Abstract
Oxidative stress is a key pathologic factor in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases. The failure of free-radical-scavenging antioxidants in clinical trials pinpoints an urgent need to identify and to block major sources of oxidative stress in neurodegenerative diseases. As a major superoxide-producing enzyme complex in activated phagocytes, phagocytic NADPH oxidase (PHOX) is essential for host defense. However, recent preclinical evidence has underscored a pivotal role of over-activated PHOX in chronic neuroinflammation and progressive neurodegeneration. Deficiency in PHOX subunits mitigates neuronal damage induced by diverse insults/stresses relevant to neurodegenerative diseases. More importantly, the suppression of PHOX activity correlates with less neuronal impairment in models of neurodegenerative diseases. The discovery of PHOX and non-phagocytic NADPH oxidases in astroglia and neurons further reinforces the critical role of NADPH oxidases in oxidative stress-mediated chronic neurodegeneration. Thus, proper modulation of NADPH oxidase activity might hold therapeutic potential for currently incurable neurodegenerative diseases.
Oxidative stress in neurodegenerative diseases
Neurodegenerative diseases are characterized by the decades-long gradual loss of subpopulations of neurons in discrete areas of the central nervous system (CNS). More than 36 million people worldwide suffer from Alzheimer's disease (AD) and Parkinson ’s disease (PD), the two most common neurodegenerative diseases. Lack of effective treatment and failure of many clinical trials targeting various pathways or molecules reinforce the need to seek new targets for drug discovery for neurodegenerative diseases. High oxygen consumption, high production of ROS, abundant oxidation-sensitive lipids, low antioxidant defense, and a restricted renewal and regenerative capacity of neurons render the brain especially susceptible to oxidative insults. In fact, a great amount of evidence has implicated oxidative stress as a central mechanism of chronic neurodegeneration in various neurodegenerative diseases, such as AD, PD, and amyotrophic lateral sclerosis (ALS) [1, 2]. The failure of antioxidants (substances that inhibit oxidation by scavenging free radicals), such as ascorbic acid (vitamin C), tocopherol (vitamin E), and coenzyme Q10, in clinical trials demands the identification and the blockage of principal sources of oxidative stress in neurodegenerative diseases. It has been well documented that excessive activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases is an important contributor to the pathogenesis of numerous peripheral inflammation-related diseases, such as atherosclerosis, diabetes, hypertension, ischemic stroke, and cardiovascular diseases [3, 4]. In this review, we summarize recent exciting progress regarding the crucial role of NADPH oxidases in neurodegenerative diseases, with an emphasis on phagocytic NADPH oxidase (PHOX) and its catalytic subunit NOX2 in neuroinflammation-mediated chronic neurodegeneration.
NADPH oxidases and their activation mechanisms
NADPH oxidases are membrane-bound, multi-subunit enzyme complexes that transfer electrons across the plasma membrane from NADPH to molecular oxygen and generate the free-radical superoxide and its downstream reactive oxygen species (ROS). Through producing ROS, activated NADPH oxidases participate in host defense, cellular signaling, regulations of gene expression, cell differentiation, and metabolism, posttranslational processing of proteins, stress response, and tissue homeostasis [5, 6]. According to the new terminology, the NOX family refers to the catalytic subunit of NADPH oxidases, which includes NOX2 (gp91phox) and its six homologs (NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2). All seven NOX isoforms are transmembrane proteins and are widely distributed in various tissues, with the particular isoforms concentrated in specific cell types or organs: NOX1 in the colon; NOX2 in phagocytes; NOX3 in the inner ear; NOX4 in the kidney and blood vessels; NOX5 in the testis and lymphoid tissue; and DUOX1 and DUOX2 in the thyroid . Many cells express several NOX isoforms; differences in subcellular distribution and activation mechanisms of different NOX isoforms might explain the nonredundancy in their functions [5, 6].
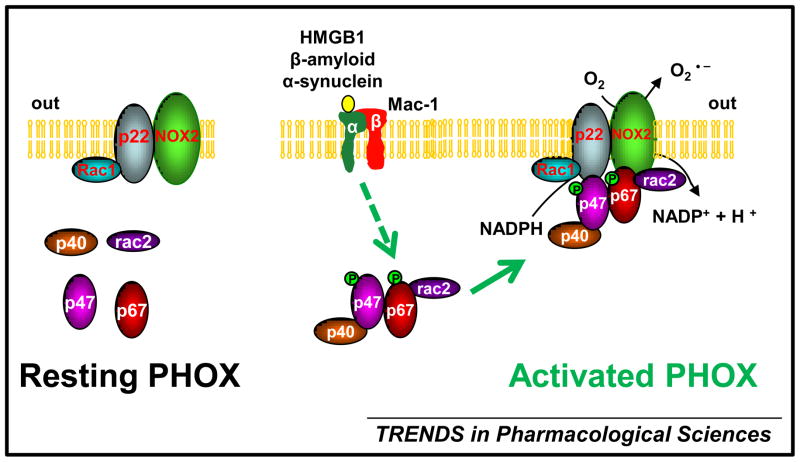
Most NADPH oxidases are activity-dependent enzyme complexes, and their activation usually requires the translocation of cytosolic subunits to the membrane-bound subunits p22phox and NOX isoforms. PHOX is the primary source of superoxide generation in various inflamed phagocytes including neutrophils, monocytes, macrophages, and microglia (the resident immune cells in the CNS). NOX2 (gp91phox), the catalytic subunit of PHOX, is the first identified and the best -characterized member of the NOX family [7]. Under normal circumstances, NOX2 is latent. Upon stimulation, the cytosolic subunits of PHOX (p47phox, p67phox, p40phox, and the small Rho GTPase, Rac1 or Rac2) translocate to the membrane-bound p22phox/ NOX2 heterodimer to assemble the active PHOX that catalyzes the reduction of oxygen to superoxide (Figure 1). Although p47phox was traditionally considered essential for PHOX activation, several recent studies have revealed a p47phox-independent mechanism of PHOX activation. In the absence of p47phox in cell-free assays or in p47phox-deficient macrophages, the PHOX activity is still preserved, but this requires the p resence of p67phox and Rac1 [8–10].
