Abstract
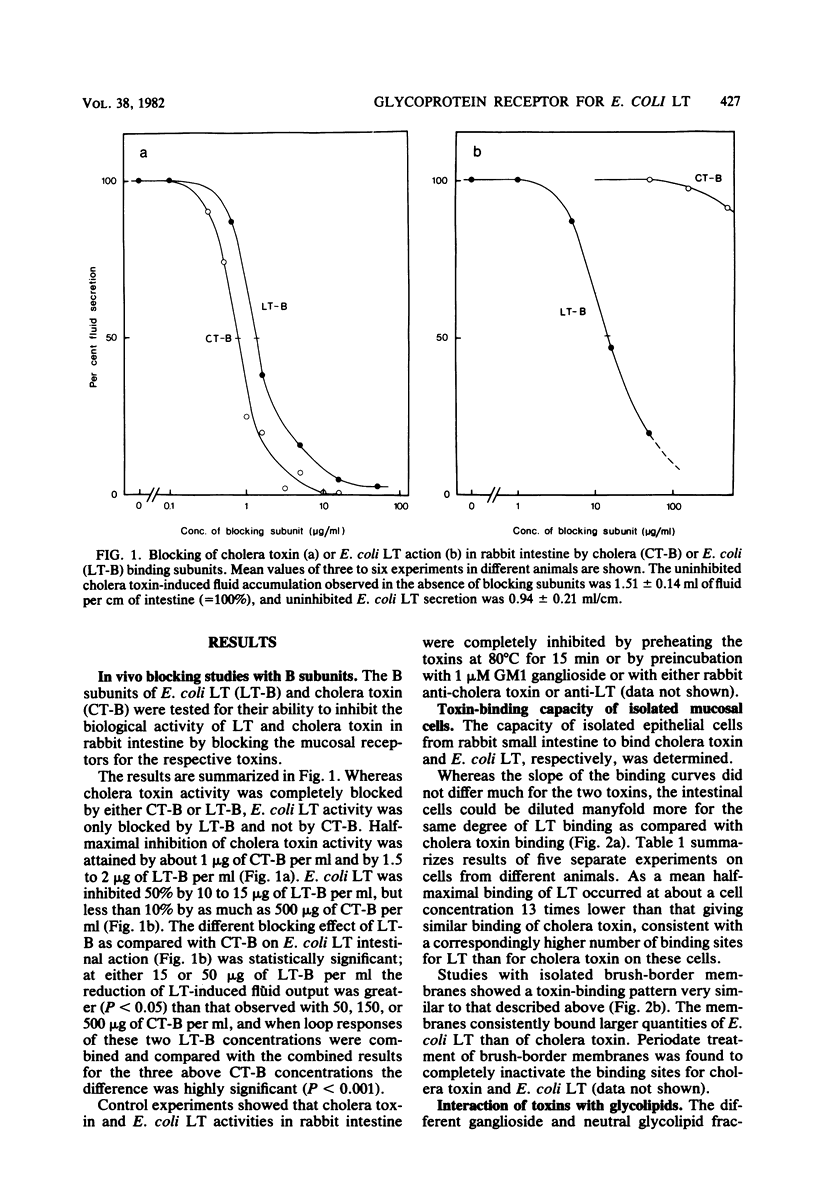
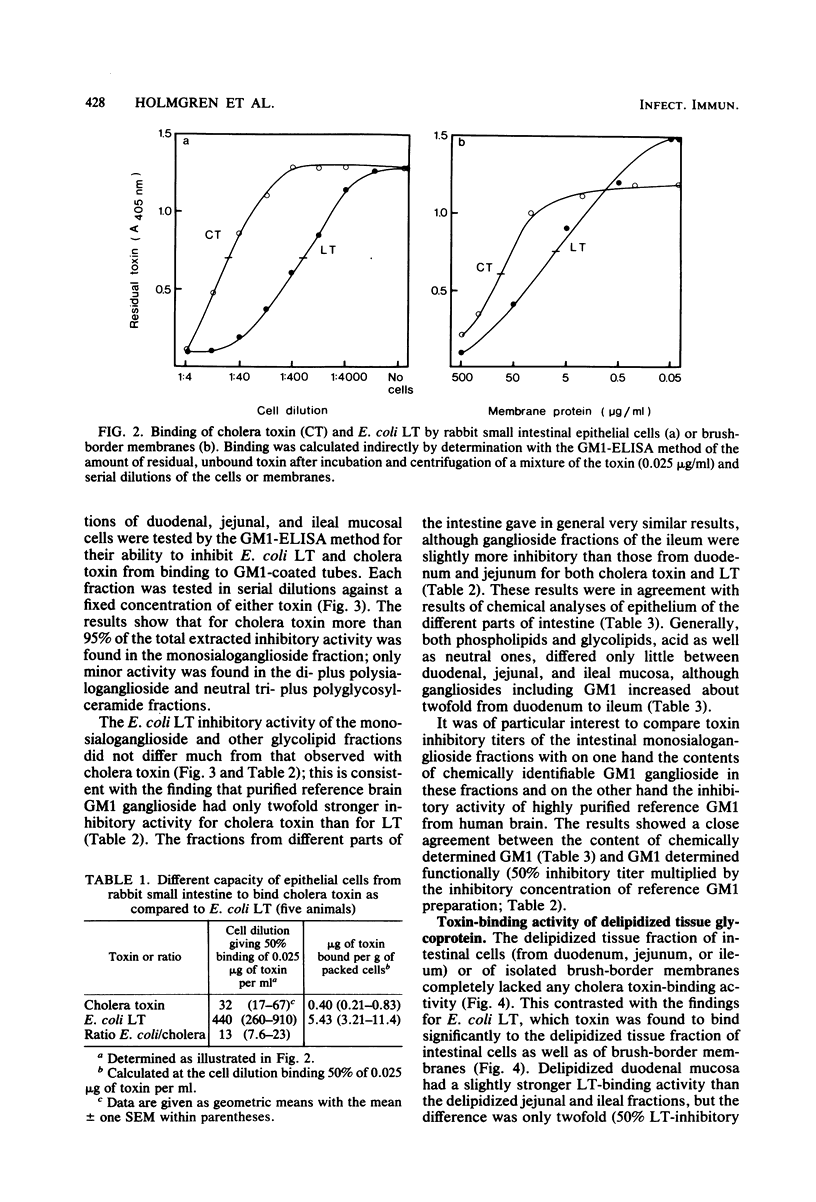
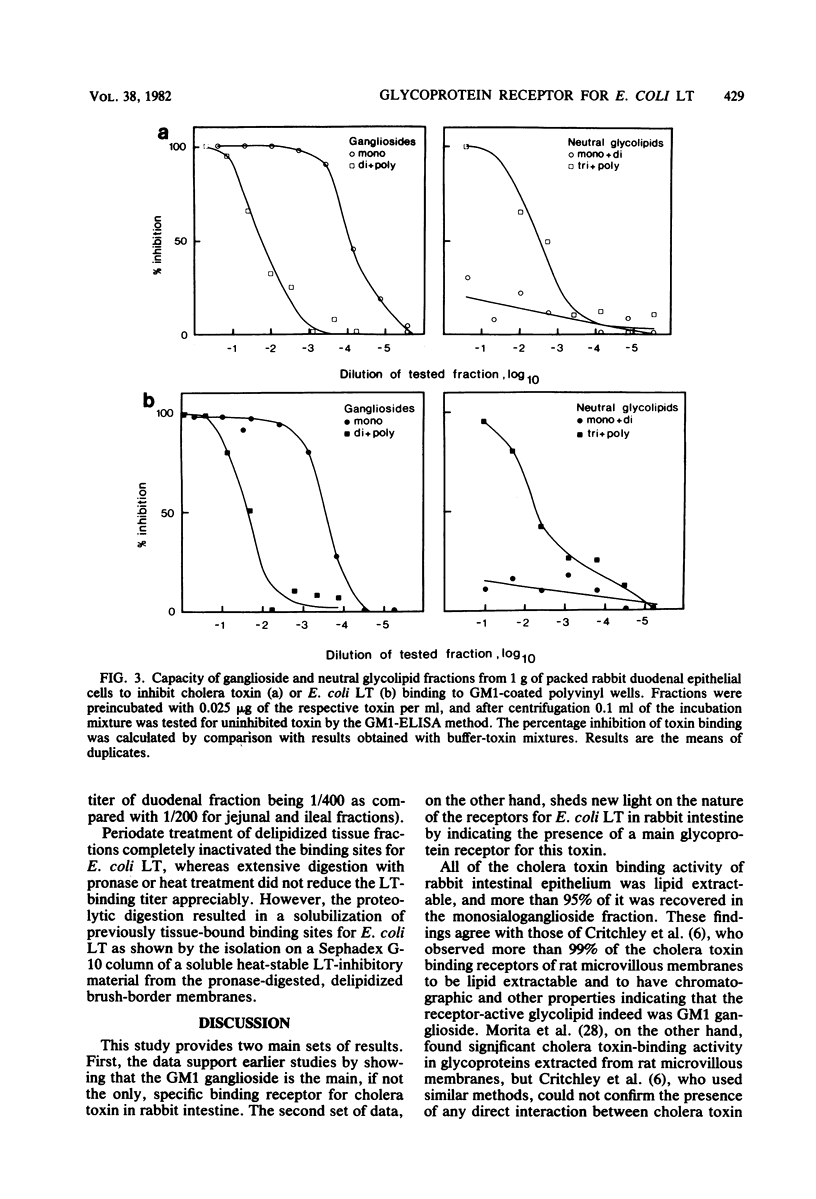
The receptors for cholera toxin and Escherichia coli heat-labile toxin (LT) in rabbit small intestinal epithelium were characterized and compared. (i) In vivo studies in ligated intestinal loops showed that whereas LT B subunits could block the fluid secretogenic action of purified LT as well as cholera toxin, cholera toxin B subunits did not inhibit the LT response even when tested in a concentration 100-fold higher than one which gave complete blocking of cholera toxin action. (ii) In vitro studies indicated that isolated intestinal epithelial cells or brush-border membranes could bind about 10-fold more of E. coli LT than of cholera toxin. (iii) All binding sites for cholera toxin in duodenal, jejunal, or ileal mucosal cells or brush-border membranes were extracted by chloroform-methanol-water (4:8:3), which removed lipids quantitatively but did not extract glycoproteins. The extracted cholera toxin binding sites were to greater than 95% recovered in a monosialoganglioside fraction; quantitatively these sites closely corresponded to the concentration of chromatographically identified mucosal GM1 ganglioside (1 nmol of cholera toxin was bound per 1 to 2 nmol of GM1). In contrast, a substantial fraction of mucosal binding sites for E. coli LT remained in the delipidized tissue residue, and these sites had properties consistent with a glycoprotein nature. Thus, whereas cholera toxin appeared to bind highly selectively to GM1 ganglioside receptor sites