Abstract
Artemisinin and its derivates are an important class of antimalarial drug and are described to possess immunomodulatory activities. Few studies have addressed the effect of artesunate in the murine malaria model or its effect on host immune response during malaria infection. Herein, we study the effect of artesunate treatment and describe an auxiliary mechanism of artesunate in modulating the inflammatory response during experimental malaria infection in mice. Treatment with artesunate did not reduce significantly the parasitemia within 12 h, however, reduced BBB breakdown and TNF-α mRNA expression in the brain tissue of artesunate-treated mice. Conversely, mefloquine treatment was not able to alter clinical features. Notably, artesunate pretreatment failed to modulate the expression of LFA-1 in splenocytes stimulated with parasitized red blood cells (pRBCs) in vitro; however, it abrogated the expression of ICAM-1 in pRBC-stimulated endothelial cells. Accordingly, a cytoadherence in vitro assay demonstrated that pRBCs did not adhere to artesunate-treated vascular endothelial cells. In addition, NF-κB nuclear translocation in endothelial cells stimulated with pRBCs was impaired by artesunate treatment. Our results suggest that artesunate is able to exert a protective effect against the P. berghei-induced inflammatory response by inhibiting NF-κB nuclear translocation and the subsequent expression of ICAM-1.
1. Introduction
Artesunate is a semisynthetic derivative of artemisinin, the principal active component of a medicinal plant Artemisia annua which has been used as a remedy for fevers and chills for centuries in China [1]. Artemisinin and its derivatives are the most important class of antimalarial drug effective for both uncomplicated and severe malaria [2]. Besides, artemisinin and its derivates have been shown to possess anticancer [3], antiviral [4], and anti-inflammatory [5–8] activities. Artesunate has been reported to block the production of IL-1β, IL-6 and IL-8 from TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes [9]. In addition, artesunate inhibits expression of toll-like receptor 4 induced by heat-killed E. coli lipopolysaccharide and production of TNF-α, IL-6, and nitric oxide (NO). Further studies also demonstrated that artesunate protects septic mice challenged with S. aureus inhibiting TLR2 and Nod2 expression, and, consequently, decreasing TNF-α release [10] probably regulating the transcription factor F-κB activation. There are also some evidences that artesunate attenuates experimental allergic airway inflammation via negative regulation of PI3 K/Akt signaling pathway [11].
Murine cerebral malaria is a condition mainly attributed to parasite and leukocytes sequestration within the brain and the systemic inflammatory response to the infection [12, 13]. Several experimental models of malaria have been adopted to understand the pathogenesis of the disease [13] as well as to discover new antimalarial drugs [14]. Among the experimental models, murine infection with P. berghei ANKA is the most commonly used animal model to study the mechanisms of cerebral malaria pathogenesis [13, 15]. In this model, the neurological syndrome is associated with severe vasculopathy and systemic inflammatory response due to activation of leukocytes, cytokine production, and increased expression of endothelial adhesion molecules [12, 13, 16, 17].
Recently, the efficacy of antimalarial drugs in this experimental model was addressed, with findings showing that artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria [15]. The anti-malarial activity of artemisinin and its derivatives has been well demonstrated in vitro and in vivo [18, 19]. Of note, the in vivo effect of artesunate on the host immune response during experimental malaria was never studied despite evidence demonstrating that this drug may present immunomodulatory activities [10, 11]. Herein, we study the effect of artesunate on inflammatory markers during malaria infection and demonstrated that artesunate is able to exert a protective effect against the P. berghei-induced inflammatory response by inhibiting NF-κB nuclear translocation and the subsequent expression of ICAM-1 in endothelial cells.
2. Material and Methods
2.1. Mice and the Model of Infection
C57BL/6 mice (18 to 20 g) were provided by the Oswaldo Cruz Foundation breeding unit (Rio de Janeiro, Brazil) and caged with free access to food and fresh water in a room at the Farmanguinhos experimental facility with a temperature ranging from 22 to 24°C and a 12 h light/dark cycle until use. All experimental procedures were performed according to The Committee on Ethical Use of Laboratory Animals of Fundação Oswaldo Cruz.
For the Plasmodium berghei ANKA infection, mice were intraperitoneally (i.p.) inoculated with 107 P. berghei pRBCs withdrawn from a previously infected mouse. Artesunate or mefloquine (200 mg/kg diluted in 10% ethanol and 90% propylene glycol, Farmanguinhos, Brazil) was orally administered by gavage (p.o.) (oral gavage needle, for mice, Thomas Scientific, USA) on the fifth day of infection. Mortality was checked daily. At the indicated time points after infection, a thick blood smear was performed for parasitemia determination by Diff-Quick staining. Finally, the mice were weighed and sacrificed in a CO2 chamber. Their lungs and spleens were excised and weighed, and the volume of these organs was evaluated by the organ/body weight ratio.
2.2. Evaluation of Blood-Brain Barrier Disruption
Blood-brain barrier (BBB) disruption was evaluated as previously described [20] and modified by Pamplona et al. [21]. Briefly, mice received an intravenous (i.v.) injection of 1% Evans blue (Sigma-Aldrich, São Paulo, Brazil) 12 h after artesunate administration on the fifth day of P. berghei infection. Mice were sacrificed 1 h later, and their brains were weighed and placed in formamide (2 mL, 37°C, 48 h) to extract the Evans blue dye from the brain tissue. Absorbance was measured at 620 nm (SpectraMax 190, Molecular Devices, California, USA). The concentration of Evans blue was calculated using a standard curve. The data are expressed as mg of Evans blue per g of brain tissue.
2.3. Histological Study
Brains were fixed and embedded in paraffin and from each 80 μm of tissue, 4 μm thin sections were obtained and stained with Hematoxylin-Eosin (Merck, Rio de Janeiro, Brazil). Congested capillaries from the superficial cerebral cortex were quantified under an optical microscope (×1000 magnification), and the percentage of congested microvessels was obtained by counting 100 vessels from each brain slide.
