagao K, et al. A selective, high affinity 5-HT 2B receptor antagonist inhibits visceral hypersensitivity in rats. Neurogastroenterol Motil. 2010;22:e69–e76. doi: 10.1111/j.1365-2982.2009.01395.x. [DOI] [PubMed] [Google Scholar]
- 37.Beattie DT, Smith JA, Marquess D, et al. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol. 2004;143:549–560. doi: 10.1038/sj.bjp.0705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appel-Dingemanse S. Clinical pharmacokinetics of tegaserod, a serotonin 5-HT(4) receptor partial agonist with promotile activity. Clin Pharmacokinet. 2002;41:1021–1042. doi: 10.2165/00003088-200241130-00002. [DOI] [PubMed] [Google Scholar]
- 39.Tonini M, Pace F. Drugs acting on serotonin receptors for the treatment of functional GI disorders. Dig Dis. 2006;24:59–69. doi: 10.1159/000090309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. As was detected in Swiss Webster mice (Figure 1A), mucosal 5-HT4R transcript levels detected in the distal colon of Balb/cJ mice were significantly greater than levels detected in all other regions. The transcript levels in the proximal colon were significantly greater than levels in the duodenum or jejunum (P < .01). ***P < .001 as compared with other regions; n = 5–6.
Supplementary Figure 2. Application of the 5-HT4R agonist, tegaserod (Teg; 1 μmol/L), to the mucosal surface of the guinea pig distal colon elicited an increase in goblet cell cavitation (yellow arrows) in periodic acid–Schiff and Alcian Blue (PAS/AB)–stained sections. The tegaserod response was blocked by the 5-HT4R antagonist, GR113808 (1 μmol/L), but not by TTX (0.3 μmol/L). n = 5 per group.
Supplementary Figure 3. Mucosal application of the 5-HT4R agonist, tegaserod (1 μmol/L), elicited an increase in ISC in distal colon preparations of (A) Swiss Webster mice and (B) guinea pigs. In both species, the response to mucosally applied tegaserod was inhibited by the 5-HT4R antagonist, GR113808 (1 μmol/L). Serosal application of tegaserod failed to induce a change in ISC. n = 4 per group. ***P < .001.
Supplementary Table 1. Composite of Data Collected From the Different Species Studied Regarding Mucosal 5-HT4 Receptors in the Colon