Figure 1. The coupling between Mac1 and PHOX in microglia-mediated ROS production.
Microglia as the resident immune cells in the CNS can sense subtle disturbances of CNS homeostasis. The phagocyte NADPH oxidase (PHOX) is dormant in resting microglia and separated into individual cytosolic and membrane-bound components. A variety of stimuli, such as chemotactic peptides can stimulate microglia an d induce PHOX activation. In particular, some cellular components (e.g. HMGB1, α-synuclein, and β-amyloid) released from activated microglia and/ or damaged neurons can act on microglial Mac1 receptor and activate downstream kinases (e.g. PI3K) leading to the phosphorylation of cytosolic subunits of PHOX, p47phox and p67phox; subsequent translocation of the cytosolic complex composed of p47phox, p67phox, p40phox, and Rac2 to the membrane-bound p22phox/ NOX2 heterodimer results in the assembly of an active PHOX. The activated NOX2 transfers electrons across the plasma membrane from NADPH to molecular oxygen and generates the free-radical superoxide. The coupling between Mac1 and PHOX represents an important mechanism underlying microgliamediated NOX2-dependent oxidative insults to neurons in the progression of neurodegenerative d iseases.
Like NOX2, NOX1 forms a heterodimer with p22phox. Rac proteins and cytosolic subunits of non-phagocytic NADPH oxidases, NOXO1 and NOXA1 (functional homologues of p47phox and p67phox, respectively) are required for NOX1 activation [5, 11, 12]. Both p22phox and NOXO1 are found to be essential for NOX3 activation, whereas the requirement of NOXA1 and Rac p roteins for NOX3 activation remains to be determined [13, 14]. NOX4, in contrast to other NOX isoforms, is constitutively active and produces large amounts of superoxide and its downstream product hydrogen peroxide [15, 16]. Although the involvement of Rac proteins is unclear, NOX4 activation appears not to require other cytosolic subunits. Collectively, for the activation of NADPH oxidases, NOX isoforms as the catalytic subunit are indispensable; p22phox is an essential component of both phagocytic and non-phagocytic NADPH oxidases; p47phox and NOXO1 serve as organizers or regulatory subunits; p67phox and NOXA1 are activators; and p40phox is nonessential, modulatory subunits [5, 17].
Distribution and physiological function of the NOX family in the CNS
The investigation of potential CNS-specific activation mechanisms and biochemical function of the NOX family in neurons, microglia, and astroglia might provide fruitful information on redox-related development, aging, and disease formation in the CNS. Although little is known about the distribution and the function of NOX5, DUOX1, and DUOX2 in the CNS, NOX1 to NOX4 have been detected in whole brain samples, specific CNS regions, and various CNS cells [6, 18]. NOX2 is expressed in microglia at much higher level than in neurons and astroglia. The cellular distribution of NOX3 in the CNS is unclear. Both NOX1 and NOX4 have been detected in neurons, astroglia, and microglia [6]. These findings mainly came from in vitro studies on cultured cells. The in vivo expression of NOX isoforms in specific cell types in the CNS has not been thoroughly examined.
Recent evidence has begun to unravel the physiological function of NADPH oxidase enzymes in the CNS. It has long been recognized that mutations in genes that encode NOX2 (gp91phox), p47phox, p67phox, or p22phox lead to chronic granulomatous disease (CGD) in human and CGD-like phenotypes in mice [6, 19]. CGD, a diverse group of hereditary immunodeficiency diseases with recurrent, persistent infections and granuloma formation in many organs, results from an inability of PHOX-deficient phagocytes to kill ingested bacteria and fungi. Interestingly, an increased prevalence rate of cognitive deficits among CGD patients and impaired memory in gp91phox- and p47phox-deficient mice [20, 21] suggest the involvement of NADPH oxidases in the development and/ or the physiological function of the CNS.
NADPH oxidase has been identified as the primary source of neuronal superoxide production induced by the activation of NMDA receptor (a specific type of ionotropic glutamate receptor whose activation is important for synaptic plasticity, neural communication, memory formation, and learning) [22, 23]. Deficiency in p47phox or blockage of the assembly of p47phox with p22phox/ NOX2 heterodimer by a NADPH oxidase inhibitor apocynin blocks NMDA-elicited superoxide production and neurotoxicity in cultured neurons and mouse hippocampus [22]. Interestingly, the NMDA receptor antagonist ketamine also increases NADPH oxidase activity; an indirect effect involving neuronal production of interleukin -6 is likely responsible for such an effect [24, 25]. Nevertheless, NOX-derived ROS appear important for neuronal death induced by excessive excitation of NMDA receptor. Moreover, neurons in cerebral areas controlling blood -pressure express NOX isoforms, and NOX activity might regulate effects of angiotensin II (an endogenous peptide regulating cardiovascular homeostasis and blood pressure) on cerebral vasculature [26, 27]. NOX-derived ROS also participate in nerve growth factor (NGF)-induced neuronal differentiation of PC12 cells and neurite outgrowth [28, 29], microglia proliferation [30], microglial induction of neuronal apoptosis during development [31], glutamate neurotransmitter release from activated microglia [32, 33], and astrocyte signaling [34]. Taken together, NOX function in microglia, astroglia, and neurons is important for the integrity and the normal function of the CNS.