2.4. TNF-α mRNA Expression by RT-PCR
Total RNA from the brains of uninfected, P. berghei-infected and artesunate-treated P. berghei-infected mice (single dose, treated 12 h before on the fifth day of infection) was collected using TRIzol Reagent (Invitrogen, São Paulo, Brazil). After DNase treatment (RQ1 RNase-Free DNase), the mRNA was reverse transcribed using Moloney murine leukemia virus (M-MLV) reverse transcriptase and an oligo(dT) 15 primer. The following primers were used to amplify TNF-α cDNA: sense, 5′-GATCTCAAAGACAACCAACATGTG-3′, and antisense, 5′-CTCCAGCTGGAAGACTCCTCCCAG-3′. PCR was performed using the following parameters: 1 cycle of 95°C for 90 sec and 27 cycles of denaturation at 95°C for 30 sec, annealing at 45°C for 1 min, and elongation at 72°C for 26 sec. β-Actin primers were used to validate the integrity of the cDNA in each reaction. PCR products were separated by 2% agarose gel electrophoresis and visualized by UV exposure on a transilluminator. The PCR products were obtained using a GeneAmp PCR System 2400 (Perkin Elmer, Massachusetts, USA). The value corresponding to the TNF-α mRNA expression was measured using the β-actin mRNA levels as a reference.
2.5. Murine Endotoxic Shock
Murine endotoxic shock was induced by intraperitoneal injection of lipopolysaccharides (LPS; 25 μg/mouse) and D-galactosamine (20 mg/mouse) diluted in PBS (200 μL) in previously artesunate-treated mice (100 mg/Kg, p.o., 1 h before). Three hours after endotoxic shock induction, mice were euthanized and blood was recovered for TNF-α evaluation. The plasma TNF-α protein levels were assayed by a standard sandwich ELISA in accordance with the manufacturer's instructions (R&D Systems, Minneapolis, USA).
2.6. Immunofluorescent Staining and Flow Cytometric Analysis
Splenocytes from normal C57BL/6 mice were isolated by Histopaque-1077 (Sigma-Aldrich) and treated with artesunate (300 ng/mL). One hour after treatment, the cells were washed, incubated with normal red blood cells (RBCs) or parasitized red blood cells (pRBCs), and maintained at 37°C in 5% CO2 for 4 h. The RBCs and pRBCs were then lysed, and the splenocytes were washed prior to immunofluorescent staining. The cells were then incubated in PBS plus 10% rat serum and 0.1% sodium azide (PBS-S, Sigma-Aldrich) and blocked with FcγIIR monoclonal antibodies (mAb; 1 : 100, CD16/CD32, BD Pharmingen, California, USA) for 30 min at 4°C. After blocking, the cells were labeled with purified mAb anti-mouse lymphocyte function-associated antigen (LFA)-1 followed by staining with secondary FITC-conjugated anti-rabbit IgG (Sigma-Aldrich) antibodies diluted in PBS-S and incubated for another 30 min at 4°C. The cells were then washed and resuspended in PBS/0.1% sodium azide for data acquisition in a FACS calibur flow cytometer (BD Biosciences, California, USA). Forward scatter (FSC) and side scatter (SSC) were set to exclude dead cells, and at least 104 lymphocytes were analyzed per sample. Control staining to determine the positive population was performed based on an irrelevant IgG isotype labeled with FITC. Once determined, the quadrants were rigorously maintained for all analyses. Data analysis was performed using CellQuest software (BD Immunocytometry Systems, California, USA).
2.7. Cytoadherence Assay
The murine vascular endothelial cell line tEnd.1 was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. For the infected erythrocyte adhesion assay, tEnd.1 cells were plated in 24-well culture chambers (Nunc, New York, USA) (105 cells/well) and cultured for 24 h. Before each experiment, the tEnd.1 cells were treated with IgG (20 μg/mL), anti-mouse ICAM-1 (20 μg/mL) or artesunate (300 ng/mL). One hour after treatment, the cells were washed, and RBCs or pRBCs were then allowed to adhere to the tEnd.1 cultures (50 erythrocytes/tEnd.1, 60% parasitemia) for 1 h. Nonadherent erythrocytes were gently washed away with PBS, and the remaining cells were subsequently fixed in ethanol and stained with Giemsa (Merck). The number of adhered erythrocytes per tEnd.1 cell was determined by direct counting. The data are expressed as an association index calculated as previously described [22]: Adhesion Index (AI) = {[(tEnd.1 with bound erythrocytes)/total tEnd.1 number] × [(erythrocytes bound to tEnd.1)/total tEnd.1 number]} × 100. The adhesion of erythrocytes was also measured essentially as previously described [23]. The erythrocytes were washed with sterile PBS and incubated with 50 μM CFSE (Invitrogen) for 30 min at 4°C. Cells were then washed, resuspended in sterile saline, and allowed to adhere for 1 h, and nonadherent erythrocytes were gently washed away with PBS as described above. The fluorescences corresponding to remaining cells were read in SpectraMax M5 Microplate Reader (Molecular Devices), and the results expressed as mean of fluorescence per well.
2.8. Immunocytochemistry
Cell pretreatment and stimulation were performed as described above. Immunofluorescent studies were performed as described previously [24]. To evaluate NF-κB translocation, cells were permeabilized with 0.1% Triton X-100 (Amersham Biosciences) after fixation with 4% (w/v) paraformaldehyde and 4% (w/v) sucrose followed by blocking with 2% bovine serum albumin. The cells were then incubated with anti-p65 (nuclear factor kappa-light-chain-enhancer of activated B cells) NF-κB (1 : 50, Santa Cruz Biotechnology) or anti-intercellular adhesion molecule (ICAM-1; 1 : 100, BD Pharmingen) mAb and subsequently incubated with the appropriate secondary FITC-conjugated antibody (Santa Cruz Biotechnology). Microscopic analysis of the fluorescent images was performed using a laser scanning confocal microscope software (Fluoview FV300 3.3 Olympus). The nuclear translocation of NF-κB was measured by immunofluorescence in nucleus using Image-Pro Plus Software (Media Cybernetic).
