Abstract
An obstacle to understanding motor pathologies of the gastrointestinal (GI) tract is that the physiology of some of the cellular components of the gut wall is not understood. Morphologists identified fibroblast-like cells in the tunica muscularis many years ago, but little is known about these interstitial cells because of inadequate techniques to identify these cells. Recent findings have shown that fibroblast-like cells express platelet-derived growth factor receptor α (PDGFRα) in mice and that antibodies for these receptors can be used to label the cells. We used immunohistochemical techniques to study the phenotype and intercellular relationships of fibroblast-like cells in the human colon. Fibroblast-like cells are labelled specifically with antibodies to PDGFRα and widely distributed through the tunica muscularis of human colon. These cells form discrete networks in the region of the myenteric plexus and within the circular and longitudinal muscle layers. Platelet-derived growth factor receptor α+ cells are distinct from c-Kit+ interstitial cells of Cajal and closely associated with varicose processes of neurons expressing substance P (excitatory motor neurons) or neuronal nitric oxide synthase (nNOS) (inhibitory motor neurons). Platelet-derived growth factor receptor α+ cells express small conductance Ca2+-activated K+ channels (SK3), which are likely to mediate purinergic neural regulation of colonic muscles. Our data suggest that PDGFRα+ cells may have an important role in transducing inputs from enteric motor neurons. This study identifies reagents and techniques that will allow investigation of this class of interstitial cells and help develop an understanding of the role of PDGFRα+ cells in the human GI tract in health and disease.
Keywords: interstitial cells of Cajal, fibroblast-like cell, enteric inhibitory neurotransmission, GI motility, purinergic neurotransmission
Introduction
There are at least two types of interstitial cells, interstitial cells of Cajal (ICC) and cells referred to as ‘fibroblast-like’ or ‘interstitial cells of Cajal-like (ICLC)’, in the tunica muscularis of the GI tract with anatomical localizations suggestive of important physiological functions [1,2]. Interstitial cells of Cajal express c-Kit [3], and exploiting this feature made it possible to isolate and study the function and pathophysiology of this population of interstitial cells [[4],[5],,[6]]. Now it is recognized that ICC are pacemaker cells, provide propagation pathways for slow waves responsible for timing phasic contractions, and constitute an interface between terminals of enteric motor neurons and smooth muscle cells to mediate a portion of the motor input from the enteric nervous system [5,7]. Several motility disorders have been linked to loss or defects in ICC, and it is likely that gastrointestinal stromal tumours (GIST) originate from transformed ICC [8]. In contrast, investigations of ‘fibroblast-like’ cells or ‘ICLC’ have been impeded by the inability to identify these cells in living tissues or amongst the mixed cell populations resulting after enzymatic dispersion. The terms ‘fibroblast-like’ and ‘ICLC’ are vague and imply that little is known about the function of these cells and why they are located in specific anatomical niches within the tunica muscularis. Iino et al. 2009 provided a critical tool for investigating these cells by showing that PDGFRα is a specific immunomarker for the ‘fibroblast-like’ cells in the murine GI tract. With this knowledge, it was possible to utilize transgenic mice with a histone-eGFP fusion protein driven by the endogenous promoter for Pdgfra to isolate and study the function of these cells [10]. We have suggested that naming these cells on the basis of chemical coding or molecular phenotype (e.g. PDGFRα+ cells) is a more precise method to refer to this class of interstitial cells [10].
Immunohistochemical studies had shown that PDGFRα+ cells in GI muscles express SK3 channels, a possible mediator of enteric inhibitory responses [11,12,,13]. This observation, coupled with findings that PDGFRα+ cells are very closely associated with enteric motor neurons and electrically coupled to smooth muscle cells [14], suggested that they, like ICC, may mediate responses to enteric inhibitory neurotransmitters. We isolated PDGFRα+ cells from mouse colon and showed that they express receptors and ion channels required to mediate purinergic inhibitory responses. We also found that single PDGFRα+ cells generated large outward currents via Ca2+-activated, apamin-sensitive K+ channels in response to purinergic neurotransmitters. In comparison, smooth muscle cells were far less responsive to purines and appear to be incapable of generating purinergic inhibitory responses in intact muscles. These results demonstrated that PDGFRα+ cells are an important component of the post-junctional receptive field for enteric motor neurotransmitters.