of rabbit small intestine, E. coli LT bound both to GM1 ganglioside and to a main glycoprotein receptor for which cholera toxin lacks affinity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Polymeric IgA is complexed with secretory component (SC) on the surface of human intestinal epithelial cells. Scand J Immunol. 1978;8(1):39–52. doi: 10.1111/j.1365-3083.1978.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Bäck E., Svennerholm A. M., Holmgren J., Möllby R. Evaluation of a ganglioside immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1979 Dec;10(6):791–795. doi: 10.1128/jcm.10.6.791-795.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Demonstration of shared and unique immunological determinants in enterotoxins from Vibrio cholerae and Escherichia coli. Infect Immun. 1978 Dec;22(3):709–713. doi: 10.1128/iai.22.3.709-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley D. R., Magnani J. L., Fishman P. H. Interaction of cholera toxin with rat intestinal brush border membranes. Relative roles of gangliosides and galactoproteins as toxin receptors. J Biol Chem. 1981 Aug 25;256(16):8724–8731. [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Viner J. P. Inhibition of the steroidogenic effects of cholera and heat-labile Escherichia coli enterotoxins by GM1 ganglioside: evidence for a similar receptor site for the two toxins. Infect Immun. 1975 May;11(5):982–985. doi: 10.1128/iai.11.5.982-985.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Modes of action of enterotoxins from Vibrio cholerae and EScherichia coli. Rev Infect Dis. 1979 Nov-Dec;1(6):918–926. doi: 10.1093/clinids/1.6.918. [DOI] [PubMed] [Google Scholar]