NOX is an important source of CNS oxidative stress
Diseased CNS regions of patients with various neurodegenerative diseases reveal oxidative stress markers, such as lipid peroxidation and reduction of glutathione levels [1, 2]. Mitochondria are generally believed to be the major source of ROS. However, recent postmortem observations show increased activation of NOX enzymes in the CNS of patients with neurodegenerative diseases. For example, brain tissues of patients with AD (a neurodegenerative disorder characterized by an irreversible loss of neurons and progressive dementia) reveal an elevation in NADPH oxidase enzyme activity, upregulation and membrane translocation of cytosolic subunits of PHOX (p67phox, p47phox, and p40phox), and an increase in mRNA transcripts of NOX1 and NOX3 [35–37], which implies the involvement of NOX-associated oxidative damage in the pathogenesis of AD.
Emerging experimental evidence has indicated that multiple NOX isoforms, especially microglial NOX2, contribute to CNS oxidative stress and neuronal damage. The prime role of microglial NOX2 in neuroinflammation-mediated chronic neurodegeneration is detailed in the next section. Recently, microglia have been found to express NOX1, NOXO1, NOXA1, and Rac1/ 2. During microglial phagocytosis of zymosan (an insoluble cell wall preparation from the yeast Saccharomyces cerevisiae), NOX1/ p22phox heterodimer is recruited to phagosome membrane where activated NOX1 produces superoxide in zymosan-loaded phagosomes [38]. In microglia treated with a common inflammagen lipopolysaccharide (LPS, the major component of the outer membrane of Gram-negative bacteria), the activation of inducible nitric oxide synthase and the release of inflammatory/ neurotoxic factors nitrite species and interleukin-1β are promoted by NOX1-mediated superoxide production. After intra-striatum LPS injection, NOX1-derived superoxide exaggerates loss of presynaptic proteins in striatal neurons [38]. These findings suggest that microglial NOX1 promotes production of neurotoxic factors during neuroinflammation (CNS inflammation) and might contribute to chronic neurodegeneration.
Recent discoveries of NOX isoforms (e.g. NOX1, NOX2, and NOX4) in neurons further support a critical role of NOX enzymes in oxidative neuronal insults in neurodegenerative diseases. Paraquat increased NOX1 expression and activated Rac1 in dopaminergic neuronal N27 cells that express NOX1, NOX2, p67phox, and Rac1 [39, 40]. Paraquat is a pesticide; it shows a positive association with increased risk of PD, a neurodegenerative movement disorder that attacks nigral dopaminergic neurons. Apocynin, diphenyleneiodonium (DPI; a nonspecific NOX2 inhibitor that reacts with flavin-containing enzymes), or siRNA-mediated NOX1 knockdown markedly attenuated ROS production and death of N27 cells induced by paraquat and PD-producing neurotoxin 1-methyl-4-phenylpyridinium (MPP+) [39, 40]. Moreover, in a mouse model of PD, the pre-administration of apocynin significantly reduces paraquat - elicited upregulation of NOX1 and death of nigral dopaminergic neurons [40]. These findings suggest the participation of NOX1, NOX2, or both in PD pathogenesis. Remarkably, NOX1 deletion slows disease progression and significantly increases lifespan of an ALS mouse model that carries mutant superoxide dismutase 1 (SOD1), indicating NOX1 plays a role in the development of ALS (a fatal paralytic neurodegenerative disorder) [41]. NOX1, NOX2, and NOX4 as well as p22phox, p40phox, p47phox, and p67phox have been detected in cultured cerebellar granule neurons. NOX2 deletion or DPI mitigates NOX activity, ROS production, and apoptotic death triggered by staurosporine in these cells, which suggests an important role of NOX2 in apoptotic death of neurons [42, 43]. Knockdown of NOX2 and NOX4 expression in cultured HT22 neuronal cells reduces glutamate-induced hydrogen peroxide accumulation and cell death, indicating NOX family contributes to oxidative glutamate toxicity [44]. NOX4 is upregulated in human and mouse brain after ischemia; post -stroke inhibition of NOX4 induction in mice prevents oxidative stress and neurodegeneration [45]. Collectively, these preclinical findings indicate an important role of activated NOX family in CNS oxidative stress and consequent neurodegeneration.
Over-activated NOX2 is a major mediator of chronic neurodegeneration
Recently, growing experimental findings have documented a pivotal role of NOX2- derived ROS from activated microglia in neuroinflammation -mediated oxidative stress and chronic neurodegeneration. Neuroinflammation, a prominent feature shared by all neurodegenerative diseases, has been increasingly accepted as a crucial contributor to chronic neurodegeneration in various neurodegenerative diseases [46–48]. While astroglia participate in neuroinflammatory response, microglial activation is the principal component of neuroinflammation. A wide range of stimuli that disrupt CNS homeostasis, such as infection, toxic insults, trauma, ischemia, or autoimmune injury, can trigger microglial activation [46–48].
