Abstract
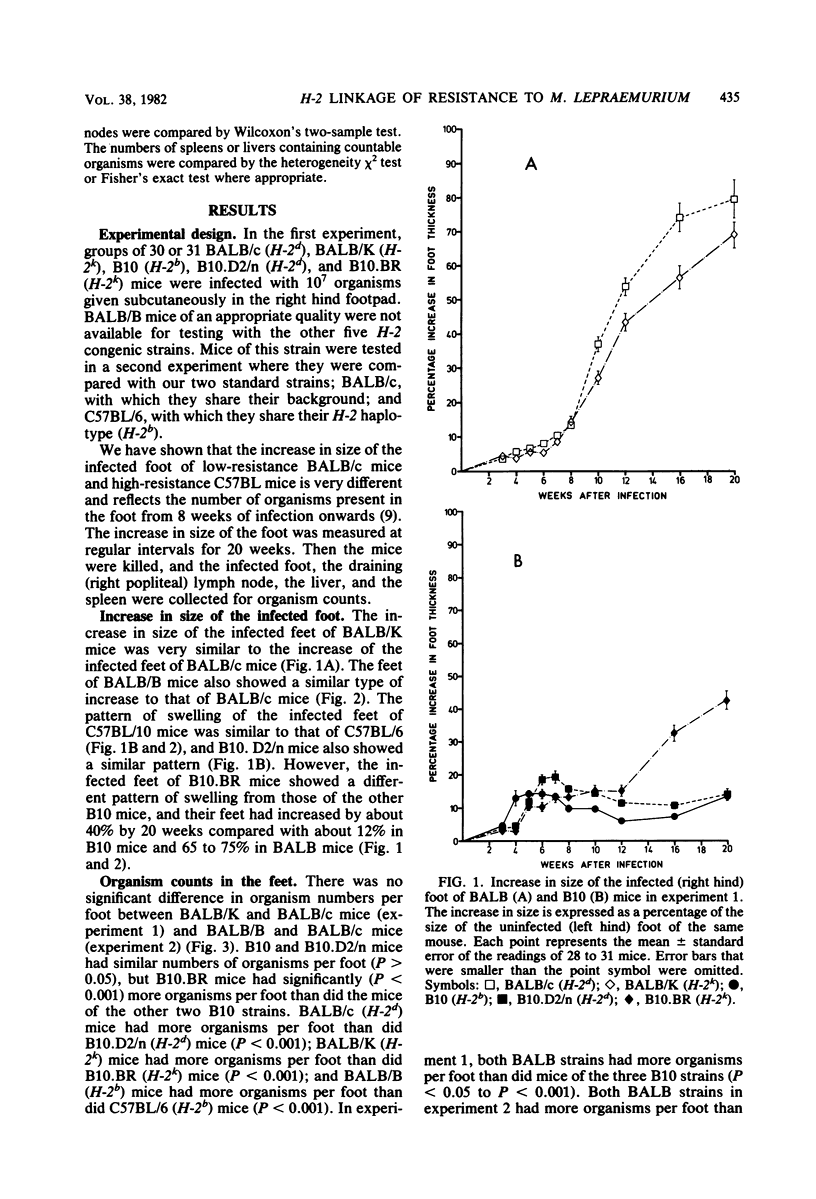
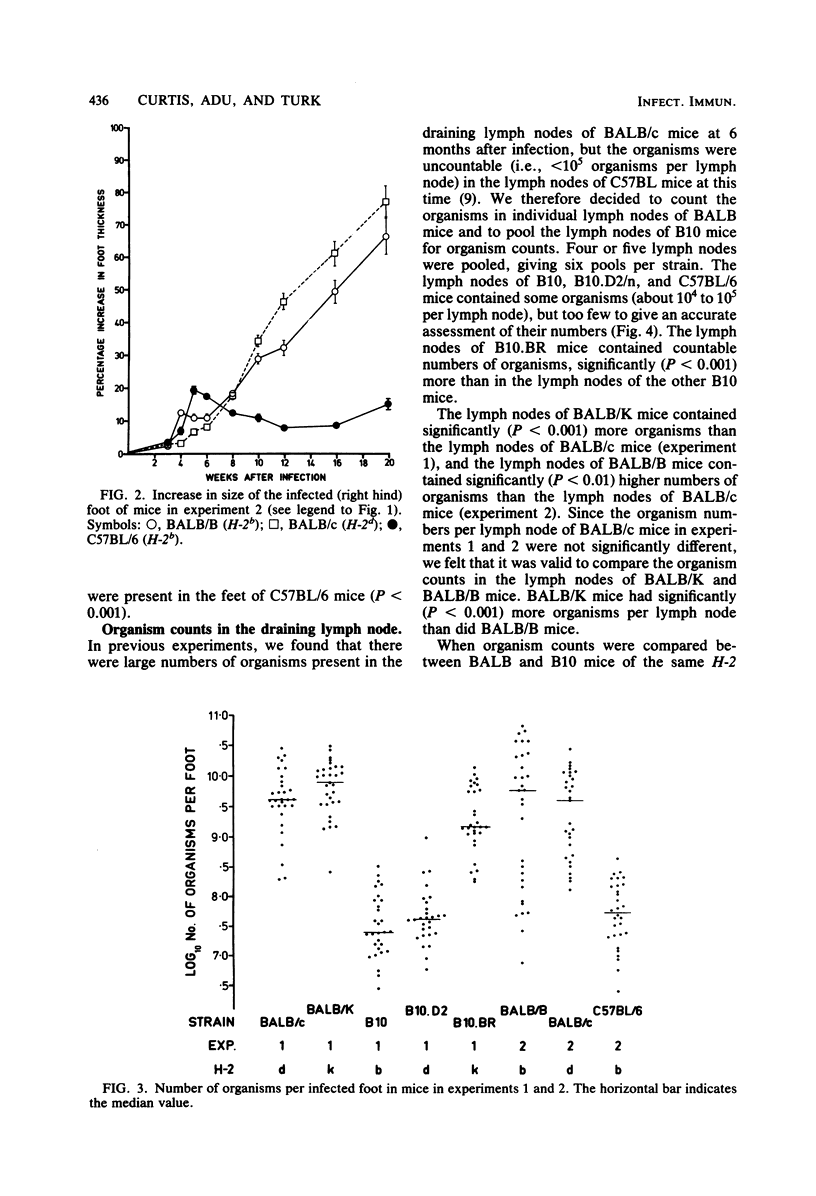
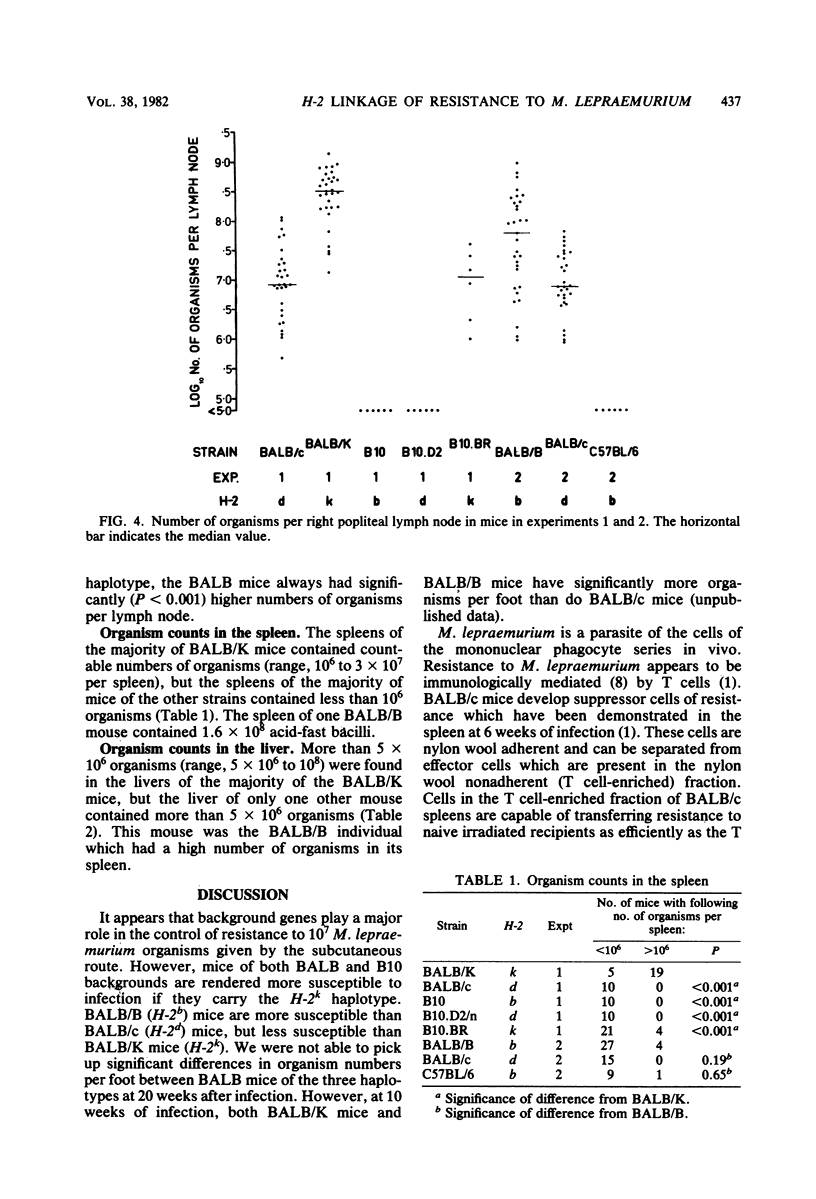
The H-2 linkage of the gene or genes controlling resistance to subcutaneous infection with 10(7) Mycobacterium lepraemurium organisms was investigated by using H-2 congenic strains on BALB and B10 backgrounds. Resistance was assessed by counting the organisms present at the infection site in the footpad and in the draining (right popliteal) lymph node 20 weeks after infection. When mice of BALB and B10 backgrounds with the same H-2 haplotype were compared, the BALB mice were always more susceptible. However, BALB/K (H-2k) mice were more susceptible than BALB/B (H-2b) mice, and BALB/B mice were more susceptible than BALB/c (H-2d) mice. There was no detectable difference in the resistance of B10.D2/n (H-2d) mice and B10 (H-2b) mice, but B10.BR (H-2k) mice were more susceptible than mice of the other two B10 