2.9. Statistical Analysis
Experiments in which three or more groups were compared statistical significance was assessed using ANOVA followed by the Newman Keuls t-test. Experiments in which two groups were compared statistical significance was assessed using t-test. The results are expressed as the mean ± SEM and the significance level in all cases was set at P < 0.05. In order to compare the percentage of survival, it was used the log-rank (Mantel-Cox) test and the significance level was set at P < 0.05.
3. Results
3.1. Evaluation of Different Aspects of Severe Malaria after Single-Dose Administration of Artesunate 5 d after P. berghei Infection
Mice developed symptoms of cerebral malaria, since altered neurological functions as reflex and sensory function, motor behaviour, autonomous function, and muscle tone and strength were observed on days 5-6 after P. berghei ANKA infection. The infected mice also exhibited elevated parasitemia and a high mortality rate (Figures 1(a) and 1(c)). Treatment with artesunate on the fifth day after infection improved the clinical outcome and prolonged survival up to 100% within 12 h of treatment (P = 0.0158; Figure 1(c)). Interestingly, at 12 hours after treatment the decrease in parasitemia was less than 30%. Notably, treatment with mefloquine did not prevent P. berghei infection-induced death (P = 0.212; Figure 1(b)) although reduced the same ratio of parasitemia as observed with artesunate treatment (25%).
Figure 1.
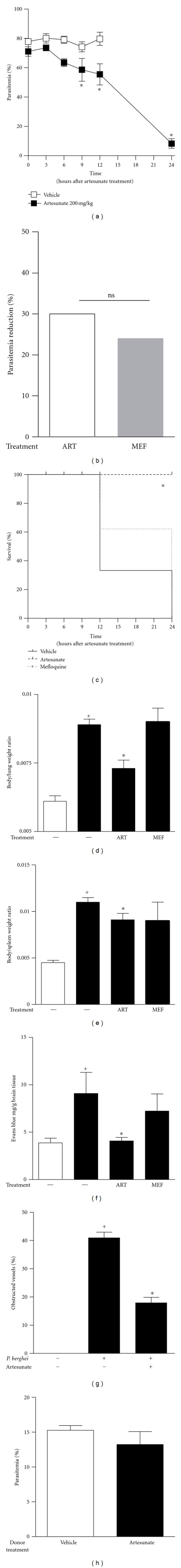
(a) Parasitemia was analyzed in a blood smear from each mouse. (b) Artesunate (200 mg/Kg; open bars) and mefloquine (200 mg/Kg; gray bars) antimalarial activity after administration on the fifth day of infection. (c) The survival of mice-treated artesunate (- - -, 200 mg/Kg), mefloquine (…, 200 mg/Kg), or vehicle (—) was administered on the fifth day of infection. (d) Body/lung weight ratio, (e) body/spleen weight ratio, and (f) BBB disruption of uninfected (open bars), infected (closed bars), or infected mice that received a single dose of artesunate (200 mg/Kg) on the fifth day of infection. BBB disruption was assessed using Evans blue. The dye quantification is shown as the mean mg of Evans blue per g of brain tissue. (g) Microvascular congestion was assessed by H&E staining of brain sections on the fifth day of infection. (h) Evaluation of infection rate of erythrocytes transferred from nontreated P. berghei-infected mice (open bars) or from artesunate-treated P. berghei-infected mice (closed bars). The results are expressed as the mean ± SEM from six animals/per group of three experiments. Statistically significant differences (P < 0.05) between the control and P. berghei-infected groups are indicated by +, and significant differences (P < 0.05) between the untreated and artesunate-treated infected mouse groups are indicated by ∗.
We also observed that P. berghei-infected mice had an increased accumulation of Evan's blue in their brains, suggesting BBB disruption (Figure 1(f)). In addition, infected mice had an increased body/lung weight ratio and body/spleen weight ratio when compared with uninfected mice (Figures 1(d) and 1(e)). Interestingly, although the single treatment of artesunate only caused a slight reduction in the body/spleen weight ratio, it led to a significant inhibition in the body/lung and dramatically decreased the accumulation of Evan's blue in the brain within 12 h (Figure 1(f)). It is important to note that in spite of parasitemia inhibition, treatment with mefloquine was not able to reduce either lung or spleen/body weight ratios and did not impair Evan's blue extravasations to brain tissue (Figures 1(d)–1(f)).
We further analyzed sections from brains taken from uninfected, P. berghei-infected, and P. berghei-infected, mice treated 12 h before with artesunate. As expected, abnormal features were observed in the brain sections from infected mice, that is, capillary congestion caused by the presence of leukocytes adhered to the endothelial cells occasionally occluding the vessel lumens, suggesting cellular activation. In accordance to the findings of Clemmer et al. [15], brain sections from infected mice that were treated with artesunate, showed a reduction in the numbers of congested capillaries, endothelial cell thickness, and leukocyte detachment from the vessel walls, suggesting a putative effect of artesunate in cell adherence.
To roll out that artesunate effect was not due to killing of parasites, erythrocytes from donor nontreated mice or from mice treated with artesunate 12 h before were transplanted into uninfected mice. This resulted in a similar infection rate (Figure 1(h)) showing the presence of live parasites after treatment with artesunate under these specific experimental conditions.