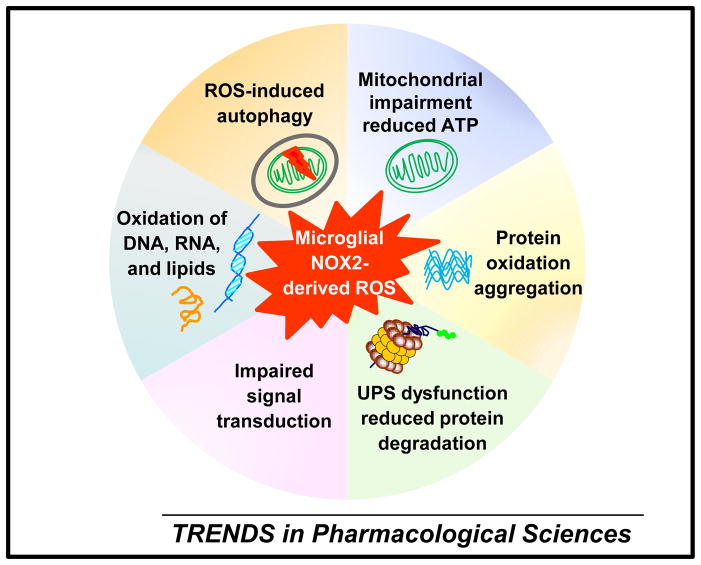
Activated microglia release a myriad of inflammatory and cytotoxic factors, such as cytokines, chemokines, eicosanoids, excitatory amino acids, proteases, and ROS. Among these factors, NOX2-derived ROS are recognized as a crucial player in chronic neurodegeneration [46–48]. H2O2 (non-radical oxidants) and peroxynitrite (a reactive product of superoxide and nitric oxide) derived from activated microglial NOX2 can enter neurons, in which they impair mitochondria integrity, reduce ATP production, and increase mitochondria-derived ROS. They also cause oxidation, nitration, aggregation, and accumulation of enzymes and other proteins (e.g. α-synuclein). ROS-induced dysfunction of the ubiquitin-proteasome system (UPS) will reduce protein degradation, which in turn exaggerates the accumulation of abnormal proteins. Impaired redox-sensitive signal transduction, oxidation of DNA, RNA, and lipids, and ROS-induced autophagy arising from over-activated NOX2 also contribute to oxidative neuronal damages during neuroinflammation [1, 2, 49] (Figure 2).
Figure 2. NOX2-derived ROS from activated microglia mediate chronic oxidative neuronal damage in neurodegenerative diseases.
NOX2-derived oxidants from activated microglia, such as H2O2 (non-radical oxidants) and peroxynitrite (a reactive product of superoxide and nitric oxide) can enter neurons and lead to 1) mitochondrial impairment and dysfunction, reduced ATP production, and increased generation of mitochondria-derived free radicals; 2) protein oxidation, nitration, aggregation, and accumulation; 3) dysfunction of the ubiquitin-proteasome system (UPS) and reduced protein degradation; 4) impaired redox-sensitive signal transduction; 5) oxidation of DNA, RNA, and lipids; and 6) ROS-induced autophagy. All these oxidative damages of neurons are integral components of chronic neurodegenerative process.
NOX2 activation in AD
Aggregated β-amyloid (the prime pathogenic mediator of AD) stimulates microglia to release NOX2-derived ROS, and the resultant oxidative stress contributes to the pathogenesis of AD [50, 51]. ATP, released from β-amyloid-stimulated microglia, induces NOX2-dependent ROS production via activating the purinergic receptor P2X7 [52, 53]. Deletion of the NOX2 gene or inhibition of NOX2 activity by DPI prevented microglia-mediated neurotoxicity triggered by β-amyloid in neuron-glia mixed cultures [54] or in a co-culture system containing microglia/ macrophages and human neuroblastoma cells that transgenically overexpress wildtype or mutated amyloid protein precursor (APP) [55]. Inhibition of NADPH oxidase by apocynin or p47phox deletion switched microglial activation from a classical to an alternative state in response to an intracerebroventricular injection of LPS or β-amyloid [56]. DPI and apocynin block β-amyloid-induced ROS generation, glutathione depletion, and mitochondrial depolarization in both neurons and astrocytes, and they also prevent β-amyloid-induced neuronal death [57]. Such findings imply NOX2 in astrocyte and neurons plays a role in β-amyloid-induced neurodegeneration. Furthermore, in an AD mouse model overexpressing mutant APP, a membrane-permeable NOX2 peptide inhibitor NOX2 ds-tat (gp91ds-tat) or genetic deletion of NOX2 attenuates neuronal oxidative stress, cerebrovascular dysfunction, and behavioral deficits [58]. These findings together indicate NOX2-mediated oxidative stress is an important contributor to AD neurodegeneration.
NOX2 activation in PD
Pathological examinations reveal upregulated microglial NOX2 in the substantia nigra of patients with PD and a mouse model of PD [59]. Moreover, ample preclinical research has demonstrated a critical role of NOX2 activation in microglia -mediated dopaminergic neurodegeneration [46, 60]. For example, aggregated α-synuclein (the key pathogenic mediator of PD) stimulates cultured microglia to release NOX2-derived ROS leading to the death of co-cultured dopaminergic neurons [61]. The presence of microglia enhances dopaminergic neurodegeneration induced by PD-producing neurotoxins 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP), MPP+, and 6-hydroxydopamine; PD-associated pesticides rotenone and paraquat; inflammagens LPS and fMLP (formylmethionyl- leucyl-phenylalanine); nanometer size diesel particles; and angiotensin II in primary mesencephalic cultures; such microglia-mediated aggravation of dopaminergic neurodegeneration is mitigated by DPI, apocynin, or NOX2 deletion [62–71]. In addition, endogenous cellular components released or leaked from stressed/ damaged neurons into the extracellular milieu , such as aggregated α-synuclein, the active form of matrix metalloproteinase-3, and high-mobility group box 1 (HMGB1), trigger reactive microgliosis (secondary microglia reactions to neuronal lesions) and NOX2-dependent ROS production, which in turn contributes to dopaminergic neurodegeneration [61, 72, 73]. In a mouse model of PD, minocycline-induced inhibition of microglial activation and membrane translocation of p67phox in mouse brains are associated with reduced dopaminergic neurodegeneration evoked by MPTP [74]. Degeneration of nigral dopaminergic neurons induced by systemic administration of MPTP or an intra -nigral injection of LPS is significantly attenuated in NOX2-deficient mice compared with wild - type mice [66, 75].