strains. BALB/K was the only strain in which a high proportion of mice showed significant dissemination of organisms to the liver and spleen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. Adoptive transfer of immunity and suppression by cells and serum in early Mycobacterium lepraemurium infections of mice. Parasite Immunol. 1979 Summer;1(2):159–166. doi: 10.1111/j.1365-3024.1979.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57/BL mice after footpad inoculation. Infect Immun. 1975 Sep;12(3):480–489. doi: 10.1128/iai.12.3.480-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Løvik M. Protective immunity and delayed-type hypersensitivity in C57BL mice after immunization with live Mycobacterium lepraemurium and sonicated bacilli. Infect Immun. 1980 Jul;29(1):17–23. doi: 10.1128/iai.29.1.17-23.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Adu H. O., Turk J. L. A lack of correlation between antigen-specific cellular reactions and resistance to Mycobacterium lepraemurium infection in mice. Immunology. 1981 Jun;43(2):293–301. [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B. Local immune response to Mycobacterium lepraemurium in C3H and C57Bl/6 mice. Clin Exp Immunol. 1979 Dec;38(3):461–474. [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Plant J. E., Blackwell J. M., O'Brien A. D., Bradley D. J., Glynn A. A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982 Jun 10;297(5866):510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A. Phenotypic expression of genetically controlled host resistance to Listeria monocytogenes. Infect Immun. 1979 Jul;25(1):345–351. doi: 10.1128/iai.25.1.345-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. M., Rosendaal M., Plant J. E. Early haemopoietic responses to Salmonella typhimurium infection in resistant and susceptible mice. Immunology. 1982 Feb;45(2):395–399. [PMC free article] [PubMed] [Google Scholar]