‘Fibroblast-like cells’ have also been identified in the human GI tract as shown by immunological and ultrastructural studies [12,15], but little is known about the role of these cells in normal GI motility or pathophysiology. Some GISTs are positive for PDGFRα and manifest gain-of-function mutations in Pdgfra [16,17,,18], so it is possible that these cells, like ICC, can be transformed. We investigated whether fibroblast-like cells in the human colon are immunoreactive for PDGFRα+ antibodies, form a population of cells distinct from ICC, display close contacts with enteric motor neurons, and express ion channels that would allow them to participate in enteric inhibitory neurotransmission. Our data show that PDGFRα+ cells are expressed widely in the tunica muscularis of the human colon, and the techniques we describe will facilitate future clinical evaluations of the status of PDGFRα+ cells in health and disease.
Materials and methods
Preparation of human colonic muscles
Segments of human colon tissues were obtained as a surgical waste from three patients (ages 62–68) who underwent colon resections for neoplasms at Renown Regional Medical Center (Reno, NV, USA). Tissues from outside the disease-affected area were placed in oxygenated Krebs solution (10°C) containing (mmol/l): 118.5 NaCl; 4.2 KCl; 1.2 MgCl2; 23.8 NaHCO3; 1.2 KH2PO4; 11.0 dextrose; 1.8 CaCl2 (pH 7.4). The Human Subjects Research Committees at Renown Regional Medical Center, and the Biomedical Institutional Review Board at University of Nevada, Reno approved the use of human tissues.
Immunohistochemistry
Colon tissues were pinned at the base of the dish coated with Sylgard elastomere (Dow Corning Inc., Midland, MI, USA), and were stretched to 150% of the original length and width. The mucosa was removed by sharp dissecting the submucosal layer. The remaining tunica muscularis was fixed in Periodate-Lysine-Paraformaldehyde fixative overnight at 4°C. After fixation, tissues were washed every 30 min. for 7 hr, then washed through a sucrose gradient (5%, 10% and 15% in PBS) for 15 min. each, and were placed in 20% sucrose in PBS overnight at 4°C. Tissues were then embedded in half-and-half mixture of OCT compound (Sakura Finetek Co., Tokyo, Japan) and 20% sucrose in PBS, frozen in liquid nitrogen, and stored at −80°C.
Frozen tissues were cut (100 μm) using a cryostat (Carl Zeiss, Thornwood, NY, USA) and were placed in PBS (15 min. × 3 washes) to remove OCT. To block non-specific antibody binding, sections were incubated in 5% donkey serum in 0.5% Triton X-100 in PBS for 1 hr at room temperature. Following blocking, sections were incubated in primary antibodies diluted with half-and-half mixture of 0.5% Triton X-100 and 1% bovine serum albumin in PBS (48 hr at 4°C). For double-labelling, sections were washed in PBS (30 min. × 4 washes) and subsequently incubated in the second primary antibody (48 hr at 4°C). After primary antibodies, sections were incubated in appropriate secondary antibodies (1:500 in PBS, Alexa; Invitrogen, Carlsbad, CA, USA) for 1 hr at room temperature in dark. Information on primary antibody sources and concentrations is shown in Table. Following incubation of antibodies, sections were washed in PBS (30 min. × 8 washes) and mounted on glass slides using Thermo Aqua-Mount (Fisher Scientific, Waltham, MA, USA). Sections were examined with a confocal microscope (Zeiss LSM510 Meta; Carl Zeiss). Micrographs were constructed using Zeiss LSM 5 Image Examiner software, and are composites of Z-series scans (0.2–0.5 μm optical sections) through a depth of 4–40 μm.
Table 1.