NOX2 in ALS
ALS is characterized by a progressive loss of motor neurons in the spinal cord, the brainstem, and the motor cortex. Enhanced NOX2-mediated ROS production by microglia has been implicated in ALS pathogenesis. Microglia in the spinal cord of animal models and patients with ALS show elevated expression of NOX2, increased ROS production, and oxidative damage [60]. In BV2 microglial cell lines, overexpression of mutant SOD1 enhances p47phox-dependent ROS production and secretion of cytokine tumor necrosis factor α (TNFα ) induced by an activator of toll-like receptor 2 (TLR2), Pam3Cys-SKKKK [76]. In addition, apocynin prevents the loss of human-embryonic-stem- cell-derived motor neurons caused by co-cultured human-primary-astroglia overexpressing mutant SOD1 [77]. Recent in vitro studies using glial cells suggest that SOD1 regulates NOX2 activity by interacting with Rac1 and that mutant SOD1 enhances Rac1-dependent NOX2 activation [78, 79]. Importantly, NOX2-mediated redox imbalance exaggerates ALS-like motor neuron disease, whereas NOX2 deficiency delays motor neurodegeneration, slows disease progression, improves neurological symptoms (e.g. muscle weakness and atrophy), and extends survival in mice carrying mutant SOD1 [41, 60]. High doses of apocynin (30–300mg/ kg) also improve survival and slow ALS progression in SOD1-mutant mice [78, 79]. Altogether, increased microglia NOX2 activity is an important disease mechanism underlying the non-cell-autonomous nature of ALS neurodegeneration.
NOX2 activation in multiple sclerosis (MS)
Multiple sclerosis (MS) is a neurodegenerative disease with widespread inflammatory axonal demyelination and neuronal injury in the brain and the spinal cord. The investigation on the role of NOX2 activation in the development of MS has generated conflicting and puzzling results. PHOX-dependent production of peroxynitrite is found to be important for microglia-mediated toxicity to cultured oligodendrocytes (a type of glial cells, which form myelin sheaths surrounding axons in the CNS) [80]. Phagocytosis of myelin by microglia leads to p47phox-mediated ROS generation and a marked suppression of microglial inflammatory responses, which are reversed by PHOX inhibition or by specific knockdown of p47phox [81]. p47phox-knockout mice develop decreased experimental autoimmune encephalomyelitis (EAE), a widely used animal model for MS, whereas p47phox-mutant mice that express a truncated and nonfunctional p47phox protein reveal enhanced EAE [82, 83]. Additionally, a low ROS-generating variant of p47phox promotes EAE development in rats [84]. NOX2-deficient mice are resistant to EAE [85]. The reasons for these seemingly discrepant findings are unclear; the interaction and the balance among superoxide, nitric oxide, and peroxynitrite might critically determine the outcome of the combined action of these reactive nitrogen and oxygen species.
The coupling of microglial surface receptor recognition and NOX2 activation
In the CNS, pattern recognition receptors (PRRs) expressed on the microglial surface including toll-like receptors (TLRs) are necessary for detection and rapid elimination of invading microorganisms, and they also respond to host-derived ligands to activate inflammatory signaling cascades [86, 87]. PRRs appear to be important for transducing diverse stress stimuli into NOX2 activation and ROS production in microglia [61, 73, 76, 88, 89]. It has been reported that overexpression of mutant SOD1 enhances TLR2 stimulation-induced activation of microglial NOX2 and subsequent secretion of TNFα in a p47phox-dependent manner [76]. Regulation mechanisms of NADPH oxidases by TLR2, TLR4, and other TLRs identified in non-immune cells and peripheral immune cells have not been thoroughly investigated in microglia. The microglial surface receptor, integrin Mac1 (macrophage antigen complex 1; also known as complement receptor 3 or CD11/ CD18) can also function as a PRR to recognize LPS and some endogenous ligands (e.g. α-synuclein, β-amyloid, or HMGB1) to induce NOX2 activation [61, 73, 88, 89]. Membrane translocation of p47phox and/ or NOX2-dependent ROS production elicited by these ligands are blunted in Mac1-null microglia; such reduced NOX2 activation is associated with less neuronal impairment [61, 73, 88, 89]. Thus, the coupling between Mac1 and PHOX might be an important mechanism underlying microglia -mediated NOX2-dependent oxidative insults to neurons in neurodegenerative diseases. Interestingly, the deletion of the prostaglandin E2 receptor EP2 (an important regulator of inflammatory oxidative injury in innate immunity) diminishes the increased neuronal oxidative stress and the elevated expression of multiple proinflammatory enzymes including subunits of PHOX (p47phox, p67phox, p40phox, and p22phox) in the spinal cord of SOD-mutant mice; EP2 deletion also improves motor strength and extends survival [90]. Thus, prostaglandin-mediated regulation of PHOX activation could be important for inflammatory oxidative injury in ALS and perhaps other neurodegenerative diseases.
The therapeutic potential of targeting NOX2 in neurodegenerative diseases
Neuroprotective effects of NOX2 inhibition in in vitro PD models
As described above, over-activated NOX2 has been implicated as a major contributor to chronic neurodegeneration in neurodegenerative diseases. Several in vitro studies using neuron-glia co-cultures reveal a correlation of NOX2 inhibition with neuroprotection by multiple anti-inflammatory drugs including sinomenine (a natural dextrorotatory morphinan analog), squamosamide derivative FLZ, pituitary adenylate cyclaseactivating polypeptides, TGF-β 1 (transforming growth factor -β 1; a known endogenous immune modulator), verapamil, and resveratrol (a nonflavonoid polyphenol with antioxidant and anti-inflammatory properties). All these drugs inhibit superoxide release from wild-type microglia; more importantly, they fail to exhibit neuroprotection in cultures from NOX2-deficient mice [91–96].