- Fredman P., Nilsson O., Tayot J. L., Svennerholm L. Separation of gangliosides on a new type of anion-exchange resin. Biochim Biophys Acta. 1980 Apr 18;618(1):42–52. doi: 10.1016/0005-2760(80)90052-1. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H. A., Holmgren J., Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M., Svennerholm L. Biosynthesis and biodegradation of rat brain gangliosides studied in vivo. J Neurochem. 1972 Mar;19(3):609–622. doi: 10.1111/j.1471-4159.1972.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Strannegård O., Svennerholm L. Gangliosides as receptors for bacterial toxins and Sendai virus. Adv Exp Med Biol. 1980;125:453–470. doi: 10.1007/978-1-4684-7844-0_40. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980 May;106(2):371–379. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Morita A., Tsao D., Kim Y. S. Identification of cholera toxin binding glycoproteins in rat intestinal microvillus membranes. J Biol Chem. 1980 Mar 25;255(6):2549–2553. [PubMed] [Google Scholar]
- Moss J., Fishman P. H., Richards R. L., Alving C. R., Vaughan M., Brady R. O. Choleragen-mediated release of trapped glucose from liposomes containing ganglioside GM1. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3480–3483. doi: 10.1073/pnas.73.10.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Garrison S., Fishman P. H., Richardson S. H. Gangliosides sensitize unresponsive fibroblasts to Escherichia coli heat-labile enterotoxin. J Clin Invest. 