Primary antibodies
| Primary antibodies | Species | Catalogue no. | Dilution | Source |
|---|---|---|---|---|
| PDGFr α | Goat | AF-307-NA | 1:100 | R & D systems, MN, USA |
| c-Kit | Rabbit | A4502 | 1:100 | Dako, CA, USA |
| SK 3 | Rabbit | APC-025 | 1:100 | Alomone Labs Ltd., Israel |
| PGP 9.5 | Rabbit | RA-95101 | 1:3000 | UltraClone Ltd., UK |
| nNOS | Rabbit | SC-648 | 1:250 | Santa Cruz Biotechnology, Inc., CA, USA |
| Substance P | Rat | MAB356 | 1:500 | Millipore, MA, USA |
Results
Distribution of PDGFRα+ cells
The distribution of PDGFRα+ cells was studied by immunolabelling using an anti-human PDGFRα antibody on muscles of ascending colon (A colon), transverse colon (T colon) and sigmoidal colon (S colon) (Figs 1 and S1). PDGFRα+ cells had multi-polar shapes and were interconnected to form a discrete network. In circular and longitudinal muscle layers (Figs 1A and B, S1A, B, D, E, G and H), cell bodies and major processes of PDGFRα+ cells ran parallel to smooth muscle cells. PDGFRα+ cells were more stellate in shape in the myenteric region (Figs 1C, S1C, F and I), and they formed a network of interconnecting cells, similar to that of ICC. There were no gross differences in the morphology of these cells or in the multi-cellular networks they formed in A, T and S colons (Fig. S1). Localization of PDGFRα+ cells within the muscle layers and within the myenteric region recapitulates the distribution of these cells in mice, and suggests that PDGFRα+ cells may have similar a functional role in regulation of GI motility.
Fig 1.
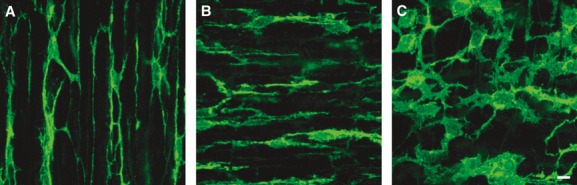
Immunolabelling of PDGFRα (green) in the muscle layer of human colon. (A) Circular muscle layer of T colon. (B) Longitudinal muscle layer of A colon. (C) Myenteric plexus region of S colon. PDGFRα+ cells were multi-polar cells and interconnected. In circular and longitudinal muscle layer, cell bodies of PDGFRα+ cells ran parallel to smooth muscle cells. In myenteric region, PDGFRα+ cells formed an extensive fine network. Scale bar is 10 μm in all panels.
Relationship between PDGFRα+ cells and ICC
Some of the networks of PDGFRα+ cells were similar in general morphology to the networks formed by ICC. Therefore, we investigated whether ICC and PDGFRα+ cells in human colon muscles are distinct populations of cells and whether they are closely associated with each other (i.e. occupy the same anatomical niches). Double immunolabelling for PDGFRα and c-Kit was performed on human A, T and S colon (Figs 2, S2, S3 and S4). The network pattern of PDGFRα+ cells was similar to that of ICC, but the cellular distribution of the cell-specific biomarkers was distinct. No obvious differences were noted in the distributions of ICC and PDGFRα+ cells or in their close proximities in A, T or S colon (Figs S2–S4). In each region, PDGFRα+ cells and ICC were heavily intertwined, forming cable-like structures within the circular and longitudinal muscle layers (Movie S1) and a complex mesh-like structure within the myenteric region between the muscle layers (Movie S2). As PDGFRα+ cells and ICC are distributed within the same anatomical regions, we suggest adopting the terminology typically used for ICC to distinguish different populations of these cells: i.e. cells running in muscle bundles as intermuscular PDGFRα+ cells (PDGFRα+-IM) and cells within the region of the myenteric plexus as PDGFRα+-MY.
Fig 2.
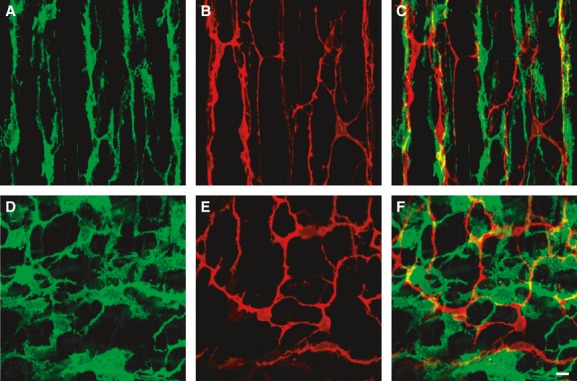
Double immunolabelling of PDGFRα (green) and c-Kit (red). (A–C) Circular muscle layer of A colon. (D–F) Myenteric plexus region of S colon. PDGFRα+ cells and c-Kit+ cells (ICC) formed distinct networks in the same anatomical regions and close relationships with each other. Scale bar is 10 μm in all panels.