Neuroprotective effects of NOX2 inhibition in animal models of PD
Several structurally and functionally different compounds that shared common properties of suppressing inflammation and inhibiting NOX2 activity provide significant neuroprotection in animal models of PD. For instance, dextromethorphan (a widely used anti-tussive agent) significantly attenuates MPTP-induced ROS production in mesencephalic neuron-glia cultures and loss of nigral dopaminergic neurons in wild-type mice; such neuroprotection is abolished in NOX2-deficient mice, which indicates NOX2 is a critical mediator of the neuroprotective property of dextromethorphan [75]. The longstanding clinical safety record makes dextromethorphan an attractive candidate for further investigation for its clinical use in PD treatment. Moreover, inhibition of microglial activation and PHOX activity by minocycline protects nigral dopaminergic neurons from thrombin- or MPTP-induced neurotoxicity [74, 97]. Through suppressing the activation of nuclear factor kappa B (NF-κB) and the membrane translocation of p47phox of microglial PHOX, compound A (a potent and selective inhibitor of IKK-β ) prevents microglia-mediated loss of nigral dopaminergic neurons induced by a stereotaxic injection of LPS [98]. Hydrogen sulfide (a biological gas) specifically inhibited NADPH oxidase activation and oxygen consumption evoked by 6-hydroxydopamine; systemic administration of NaHS (a hydrogen sulfide donor) prevent dopaminergic neurodegeneration induced by 6-hydroxydopamine and rotenone via suppression of NADPH oxidase activity and microglial activation [99].
Neuroprotective effects of NOX2 inhibition in other neurodegenerative diseases
Although the neuroprotective effect of inhibition of microglial activation and suppression of NOX2 activity has mostly been demonstrated in PD models, the potential benefit of NOX2 inhibition will not be limited to PD. For instance, an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation and superoxide production after 17-beta-estradiol administration are responsible for estrogen-induced attenuation of ischemic oxidative damage in stroke [100]. Galantamine, an alkaloid d rug currently used to treat AD, offers neuroprotection through the suppression of inducible nitric oxide synthase, NADPH oxidase, and ROS production in hippocampal slices subjected to anoxia/ reoxygenation (an in vitro brain ischemia-reperfusion model) [101]. Treatment of microglia or monocytes with ibuprofen (a nonsteroidal anti-inflammatory drug) inhibits β-amyloid-elicited Vav tyrosine phosphorylation, PHOX assembly, and superoxide production; more importantly, a 9-month treatment of aged R1.40 mice (a model of AD) with ibuprofen leads to a marked reduction in lipid peroxidation, tyrosine nitration, protein oxidation, microglial activation, and plaque burden. Thus, ibuprofen reduces oxidative damage and enhances plaque clearance through the inhibition of microglial NOX2 in AD [102]. Additionally, an intraperitoneal injection of a low dose of dextromethorphan (0.1 mg/ kg) attenuates moderate EAE (an animal model of MS) through the inhibition of NOX2 expression and the suppression of the infiltration of monocytes and lymphocytes into the spinal cord [103]. Taken together, the correlation of pharmacological inhibition of NOX2 activity with attenuated neurodegeneration in models of various neurodegenerative diseases strongly implicates NOX2 as a promising therapeutic target for neurodegenerative diseases.
Potential drawbacks to targeting NOX2
Given the indispensable role of NOX2 in host defense and the important physiological function of NOX-derived ROS, the long-term administration of d rugs targeting NADPH oxidases in an attempt to retard chronic neurodegeneration and disease progression in neurodegenerative diseases might compromise the immune system or other ROS-regulated function. It is well-established that mutations in genes encoding NOX2, p47phox, p67phox, or p22phox predispose individuals to a markedly increased susceptibility to severe bacterial and fungal infections, leading to CGD in human [6]. Of more than 400 causative mutations identified so far , approximately 95% result in complete or partial loss of protein expression, whereas only 5% are loss-of-function mutations that lead to normal protein expression and impaired PHOX function. More than two-thirds of CGD cases are X-linked recessive, which result from mutations in X-linked CYBB gene that encodes NOX2, the remaining CGD cases are autosomal recessive. Importantly, female subjects with only one copy of causative genes exhibit reduced NOX2 activity but no CGD pathology or other ROS-associated illness. This gene-dosing effect, combined with the finding that only CGD patients whose phagocytic ROS generation is more than two orders of magnitude lower than that in healthy controls develop severe illness and poor long-term survival [104], suggests it is feasible to treat neurodegenerative diseases through modulating NADPH oxidase activity and to avoid compromising the immune system and other physiological function. In addition, the clinical safety record of several “old” drugs (e.g. dextromethorphan and galantamine) that show novel functions in NOX2 inhibition and neuroprotection in experimental settings [75, 101, 103] further hints at the feasibility of pharmacological modulation of over -activated NOX2 for the treatment of neurodegenerative diseases. Furthermore, the newly-discovered p47phox- independent mechanism of PHOX activation in in vitro studies [8–10] implies that it might be clinically safer to modulate NOX2 activity through targeting p47phox. Nonetheless, great caution must be exercised when developing therapeutic compounds to decrease — but not to abolish — the excessive activity of the NADPH oxidase enzyme for neurodegenerative d iseases.