3.2. Production of TNF-α after Artesunate Treatment
Because artesunate caused a reduction of endothelial cell thickness and the detachment of leukocytes from the brain vasculature during experimental severe malaria, we investigated the mechanism by which artesunate affected endothelial cell activation. The expression of TNF-α, a well-described endothelial cell activator [25], was investigated first in a qualitative PCR assay. As expected, TNF-α mRNA expression was increased in the brain tissue of P. berghei-infected mice, whereas TNF-α expression was decreased in the brain tissue of artesunate-treated infected mice (Figure 2(a)). We further observed that artesunate treatment was also able to reduce systemic TNF-α production, by means of ELISA assay, in a murine endotoxic shock model (Figure 2(b)) suggesting that artesunate could exhibit activity in the systemic inflammatory response in vivo.
Figure 2.
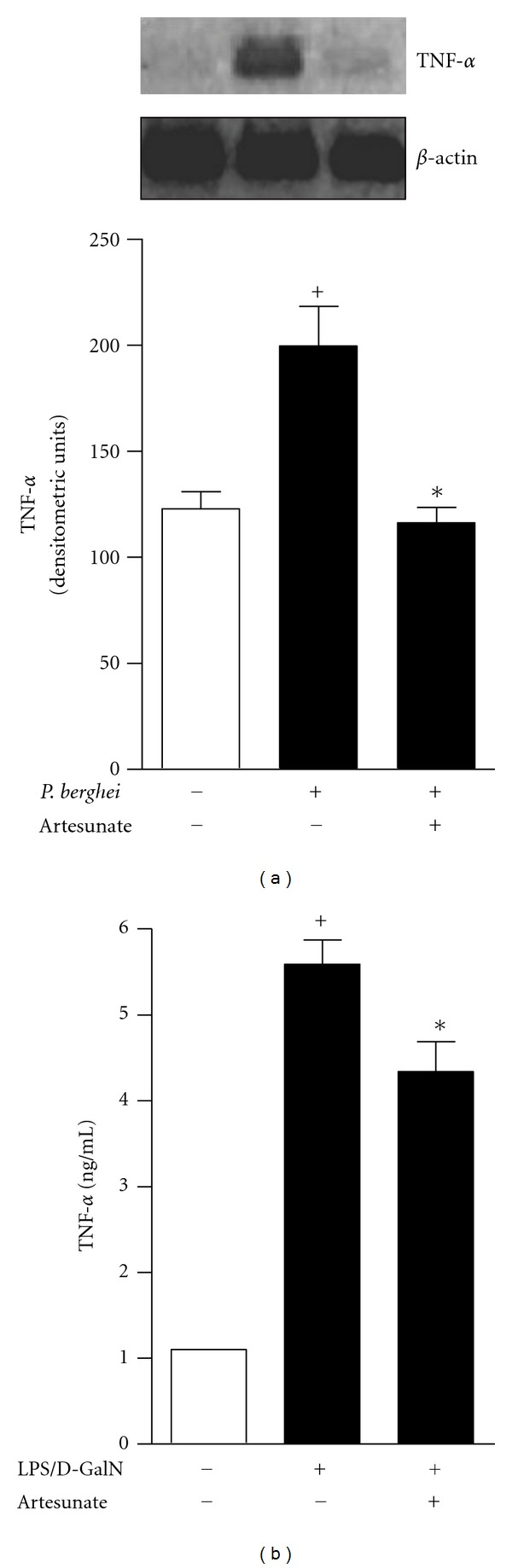
(a) TNF-α mRNA expression in the brains of uninfected (open bars), infected (closed bars), or infected mice that received a single dose of artesunate (200 mg/Kg) on the fifth day of infection. (b) Inhibition of TNF-α production during murine endotoxic shock after treatment with artesunate. The results are expressed as the mean ± SEM from three animals per group of three experiments. Statistically significant differences (P < 0.05) between the control and P. berghei-infected groups are indicated by +, and significant differences (P < 0.05) between the untreated and artesunate-treated infected mouse groups are indicated by ∗.
3.3. LFA-1 Expression in Splenocytes Stimulated In Vitro by P. berghei-Infected Erythrocytes
To assess whether artesunate treatment could modulate the attachment of leukocytes to endothelium we first addressed the effect of artesunate in the expression the integrin CD11a/CD18 (also known as LFA-1; Lymphocyte function-associated antigen 1), the adhesion molecule described as responsible for the attachment of leukocytes in cerebral malaria. The expression of LFA-1 was measured in naive splenocytes stimulated with pRBCs in vitro. Stimulation with pRBCs leads to an increased expression of LFA-1 on the cell membrane of splenocytes when compared with cells stimulated with RBCs. Interestingly, pre-treatment with artesunate (300 ng/mL) does not modulate the expression of LFA-1 in pRBC-stimulated splenocytes (Figure 3).
Figure 3.