Proximity of PDGFRα+ cells to motor nerves
The close relationship of PDGFRα+-IM with ICC-IM suggests that this class of PDGFRα+ cells may be closely associated with terminals of enteric neurons. We studied the relationship between PDGFRα+ cells and enteric neurons, using double immunolabelling with antibodies for PDGFRα and protein gene product 9.5 (PGP9.5) (Figs 3, S5), nNOS (Figs 4, S6) and Substance P (Figs 5, S7). PGP9.5 labels all enteric neurons, and nNOS and substance P are markers for enteric inhibitory and excitatory motor neurons that innervate GI muscles [19]. PDGFRα+ cells ran parallel to and were closely associated with both excitatory and inhibitory enteric neurons, and we could find no obvious differences in this pattern in A, T and S colon (Figs S5–S7). Processes of PDGFRα+-IM form a basket around neural processes (Fig.C), and when fine varicose processes of inhibitory or excitatory fibres were observed, a PDGFRα+-IM process was usually in close proximity [Figs 4C (arrow) and 5C (arrows)]. These results suggest that neurotransmitters released from motor neurons will be highly concentrated near PDGFRα+ cells, and these cells may play a functional role in regulation of colonic motility.
Fig 3.
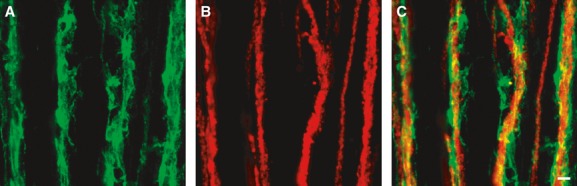
Double immunolabelling of PDGFRα (green) (A) and PGP9.5 (red) (B) in the circular muscle layer of T colon. PDGFRα+ cells were closely associated with processes of enteric neurons, which express PGP9.5-like immunoreactivity (C). Scale bar is 10 μm in all panels.
Fig 4.
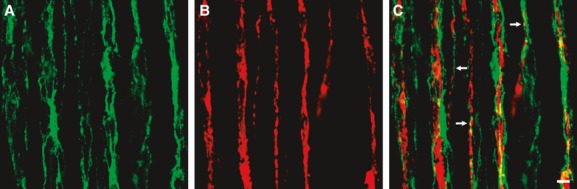
Double immunolabelling of PDGFRα (green) (A) and nNOS (red) (B) in the circular muscle of S colon. PDGFRα+ cells were closely associated with inhibitory enteric neurons, denoted by the expression of nNOS-like immunoreactivity (C). Fine varicose processes of inhibitory enteric neurons were in close proximity to PDGFRα+ cell processes (arrows in C), suggesting that PDGFRα+ cells may have synapse-like communication with these neurons. Scale bar is 10 μm in all panels.
Fig 5.
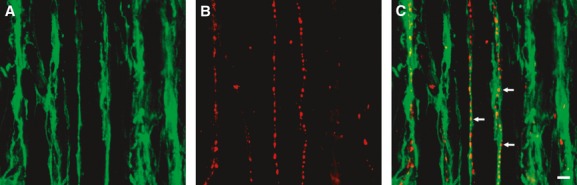
Double immunolabelling of PDGFRα (green) (A) and substance P (red) (B) in the circular muscle layer of A colon. PDGFRα+ cells were closely associated with excitatory enteric neurons, denoted by expression of Substance P-like immunoreactivity (C). Fine varicose processes of excitatory enteric neurons were in close proximity to PDGFRα+ cell processes (arrows in C), suggesting that PDGFRα+ cells may have synapse-like communication with these neurons. Scale bar is 10 μm in all panels.
Expression of SK3 in PDGFRα+ cells
It is well known that the purinergic component of enteric inhibitory regulation of GI motility is mediated via apamin-sensitive, SK3 channels. Opening of these channels results in K+ efflux and causes hyperpolarization of muscles and relaxation. The robust expression of SK3 channels in fibroblast-like cells has suggested that these cells could mediate purinergic inputs within the gut [10,11,12,13,20]. We investigated SK3 expression in PDGFRα+ cells of human colon, using double immunolabelling. PDGFRα+ cells in human A, T and S colon muscle layer expressed SK3 (Figs 6, S8–S10), and labelling of other cells was not resolved. Expression of SK3 in PDGFRα+ cells confirms that these cells are the fibroblast-like cells described in previous studies. These data are consistent with a role of PDGFRα+ cells in enteric inhibitory hyperpolarization responses in human colon.