Limitations of known NOX2 inhibitors in clinical translation
Of several known NADPH oxidase inhibitors, apocynin and DPI are most widely used and are invaluable pharmacological tools in NADPH oxidase research . Apocynin (a methoxy-substituted catechol) blocks the assembly of p47phox with membrane-bound p22phox/ NOX2 heterodimer. It has also been reported to affect redox status in nonphagocytic cells acting as a radical scavenger [105] or a pro-oxidant under certain conditions [106]. DPI as an irreversible noncompetitive flavoprotein inhibitor abstracts electrons from the reduced flavin and prevents electron flow through the flavoprotein conduit. DPI has been widely used as a nonspecific NOX2 inhibitor because it shows more potent inhibition of NOX2 than of other flavin-containing enzymes, such as nitric oxide synthases, NADH dehydrogenase, xanthine oxidase, cytochrome p450 oxidoreductase, and NADH-ubiquinone oxidoreductase [3]. The lack of selectivity of DPI for NADPH oxidases and the pro-oxidant potential of apocynin might limit their clinical translation. The newly-developed NADPH oxidase inhibitors, 4-(2- aminoethyl)benzenesulfonyl fluoride (AEBSF) and the synthetic peptide NOX2ds-tat, inhibit the association of p47phox with p22phox/ NOX2 heterodimer. However, the irreversible off-target effect on serine proteases and the very low efficacy (IC50 >1 mM) of AEBSF and the unfavorable administration routes (non-oral routes) of NOX2ds-tat peptide might exclude them from clinical translation. The action mechanism and the selectivity of other putative NADPH oxidase inhibitors remain to be investigated thoroughly. More detailed information on NOX inhibitors has been discussed in recent comprehensive review articles [3, 107]. In view of limitations of known NOX2 inhibitors in clinical translation, novel blood-brain-barrier permeable NADPH oxidase inhibitors with improved efficacy, specificity and pharmacokinetic profiles might have potential for development into clinical therapeutics for various neurodegenerative d iseases.
Concluding remarks
Oxidative stress and neuroinflammation are the two most common features shared by various neurodegenerative diseases. Over-activated NADPH oxidases, especially PHOX and its catalytic subunit NOX2 have been recognized as a major mediator of inflammatory oxidative neurodegeneration in these diseases. Pharmacological inhibition of NOX2 activity with several structurally and functionally different compounds attenuates the neurodegeneration in models of neurodegenerative diseases. Such preclinical evidence provides a strong rationale for pharmacological modulation of NADPH oxidase activity in combating oxidative stress-mediated neurodegeneration in these diseases. Given the limitation of existing NOX2 inhibitors in clinical translation and the potential drawbacks to targeting NOX2, the next step is to develop novel blood-brainbarrier permeable NADPH oxidase inhibitors with improved specificity, efficacy, and pharmacokinetic profiles. Further examination of anti-inflammatory and NOX-inhibitory action as well as neuroprotective effects of a new generation of NADPH oxidase inhibitors in multiple animal models of neurodegenerative diseases will identify promising drug candidates for future clinical trials. Thus, with increasing awareness of the prime role of NADPH oxidases, especially NOX2, in oxidative stress-mediated chronic neurodegeneration, proper modulation of NOX activity might present a promising therapeutic strategy to retard the progression of many neurodegenerative diseases.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. We apologize for limiting citation of many excellent original contributions due to space l imitations. The authors declare they have no actual or potential competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnham KJ, et al. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, et al. Oxidative stress in Parkinson's disease: a mechanism of pathogenic and therapeutic significance. Annals of the New York Academy of Sciences. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond GR, et al. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatehi-Hassanabad Z, et al. Reactive oxygen species and endothelial function in diabetes. European journal of pharmacology. 2010;636:8–17. doi: 10.1016/j.ejphar.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxidants & redox signaling. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, et al. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. J Biol Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 9.Mizrahi A, et al. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. J Leukoc Biol. 2006;79:881–895. doi: 10.1189/jlb.1005553. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, et al. Rotenone activates phagocyte NADPH oxidase by binding to its membrane subunit gp91phox. Free radical biology & medicine. 2012;52:303–313. doi: 10.1016/j.freeradbiomed.2011.10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 12.Valente AJ, et al. NOX1 NADPH oxidase regulation by the NOXA1 SH3 domain. Free radical biology & medicine. 2007;43:384–396. doi: 10.1016/j.freeradbiomed.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G, et al. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem. 2004;279:34250–34255. doi: 10.1074/jbc.M400660200. [DOI] [PubMed] [Google Scholar]
- 14.Ueno N, et al. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 15.Nisimoto Y, et al. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Loehneysen K, et al. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C-terminus. J Biol Chem. 2012 doi: 10.1074/jbc.M111.332494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Gao HM, et al. Neuroinflammation and alpha-Synuclein Dysfunction Potentiate Each Other, Driving Chronic Progression of Neurodegeneration in a Mouse Model of Parkinson's Disease. Env iron Health Perspect. 2011;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano Y, et al. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pao M, et al. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:230–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- 21.Kishida KT, et al. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan AM, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girouard H, et al. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 25.Behrens MM, et al. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman MC, et al. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, et al. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractussolitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibi M, et al. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free radical biology & medicine. 2006;40:1785–1795. doi: 10.1016/j.freeradbiomed.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Munnamalai V, Suter DM. Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. Journal of neurochemistry. 2009;108:644–661. doi: 10.1111/j.1471-4159.2008.05787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mander PK, et al. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol. 2006;176:1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- 31.Marin-Teva JL, et al. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 32.Harrigan TJ, et al. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. Journal of neurochemistry. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barger SW, et al. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. Journal of neurochemistry. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramov AY, et al. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free radical biology & medicine. 2011;51:171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimohama S, et al. Activation of NADPH oxidase in Alzheimer's disease brains. Biochemical and biophysical research communications. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 37.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J Alzheimers Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 38.Cheret C, et al. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anantharam V, et al. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–997. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cristovao AC, et al. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxidants & redox signaling. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marden JJ, et al. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coyoy A, et al. Role of NADPH oxidase in the apoptotic death of cultured cerebellar granule neurons. Free radical biology & medicine. 2008;45:1056–1064. doi: 10.1016/j.freeradbiomed.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 43.Guemez-Gamboa A, Moran J. NOX2 mediates apoptotic death induced by staurosporine but not by potassium deprivation in cerebellar granule neurons. J Neurosci Res. 2009;87:2531–2540. doi: 10.1002/jnr.22079. [DOI] [PubMed] [Google Scholar]