1979 Aug;64(2):381–384. doi: 10.1172/JCI109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Nalin D. R., McLaughlin J. C. Effects of choleragenoid and glucose on the response of dog intestine to escherichia coli enterotoxins. J Med Microbiol. 1978 May;11(2):177–186. doi: 10.1099/00222615-11-2-177. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Huda S., Neogi P. K., Daniel R. R., Spira W. M. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol. 1980 Jan;11(1):35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Enterotoxigenic Escherichia coli: identification and characterization. J Infect Dis. 1980 Aug;142(2):279–286. doi: 10.1093/infdis/142.2.279. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Svennerholm A. M. Experimental studies on cholera immunization. 4. The antibody response to formalinized Vibrio cholerae and purified endotoxin with special reference to protective capacity. Int Arch Allergy Appl Immunol. 1975;49(4):434–452. [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Tayot J. L., Holmgren J., Svennerholm L., Lindblad M., Tardy M. Receptor-specific large-scale purification of cholera toxin on silica beads derivatized with lysoGM1 ganglioside. Eur J Biochem. 1981 Jan;113(2):249–258. doi: 10.1111/j.1432-1033.1981.tb05060.x. [DOI] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Metzger J. F. Comparison of the action of Escherichia coli enterotoxin on the thymocyte adenylate cyclase-cyclic adenosine monophosphate system to that of cholera toxin and prostaglandin E1. Infect Immun. 1974 Sep;10(3):503–509. doi: 10.1128/iai.10.3.503-509.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