Fig 6.
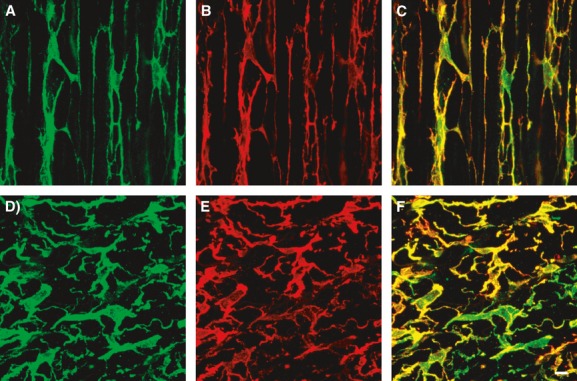
Double immunolabelling of PDGFRα (green) and SK3 (red). (A–C) Circular muscle layer of T colon. (D–F) Myenteric plexus region of T colon. SK3-like immunoreactivity was expressed in all PDGFRα+ cells and was not resolved in other cell types. Scale bar is 10 μm in all panels.
Discussion
Discovering that PDGFRα antibodies can label cells previously referred to as ‘fibroblast-like’ interstitial cells in GI muscles has opened the door to investigations of these cells [9,10]. Here, we verified that this approach is also useful for identification of this class of interstitial cells in the human GI tract. We showed that these cells, referred to here as PDGFRα+ cells because of their chemical coding, are widely distributed within the myenteric region and in circular and longitudinal muscle layers throughout the colon. PDGFRα+ cells are closely associated with, but distinct from ICC. We suggest referring to the morphologically distinct populations of cells as PDGFRα+-MY and PDGFRα+-IM, respectively, because they occupy the same anatomical niches as ICC-MY and ICC-IM. PDGFRα+-IM networks form baskets around the processes of excitatory and inhibitory motor neurons, and ultrastructural studies have shown that this type of cell forms gap junctions with smooth muscle cells [14]. We also showed that PDGFRα+ cells express SK3, a potential mediator for purineric regulation of colonic motility. Establishing a biomarker for this class of cells makes it possible for pathologists and neurogastroenterologists to characterize their status in pathophysiological conditions and in animal models. Just as occurred with ICC after development of c-Kit labelling, this is a critical step in more fully understanding the role of these unique cells in health and disease.
The question of whether ICC and the class of interstitial cells referred to by others as ‘fibroblast-like’ cells or ‘ICLC’ are discrete classes of cells has been debated previously. In the seminal article showing that GIST are c-Kit+ cells, it was also noted that GIST cells express CD34, a sialylated transmembrane glycoprotein [21]. Others agreed that CD34 and c-Kit labelled the same population of cells. For example, sequential immunohistochemical labelling of 3 μm sections of human small intestine with antibodies for c-Kit and CD34 suggested that ICC near the myenteric plexus and within the circular muscle layer of human small intestine were positive for both antigens [22]. Subsequent studies disputed these findings, however, and showed that c-Kit and CD34 labelled distinct cells in close proximity to one another [2,23]. Pieri et al. 2008 chose to refer to these cells as ICLC. It seems likely that some of the cells labelled by CD34 are PDGFRα+ cells, but additional cell types, including endothelial cells, are CD34+ [2], making this antigen a less specific target for cellular identification. In the present study, we showed that PDGFRα+ cells are distinct from c-Kit+ ICC in the human colon.
The current study showed that the organization and anatomy of PDGFRα+ cells in human colon are similar to that in mice [9,10]. These data suggest that mice might be good models for investigating the role of PDGFRα+ cells. In recent studies, we utilized animals with expression of an eGFP-histone fusion protein driven by endogenous promoters for Pdgfra. We showed that cellular expression of the fusion protein in the gut co-localized with PDGFRα by immunohistochemistry, and that PDGFRα+ cells could be found in enzymatic dispersions of colonic muscles. These cells expressed P2ry1 and Kcnn3 (P2Y1 receptor and SK3 channel transcripts), and, when studied with patch clamp techniques, displayed spontaneous outward currents and large outward current responses to P2Y1 receptor agonists. It should be noted that responses of smooth muscle cells to purine agonists were minor or unresolvable in comparison with responses of PDGFRα+ cells. ATP and β-NAD, a new candidate for the purinergic enteric inhibitory neurotransmitter, elicited inward currents in human and non-human primate colonic smooth muscle cells [24]. Thus, it is unlikely that smooth muscle cells can mediate post-junctional responses to purinergic neurotransmitters in the human colon. Taken together, the anatomical features of PDGFRα+ cells (i.e. proximity to varicosities of enteric motor neurons; gap junction with smooth muscle cells), molecular apparatus (i.e. receptors and effector channels) and outward current responses to purines (as opposed to minimal or even contradictory responses of smooth muscle cells) suggest that PDGFRα+ cells are the primary post-junctional mediators of the purinergic component of enteric inhibitory neurotransmission. Currently, we are unable to identify human PDGFRα+ cells in the heterogenous mixture of cells resulting from enzymatic dispersion of tissues. We are now looking for an effective technique to make physiological studies of these cells possible.