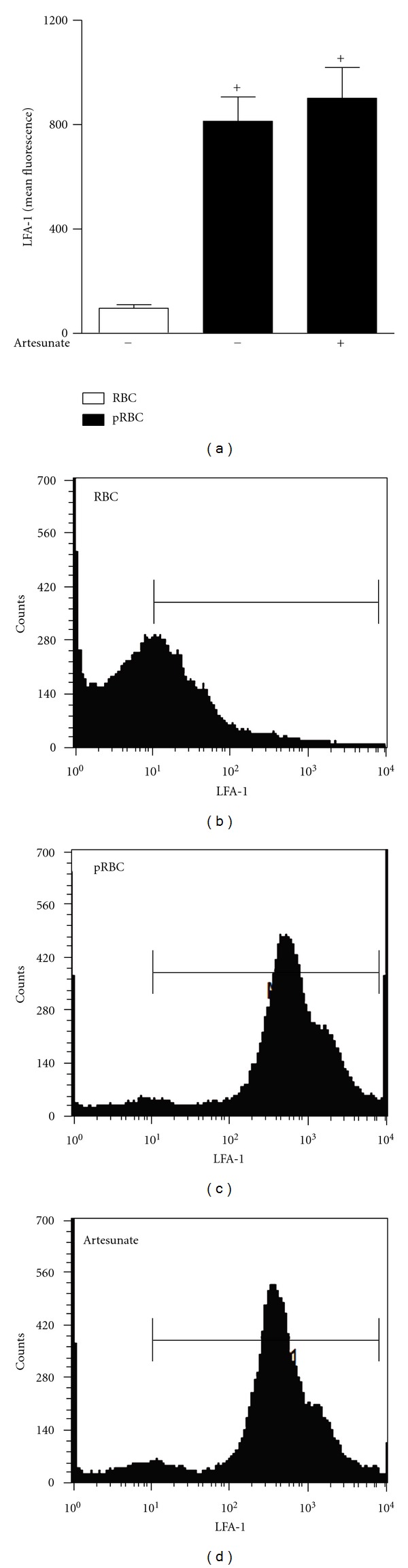
LFA-1 expression by splenocytes treated with artesunate and activated by pRBC. Representative histograms demonstrate the increase in fluorescence indicated by increase in LFA-1 expression in splenocytes. The results are expressed as the mean ± SEM from at least six animals per group from two different experiments. Statistically significant differences (P < 0.05) between the control and P. berghei-infected groups are indicated by +.
3.4. ICAM-1 Expression and pRBC Adhesion to Endothelial Cells
We next investigated the effect of artesunate in the ligant of LFA-1, the intracellular adhesion molecule-1 (ICAM-1). The coculture of pRBCs and endothelial cells leads to an increase of ICAM-1 expression in endothelial cells, whereas RBC stimulation did not. We observed that pretreatment with artesunate (300 ng/mL) inhibited the expression of ICAM-1 in these cells (Figure 4(a)). Interestingly, blockade of ICAM-1 inhibit pRBC adhesion to endothelial cells (P = 0.0007; Figure 4(b)). In agreement with these findings, pRBCs were able to adhere to endothelial cells and the treatment of tEnd.1 cells with artesunate (300 ng/mL) was able to inhibit pRBC adhesion (Figure 4(c)). We further studied the spontaneous adhesion of RBC to TNF-α-activated endothelial cells and observed that the pretreatment of endothelial cells with artesunate and further stimulation with TNF-α led to a substantial inhibition of the RBC adhesion to the endothelial cells (>60%) (Figure 4(d)). Of note, treatment with 300 ng/mL artesunate was not toxic to the vascular endothelial cell line tEnd.1 (99% viability).
Figure 4.
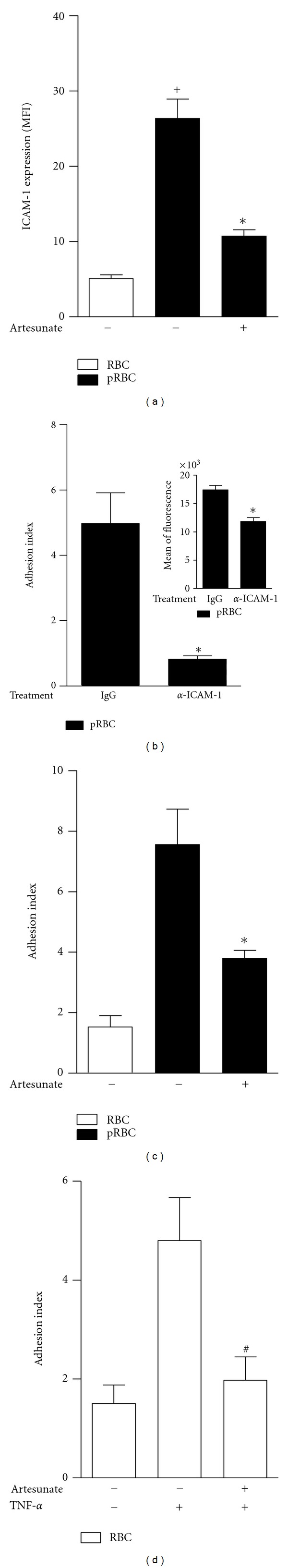
ICAM-1 expression and cytoadhesion of pRBC in the t.End.1 vascular endothelial cell line. (a) ICAM-1 expression was evaluated in t.End.1 vascular endothelial cells pretreated with artesunate (300 ng/mL) and stimulated with RBCs or pRBCs. (b) Endothelial cells were pretreated with IgG or anti-mouse ICAM-1 (20 μg/mL) and incubated with pRBCs. The adhesion of pRBC was calculated by the adhesion index or by fluorescence (insert) as described in the Material and Methods. (c–d) Endothelial cells were pretreated or not with artesunate (300 ng/mL), followed by incubation with RBCs (c) or pRBCs (d) for 1 h. The results are expressed as the mean ± SEM from three different experiments. Statistically significant differences (P < 0.05) between the RBC and pRBC groups are indicated by +, significant differences between pRBCs and pRBCs cultured with t.End.1 pretreated with artesunate are indicated by ∗, and significant differences between RBCs and RBCs cultured with t.End.1 pretreated with artesunate are indicated by #.
3.5. NF-κB Nuclear Translocation in Artesunate Pretreated Endothelial Cells Activated by P. berghei-Infected Erythrocytes
RBCs recovered from uninfected mice were not able to induce NF-κB nuclear translocation in endothelial cells. On the other hand, pRBC activated endothelial cells, as shown by an increased fluorescence in the endothelial nuclei (Figure 5). In contrast, pretreatment with artesunate impaired the nuclear translocation of NF-κB in the endothelial cells activated by P. berghei-infected erythrocytes.
Figure 5.