- 44.Ha JS, et al. Extracellular hydrogen peroxide contributes to oxidative glutamate toxicity. Brain Res. 2010;1359:291–297. doi: 10.1016/j.brainres.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 45.Kleinschnitz C, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 48.Glass CK, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milton RH, et al. CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J Neurosci. 2008;28:11488–11499. doi: 10.1523/JNEUROSCI.2431-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson B, et al. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 52.Kim SY, et al. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med. 2007;39:820–827. doi: 10.1038/emm.2007.89. [DOI] [PubMed] [Google Scholar]
- 53.Parvathenani LK, et al. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 54.Qin L, et al. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. Journal of neurochemist ry. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 55.Qin B, et al. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging. 2006;27:1577–1587. doi: 10.1016/j.neurobiolaging.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Choi SH, et al. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. Journal of neurochemistry. 2012;120:292–301. doi: 10.1111/j.1471-4159.2011.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abramov AY, et al. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park L, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu DC, et al. NADPH oxidase mediates oxidative stress in the 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu DC, et al. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. The FASEB journal : official publicat ion of the Federation of American Societies for Experimental Biology. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 62.Gao HM, et al. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci. 2003;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao HM, et al. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. Journal of neurochemist ry. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 64.Gao HM, et al. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao HM, et al. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. The FASEB journal : official publicat ion of the Federation of American Societies for Experimental Biology. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 66.Qin L, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Pallares J, et al. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6- hydroxydopamine-induced degeneration of dopaminergic neurons. Journal of neurochemist ry. 2007;103:145–156. doi: 10.1111/j.1471-4159.2007.04699.x. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Pallares J, et al. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Wu XF, et al. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Ant ioxidant s & redox signaling. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 70.Block ML, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. The FASEB journal : official publicat ion of the Federat ion of American Societies for Experimental Biology. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 71.Gao X, et al. Formyl-methionyl-leucyl-phenylalanine-induced dopaminergic neurotoxicity via microglial activation: a mediator between peripheral infection and neurodegeneration? Environ Health Perspect. 2008;116:593–598. doi: 10.1289/ehp.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim YS, et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. The FASEB journal : official publicat ion of the Federat ion of American Societies for Experimental Biology. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 73.Gao H, et al. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu DC, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. The FASEB journal : official publicat ion of the Federat ion of American Societies for Experimental Biology. 2004;18:589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, et al. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J Biol Chem. 2009;284:3691–3699. doi: 10.1074/jbc.M804446200. [DOI] [PubMed] [Google Scholar]
- 77.Marchetto MC, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Harraz MM, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Q, et al. ALSIN and SOD1G93A regulate endosomal ROS production by glial cells and pro-inflammatory pathways responsible for neurotoxicity. J Biol Chem. 2011 doi: 10.1074/jbc.M111.279711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, et al. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, et al. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47–PHOX-mediated generation of reactive oxygen species. J Neurosci. 2006;26:12904–12913. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hultqvist M, et al. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Nat l Acad Sci U S A. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Veen RC, et al. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J Immunol. 2000;164:5177–5183. doi: 10.4049/jimmunol.164.10.5177. [DOI] [PubMed] [Google Scholar]
- 84.Becanovic K, et al. Advanced intercross line mapping of Eae5 reveals Ncf-1 and CLDN4 as candidate genes for experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6055–6064. doi: 10.4049/jimmunol.176.10.6055. [DOI] [PubMed] [Google Scholar]
- 85.Li S, et al. Distinct role of nitric oxide and peroxynitrite in mediating oligodendrocyte toxicity in culture and in experimental autoimmune encephalomyelitis. Neuroscience. 2011;184:107–119. doi: 10.1016/j.neuroscience.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 87.Block ML, et al. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 88.Zhang D, et al. Microglial MAC1 receptor and PI3K are essential in mediating beta-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J Neuroinflammation. 2011;8:3. doi: 10.1186/1742-2094-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu X, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J Immunol. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang X, et al. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008;64:304–314. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qian L, et al. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008;181:660–668. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qian L, et al. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang S, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther. 2006;319:595–603. doi: 10.1124/jpet.106.102236. [DOI] [PubMed] [Google Scholar]
- 94.Zhang D, et al. Squamosamide derivative FLZ protects dopaminergic neurons against inflammation-mediated neurodegeneration through the inhibition of NADPH oxidase activity. J Neuroinflammation. 2008;5:21. doi: 10.1186/1742-2094-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang F, et al. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti -inflammatory actions. Mol Pharmacol. 2010;78:466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, et al. Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology. 2011;60:373–380. doi: 10.1016/j.neuropharm.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi SH, et al. Inhibition of thrombin-induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. Journal of neurochemistry. 2005;95:1755–1765. doi: 10.1111/j.1471-4159.2005.03503.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhang F, et al. Inhibition of IkappaB kinase-beta protects dopamine neurons against lipopolysaccharide-induced neurotoxicity. J Pharmacol Exp Ther. 2010;333:822–833. doi: 10.1124/jpet.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu LF, et al. Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell. 2010;9:135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 100.Zhang QG, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Egea J, et al. Galantamine elicits neuroprotection by inhibiting iNOS, NADPH oxidase and ROS in hippocampal slices stressed with anoxia/reoxygenation. Neuropharmacology. 2012;62:1082–1090. doi: 10.1016/j.neuropharm.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 102.Wilkinson BL, et al. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease. Neurobiol Aging. 2012;33:197 e121–132. doi: 10.1016/j.neurobiolaging.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chechneva OV, et al. Low dose dextromethorphan attenuates moderate experimental autoimmune encephalomyelitis by inhibiting NOX2 and reducing peripheral immune cells infiltration in the spinal cord. Neurobiol Dis. 2011;44:63–72. doi: 10.1016/j.nbd.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuhns DB, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. The New England journal of medicine. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heumuller S, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 106.Vejrazka M, et al. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochimica et biophysica acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 107.Jaquet V, et al. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxidants & redox signaling. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]