The anatomy, distribution and spatial relationships with other cells suggest that PDGFRα+ cells have a role in enteric inhibitory responses in the human colon similar to that in mouse. Proximity of these cells to neural processes with nNOS-like immunoreactivity suggests that neurotransmitters released from inhibitory neurons might activate post-junctional responses in PDGFRα+ cells. Purinergic responses in the human colon are characterized by rapid hyperpolarization transients (fast inhibitory junction potentials) that are inhibited by P2Y1 receptor antagonists and apamin [25,26,27,28]. We have not identified an antibody suitable for P2Y1 receptor labelling in human colon, but expression of SK3, an apamin-sensitive K+ channel, PDGFRα+ cells would enable these cells to generate outward currents in response to purines. Spontaneous activation of SK3 channels, as observed in mouse PDGFRα+ cells is also suggestive of a contribution of these cells to resting membrane potentials in colonic muscles. Thus, these cells could generally participate in the regulation of excitability responses of colonic muscles to other stimuli. It should also be noted that excitatory neurons, labelled by immunoreactivity to substance P, are also closely associated with PDGFRα+ cells. Little is known about a potential role for these cells in excitatory neurotransmission.
How activation of ion channels in PDGFRα+ cells can influence the behaviour of colonic muscles should also be discussed. Cells previously identified as ‘fibroblast-like’ cells in GI muscles form gap junctions with surrounding smooth muscle cells as shown by ultrastructural studies [14,29]. Gap junctions provide electrical coupling between cells, such that induction of a K+ current (i.e. outward current) in PDGFRα+ cells results in hyperpolarization that is conducted to coupled cells. Thus, hyperpolarization of PDGFRα+ cells results in net hyperpolarization of the syncytium of cells formed by smooth muscle, PDGFRα+ cells, and ICC. Colonic contractions are initiated by entry of Ca2+ through voltage-dependent Ca2+ channels in smooth muscle cells. Thus, hyperpolarization of smooth muscle cells will result in lowered open probability of Ca2+ channels and reduce contraction.
At present, little is known about the involvement, fate or loss-of-function of PDGFRα+ cells in GI motor dysfunction. Certainly, changes in purinergic neural inputs could have profound effects on colonic motility. Mice with enhanced activation of PDGFRα develop GI fibrosis and sarcoma [30], and PDGFRα plays a role in the development of fibrotic diseases in several organs [31]. Gain-of-function mutations in Pdgfra are present in about 5% of c-Kit-negative and PDGFRα-positive GISTs [17,18].
In summary, we demonstrated that the ‘fibroblast-like’ cells in the tunica muscularis of human GI tract can be specifically labelled with antibodies raised against PDGFRα, and these cells are widely distributed in the colon and distinct from ICC. PDGFRα+ cells are closely associated with enteric motor neurons and express important cellular effectors that mediate responses to enteric inhibitory neural regulation of the gut. This study identifies reagents and techniques that provide new opportunities for understanding the role of fibroblast-like cells in the human GI tract.
Acknowledgments
This study was supported by a Program Project Grant from NIDDK (P01 DK41315). Immunohistochemistry was performed with reagents and equipment of Core C of the PPG. We are grateful to Dr. Harold L. Kennedy (Western Surgical Group, Reno Nevada) for assisting us in obtaining colon samples from patients undergoing surgery for non-obstructive neoplasms.
Conflicts of interest
MK, YN, GWH, SMW and KMS have no commercial investments or conflicts to disclose.