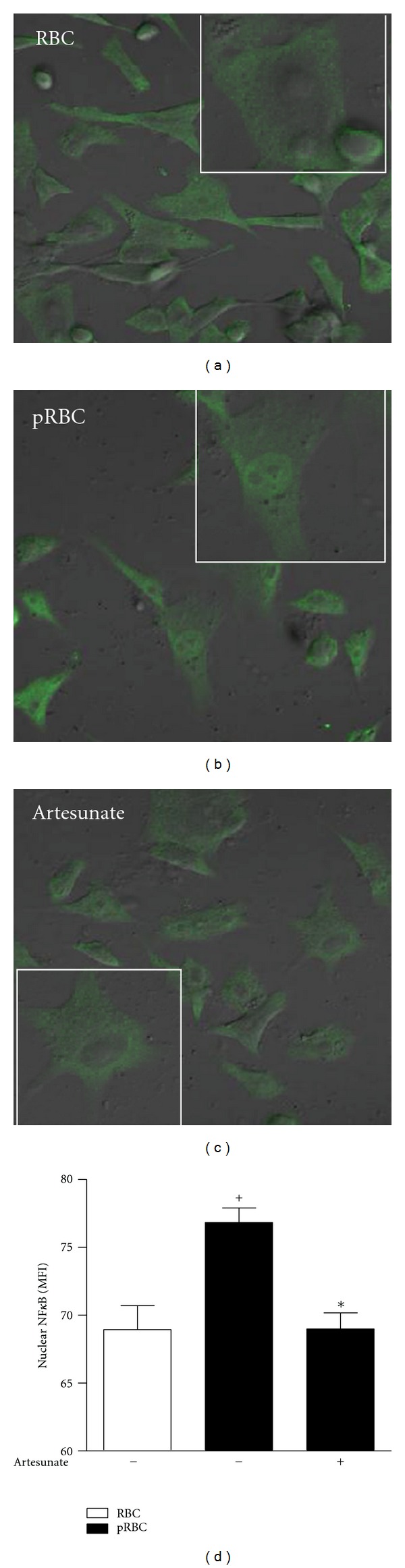
NF-κB translocation into the nucleus of pRBC-stimulated t.End.1 vascular endothelial cells. t.End.1 cells were pretreated or not with artesunate (300 ng/mL) and stimulated with RBCs or pRBCs as described in the Material and Methods section. Translocation of p65 NF-κB was observed by laser scanning confocal microscopy and measured as fluorescence intensity in endothelial cell nucleus. These images are representative of five different fields. The results are expressed as the mean ± S.E.M. from at least five different fields per group from two different experiments. Statistically significant differences (P < 0.05) between the control and pRBC groups are indicated by +, and significant differences between pRBCs and pRBCs cultured with t.End.1 pretreated with artesunate are indicated by ∗.
4. Discussion
Artemisinin derivatives are the most important antimalarial treatment available. The mechanism of action of these drugs depends on the alkylation of plasmodium-specific proteins, which prevents the formation of hemozoin and consequently causes the release of free iron that is toxic to the parasite [26]; however the literature has shown that artesunate also presents immunomodulatory effects in mammalian cells [10, 11, 27, 28]. Of note, few studies have addressed the effect of artesunate in the murine malaria model and its effect on host immune response. Recently, it was described for the first time the efficacy of artesunate in rescuing mice during severe malaria associated with the decrease in leukocyte accumulation in the brain. Herein, we proposed an artesunate mechanism to modulate endothelial cells activation and avoiding the microvascular congestion.
We observed that 24 h after treatment with artesunate, the parasitemia levels and the symptoms of cerebral malaria were reduced, which are in accordance with results described by Clemmer et al. [15]. Such results are also in agreement with Baptista et al. [29] and Amante et al. [30] who recently demonstrated that during experimental cerebral malaria, increased levels of parasitemia are crucial for clinical symptoms, BBB breakdown, and leukocyte sequestration in the brain microvasculature [31]. Furthermore, we observed a significant decrease in pulmonary edema, splenomegaly, and BBB breakdown during murine severe malaria 12 h after the administration of a single dose of artesunate, in spite of sustained high parasitemia. Consistently, parasites recovered from artesunate-treated mice were able to infect healthy mice to the same extent as parasites recovered from untreated P. berghei-infected mice. Moreover, we demonstrated that treatment with mefloquine (another antimalarial drug) was neither able to significantly alter the parasitemia levels nor resulted in the reversion of clinical features, such as disruption of BBB or splenomegaly. These results suggest an auxiliary mechanism of action of artesunate that would explain its early effect during severe malaria.
Artesunate and artemisinin derivatives have activities demonstrated in different pathologies, such as microbial infections, tumor growth, and inflammatory diseases [3, 4, 8, 32, 33]. In vitro studies demonstrated that artesunate treatment impaired the proliferation of phytohemagglutinin-stimulated peripheral blood mononuclear cells [7] and decreased the neutrophil capacity to phagocytose Escherichia coli in vitro [32]. Moreover, Mirshafiey and coworkers [5] showed a reduction in paw edema and the infiltration of inflammatory cells in the knee joints of artesunate-treated rats with rheumatoid arthritis.
TNF-α is well described as an important cytokine involved in systemic diseases, such as rheumatoid arthritis, septic shock and severe malaria BBB breakdown. In accordance, we observed that treatment with artesunate led to a significant inhibition of TNF-α production in endotoxemic shock assays and in the brain tissue of P. berghei-infected mice suggesting that artesunate exhibits an immunomodulatory effect.
To further investigate a putative cell target to artesunate activity, we evaluated the in vitro effect of artesunate on leukocytes and endothelial cells activation after stimulation with pRBCs. The increased expression of adhesion molecules, such as LFA-1 [34] and ICAM-1 [35], is well described during severe malaria. Interestingly, pretreatment with artesunate did not modulate the expression of LFA-1 in pRBC-stimulated splenocytes in vitro and, conversely significantly inhibited ICAM-1 expression in pRBC-stimulated endothelial cells. Moreover, RBCs failed to adhere to endothelial cells pretreated with artesunate and stimulated with TNF-α, suggesting a direct effect of this drug on endothelial cell activation in a mechanism independent of the parasite. It has already been reported that pRBCs are able to adhere to endothelial cells [36, 37], and our results showed that pretreatment with artesunate is also able to inhibit this phenomenon independent of parasite killing.
It is known that ICAM-1, but not LFA-1, expression [38] depends on NF-κB nuclear translocation [39, 40], which can be triggered by TNF-α or pRBC. Accordingly, our results demonstrate that artesunate inhibited the NF-κB pathway in endothelial cells stimulated with pRBCs. Studies have already demonstrated that artemisinin exhibits the ability inhibit the PI3K/Akt signaling pathway and the translocation of NF-κB to the nucleus of TNF-α-stimulated cells, thereby diminishing the production of pro-inflammatory cytokines [9, 41]. Compelling evidence has demonstrated that other sesquiterpene lactones similar to artesunate can inhibit NF-κB activation and TNF-α production [42]. In fact, sesquiterpene lactones alkylate the p65 subunit of NF-κB, which prevents its binding to DNA [43] and may be a possible mechanism by which artesunate exerts its immunomodulatory activity.
Indeed, study of the anti-inflammatory property of artesunate during Plasmodium infection are quite a challenge; however the sum of evidences concerning its anti-inflammatory effect in several models [3, 4, 8, 32, 33] and further experiments in vitro and ex vivo with Plasmodium sp.-activated leukocytes and endothelial cells would provide more details of artesunate's effects on inflammatory response during malaria infection. Together, our results suggest that artesunate, in addition to its antimalarial properties, would exert a protective effect against severe malaria via its immunomodulatory properties by inhibiting NF-κB nuclear translocation and the subsequent expression of ICAM-1.
Acknowledgments
The authors thank Dr. Carmen Penido of Laboratório de Farmacologia Aplicada-Farmanguinhos FIOCRUZ, for her critical reading of the manuscript. Fausto Ferraris is a Ph.D. student of the Post-Graduation Program in Cellular and Molecular Biology of Instituto Oswaldo Cruz and a fellow of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This work was supported by grants from CNPq, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), CAPES, and Fundação Oswaldo Cruz (FIOCRUZ).
References
- 1.Woodrow CJ, Haynes RK, Krishna S. Artemisinins. Postgraduate Medical Journal. 2005;81(952):71–78. doi: 10.1136/pgmj.2004.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude RJ, Woodrow CJ, White LJ. Artemisinin antimalarials: preserving the “magic bullet”. Drug Development Research. 2010;71(1):12–19. doi: 10.1002/ddr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. International Journal of Oncology. 2001;18(4):767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 4.Kaptein SJF, Efferth T, Leis M, et al. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Research. 2006;69(2):60–69. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Mirshafiey A, Saadat F, Attar M, Di Paola R, Sedaghat R, Cuzzocrea S. Design of a new line in treatment of experimental rheumatoid arthritis by artesunate. Immunopharmacology and Immunotoxicology. 2006;28(3):397–410. doi: 10.1080/08923970600927447. [DOI] [PubMed] [Google Scholar]
- 6.Tawfik AF, Bishop SJ, Ayalp A, El-Feraly FS. Effects of artemisinin, dihydroartemisinin and arteether on immune responses of normal mice. International Journal of Immunopharmacology. 1990;12(4):385–389. doi: 10.1016/0192-0561(90)90019-j. [DOI] [PubMed] [Google Scholar]
- 7.Veerasubramanian P, Gosi P, Limsomwong C, Walsh DS. Artesunate and a major metabolite, dihydroartemisinin, diminish MITOGEN-induced lymphocyte proliferation and activation. Southeast Asian Journal of Tropical Medicine and Public Health. 2006;37(5):838–847. [PubMed] [Google Scholar]
- 8.Wang J, Zhou H, Zheng J, et al. The antimalarial artemisinin synergizes with antibiotics to protect against lethal live Eschenchia coli challenge by decreasing proinflammatory cytokine release. Antimicrobial Agents and Chemotherapy. 2006;50(7):2420–2427. doi: 10.1128/AAC.01066-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, He Y, Yang X, et al. Anti-malarial agent artesunate inhibits TNF-α-induced production of proinflammatory cytokines via inhibition of NF-κB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology. 2007;46(6):920–926. doi: 10.1093/rheumatology/kem014. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Li J, Pan X, et al. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-α release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-κB activation. International Immunopharmacology. 2010;10(3):344–350. doi: 10.1016/j.intimp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C, Ho WE, Goh FY, et al. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0020932.e20932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends in Parasitology. 2006;22(11):503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 13.de Souza JB, Hafalla JCR, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137(5):755–772. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 14.Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nature Reviews Drug Discovery. 2004;3(6):509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 15.Clemmer L, Martins YC, Zanini GM, Frangos JA, Carvalho LJM. Artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria and rapidly decrease leukocyte accumulation in the brain. Antimicrobial Agents and Chemotherapy. 2011;55(4):1383–1390. doi: 10.1128/AAC.01277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer PR, van der Heyde HC, Sun G, Specian RD, Granger DN. Regulation of endothelial cell adhesion molecule expression in an experimental model of cerebral malaria. Microcirculation. 2002;9(6):463–470. doi: 10.1038/sj.mn.7800159. [DOI] [PubMed] [Google Scholar]