Contributions of authors
MK and YN acquired and analysed data; GWH analysed and interpreted data and developed three-dimensional movies from confocal slices; KMS, MK and SMW planned study, analysed data and wrote and critically revised manuscript.
Supporting Information
Immunolabelling of PDGFRα (green) in the muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). (A, D, G) Circular muscle layer. (B, E, H) Longitudinal muscle layer. (C, F, I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and c-Kit (red) in the muscle layer of human A colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and c-Kit (red) in the muscle layer of human T colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and c-Kit (red) in the muscle layer of human S colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and PGP9.5 (red) in the circular muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and nNOS (red) in the circular muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and substance P (red) in the circular muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and SK3 (red) in the muscle layer of human A colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and SK3 (red) in the muscle layer of human T colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and SK3 (red) in the muscle layer of human S colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Three-dimensional image of PDGFRα+-IM (green) and c-Kit+ cells (red) in circular muscle layer of human T colon.
Three-dimensional picture of PDGFRα+-MY (green) and c-Kit+ cells (red) in myenteric plexus region of human S colon.
References
- Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- Pieri L, Vannucchi MG, Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008;12:1944–55. doi: 10.1111/j.1582-4934.2008.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;1:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137:1548–56. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Kim TW, Ro S, et al. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587(Pt 20):4905–18. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621–39. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumours. Science. 1998;278:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Horiguchi S, et al. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor α in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, et al. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm MF, Lang RJ. Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin Exp Pharmacol Physiol. 2002;29:18–25. doi: 10.1046/j.1440-1681.2002.03601.x. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, de Kerchove d'Exaerde A, Jr, et al. Kit-negative fibroblast-like cells expressing SK3, a Ca2 activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–58. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- Fujita A, Takeuchi T, Hanai J, et al. Localization of Ca2+-activatied K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/Wv mouse small intestine. J Auton Nerv Syst. 2000;80:142–7. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Vanderwinden JM, Rasmussen H, et al. Ultrastructure of interstitial cells of Cajal in myenteric plexus of human colon. Cell Tissue Res. 2009;337:197–212. doi: 10.1007/s00441-009-0818-6. [DOI] [PubMed] [Google Scholar]
- Joensuu H. Gastrointestinal stromal tumour (GIST) Ann Oncol. 2006;17:x280–6. doi: 10.1093/annonc/mdl274. [DOI] [PubMed] [Google Scholar]
- Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutation of platelet-derived growth factor receptor α gene in gastrointestinal stromal tumours. Gastroenterology. 2003;125:660–7. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumours. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- Furness JB, Young HM, Pompolo S, et al. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology. 1995;108:554–63. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107–15. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumours. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Robinson TL, Sircar K, Hewlett BR, et al. Gastrointestinal stromal tumours may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000;156:1157–63. doi: 10.1016/S0002-9440(10)64984-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, De Laet MH, et al. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000;302:145–53. doi: 10.1007/s004410000264. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, et al. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–17. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, et al. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008;295:G522–33. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA. 2007;104:16359–64. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, et al. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–17. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther. 2010;333:602–11. doi: 10.1124/jpet.109.160978. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Komuro T, Hirota S, et al. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res. 1997;289:137–43. doi: 10.1007/s004410050859. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–13. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunolabelling of PDGFRα (green) in the muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). (A, D, G) Circular muscle layer. (B, E, H) Longitudinal muscle layer. (C, F, I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and c-Kit (red) in the muscle layer of human A colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and c-Kit (red) in the muscle layer of human T colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and c-Kit (red) in the muscle layer of human S colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and PGP9.5 (red) in the circular muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and nNOS (red) in the circular muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and substance P (red) in the circular muscle layer of human A colon (A–C), T colon (D–F) and S colon (G–I). Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and SK3 (red) in the muscle layer of human A colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and SK3 (red) in the muscle layer of human T colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Double immunolabelling of PDGFRα (green) and SK3 (red) in the muscle layer of human S colon. (A–C) Circular muscle layer. (D–F) Longitudinal muscle layer. (G–I) Myenteric plexus region. Scale bar is 10 μm in all panels.
Three-dimensional image of PDGFRα+-IM (green) and c-Kit+ cells (red) in circular muscle layer of human T colon.
Three-dimensional picture of PDGFRα+-MY (green) and c-Kit+ cells (red) in myenteric plexus region of human S colon.