- 17.Desruisseaux MS, Machado FS, Weiss LM, Tanowitz HB, Golightly LM. Cerebral malaria: a vasculopathy. American Journal of Pathology. 2010;176(3):1075–1078. doi: 10.2353/ajpath.2010.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janse CJ, Waters AP, Kos J, Lugt CB. Comparison of in vivo and in vitro antimalarial activity of artemisinin, dihydroartemisinin and sodium artesunate in the Plasmodium berghei-rodent model. International Journal for Parasitology. 1994;24(4):589–594. doi: 10.1016/0020-7519(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 19.de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52(6):818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 20.van der Heyde HC, Bauer P, Sun G, et al. Assessing vascular permeability during experimental cerebral malaria by a radiolabeled monoclonal antibody technique. Infection and Immunity. 2001;69(5):3460–3465. doi: 10.1128/IAI.69.5.3460-3465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamplona A, Ferreira A, Balla J, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nature Medicine. 2007;13(6):703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 22.Roffê E, Silva AA, Marino APMP, dos Santos PVA, Lannes-Vieira J. Essential role of VLA-4/VCAM-1 pathway in the establishment of CD8+ T-cell-mediated Trypanosoma cruzi-elicited meningoencephalitis. Journal of Neuroimmunology. 2003;142(1-2):17–30. doi: 10.1016/s0165-5728(03)00254-6. [DOI] [PubMed] [Google Scholar]
- 23.Walther BT, Ohman R, Roseman S. A quantitative assay for intercellular adhesion. Proceedings of the National Academy of Sciences of the United States of America. 1973;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Assis MC, Plotkowski MC, Fierro IM, Barja-Fidalgo C, de Freitas MS. Expression of inducible nitric oxide synthase in human umbilical vein endothelial cells during primary culture. Nitric Oxide. 2002;7(4):254–261. doi: 10.1016/s1089-8603(02)00123-4. [DOI] [PubMed] [Google Scholar]
- 25.Viebig NK, Wulbrand U, Förster R, Andrews KT, Lanzer M, Knolle PA. Direct activation of human endothelial cells by Plasmodium falciparum-infected erythrocytes. Infection and Immunity. 2005;73(6):3271–3277. doi: 10.1128/IAI.73.6.3271-3277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamchonwongpaisan S, Meshnick SR. The mode of action of the antimalarial artemisinin and its derivatives. General Pharmacology. 1996;27(4):587–592. doi: 10.1016/0306-3623(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 27.Li WD, Dong YJ, Tu YY, Lin ZB. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-α and blocking the signaling pathway NF-κB translocation. International Immunopharmacology. 2006;6(8):1243–1250. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Zhang R, Li J, et al. Antimalarial artesunate protects sepsis model mice against heat-killed Escherichia coli challenge by decreasing TLR4, TLR9 mRNA expressions and transcription factor NF-κB activation. International Immunopharmacology. 2008;8(3):379–389. doi: 10.1016/j.intimp.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigário AM. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infection and Immunity. 2010;78(9):4033–4039. doi: 10.1128/IAI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amante FH, Haque A, Stanley AC, et al. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. Journal of Immunology. 2010;185(6):3632–3642. doi: 10.4049/jimmunol.1000944. [DOI] [PubMed] [Google Scholar]
- 31.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJM. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. Journal of Infectious Diseases. 2011;203(10):1454–1463. doi: 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarciron ME, Saccharin C, Petavy AF, Peyron F. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. American Journal of Tropical Medicine and Hygiene. 2000;62(1):73–76. doi: 10.4269/ajtmh.2000.62.73. [DOI] [PubMed] [Google Scholar]
- 33.Chen HH, Zhou HJ, Wu GD, Lou XE. Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology. 2004;71(1):1–9. doi: 10.1159/000076256. [DOI] [PubMed] [Google Scholar]
- 34.Ohayon A, Golenser J, Sinay R, et al. Protein kinase C θ deficiency increases resistance of C57BL/6J mice to Plasmodium berghei infection-induced cerebral malaria. Infection and Immunity. 2010;78(10):4195–4205. doi: 10.1128/IAI.00465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favre N, Da Laperousaz C, Ryffel B, et al. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes and Infection. 1999;1(12):961–968. doi: 10.1016/s1286-4579(99)80513-9. [DOI] [PubMed] [Google Scholar]
- 36.Joergensen L, Bengtsson DC, Bengtsson A, et al. Surface co-expression of two different PfEMP1 antigens on single Plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathogens. 2010;6(9) doi: 10.1371/journal.ppat.1001083.e01083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedosov DA, Caswell B, Suresh S, Karniadakis GE. Quantifying the biophysical characteristics of Plasmodium-falciparum- parasitized red blood cells in microcirculation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):35–39. doi: 10.1073/pnas.1009492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semmrich M, Smith A, Feterowski C, et al. Importance of integrin LFA-1 deactivation for the generation of immune responses. Journal of Experimental Medicine. 2005;201(12):1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripathi AK, Sullivan DJ, Stins MF. Plasmodium falciparum-infected erythrocytes increase intercellular adhesion molecule 1 expression on brain endothelium through NF-κB. Infection and Immunity. 2006;74(6):3262–3270. doi: 10.1128/IAI.01625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ., Jr. Plasmodium falciparum-infected erythrocytes induce NF-κB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114(19):4243–4252. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aldieri E, Atragene D, Bergandi L, et al. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-κB activation. FEBS Letters. 2003;552(2-3):141–144. doi: 10.1016/s0014-5793(03)00905-0. [DOI] [PubMed] [Google Scholar]
- 42.Hehner SP, Heinrich M, Bork PM, et al. Sesquiterpene lactones specifically inhibit activation of NF-κB by preventing the degradation of IκB-α and IκB-β. The Journal of Biological Chemistry. 1998;273(3):1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 43.Ly G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. The Journal of Biological Chemistry. 1998;273(50):33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]