Abstract
Adipose tissue is a known reservoir of multipotent mesenchymal stem cells which can be manipulated in culture to produce cells with different phenotypes. The goal of this study was to determine if the addition of these multipotential cells to organotypic, human skin equivalent cultures would accelerate wound healing after laser injury. For our initial studies, we were able to obtain 3-dimensional raft cultures from adult skin explanted directly onto the dermal equivalent containing human fibroblasts with or without adipose-derived stromal cells (ADSCs). Two days after laser injury the raft cultures of skin explants that contained adipose-derived stromal cells had a completely healed multilayered epidermis, whereas, the control raft culture without the adipose-derived cells still had areas of injury. With this encouraging outcome, these experiments were then repeated in a raft culture system initiated from dissociated primary adult human keratinocytes on the humanized dermal equivalent. Again, the cultures containing ADSCs healed faster than the control cultures. In conclusion, these data provide support to our hypothesis that ADSCs are an excellent and readily available source of factors necessary for accelerated wound healing and tissue regeneration.
Introduction
Wound healing is a complex process and its delay can cause a number of problems including infection with increased area of injury, scarring with visible deformity, pain, and loss of function. Wound healing involves keratinocyte migration as an initial feature of the healing process. Healing involves cross talk between the keratinocytes and dermal fibroblasts and subcutaneous adipose tissue cells through growth factors and cytokines, as well as interactions among syndecans, laminins, and integrins. Some of the factors that promote keratinocyte migration, proliferation, stratification and differentiation include fibroblast growth factor1,2,3, wnt4,5,6,7, transforming growth factor-β (TGFβ)8,9, and heparin binding EGF-like growth factor (HB-EGF)10.
Adipose tissue is a known reservoir of mesenchymal stem cells which can be manipulated in culture to produce cells such as osteocytes11,12, endothelial progenitor cells13, adipocytes12,14, and chondrocytes12. Lu et al. showed that adipose-derived stem cells significantly increased the flap viability of random pattern skin flaps15. In the study by Stoff et. al., human derived mesenchymal cells were added to incisional wounds in rabbits, and at 14, 21, and 80 days the wounds had a greater tensile strength than the controls16. Previously, wound healing has been studied in conventional organotypic skin cultures on a DE with fibroblasts only17,18,19. And more recently, fibroblasts derived from human embryonic stem cells showed improved wound healing in 3-D human skin equivalents20.
The essence of the 3-D system is to culture human keratinocytes or selected human epithelial cell lines at the air-medium interface on top of a DE (type 1 collagen and murine 3T3J2 or human fibroblasts). In 9 days or longer, keratinocytes stratify and differentiate into a squamous epithelium21,22. When primary human keratinocytes are used, the squamous epithelium has been faithfully recapitulated. Differentiation proceeds in normal orderly fashion and the proliferating and differentiation markers are appropriately expressed in the basal and suprabasal cells, respectively. Importantly, only the basal cells proliferate, as revealed by the incorporation of tritiated thymidine or BrdU, a thymidine analog23,24.Thus, organotypic cultures are an excellent model system for the study of skin injury.
In our studies, we were able to incorporate ADSCs into a humanized collagen bed containing adult human fibroblasts (rather than 3T3J2 cells). During the preparation of this article, Lu and colleagues25 reported developing organotypic cultures on an ADSC containing DE (dermal equivalent). However, there were no wound healing studies carried out in that study. To date, there have been no publications demonstrating improved wound healing with the addition of ADSCs in organotypic cultures. We will demonstrate that we have successfully incorporated ADSCs into our humanized dermal equivalent and have accelerated wound healing with the addition of the ADSCs.
Methods
Abdominal adipose tissues that were routinely discarded at the time of panniculectomy or abdominoplasty, were harvested using tumescent liposuction with the toomey syringes, a sterile-enclosed negative pressure system. The adipose tissue was processed using the protocols of Wang et al., Zuk et al., Lu et al., and Traktuev et al., for preparation of ADSCs11,12,13,26. In brief, the liposuctioned fat was digested with an equal volume of collagenase Type 1 (Worthington, Lakewood, N.J.) (1.5 mg/ml in phosphate-buffered saline), washed with Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) (Hyclone Laboratories Inc., Logan, Utah), and centrifuged following standard protocol to obtain a cell pellet of stromal-vascular fraction (SVF). The recovered cells were cultured in DMEM supplemented with 10 % FBS at 37°C in CO2. The cultures were split when the cells were about 80% confluent. During culturing, most non-adherent cells (such as endothlelial cells, leukocytes, and RBC’s) were lost26. The early passage ADSCs were then incorporated into a collagen bed along with adult human fibroblasts.
Primary human keratinocytes and fibroblasts were isolated from discarded adult skin following standard procedures. Briefly, the skin was minced and digested with 0.25% trypsin for 1 hour at 37°C using a stirring bar during the digestion. The neutralization was carried out with DMEM containing 10% FBS. The cells were spun down in a microcentrifuge at 1000 rpm for 5 minutes. Keratinocytes and fibroblasts were then selectively recovered by culturing in keratinocyte serum free medium (Invitrogen/Gibco, Grand Island, N.Y.) or DMEM with 10% FBS, respectively.
The standard protocol for the organotypic raft cultures27 was modified by incorporating (60,000–90,000) ADSCs in the DE. Primary keratinocytes were seeded on the DE and grown for 36 hours as submerged cultures as described previously21,22. Subsequently, the assembly was then raised to the air-medium interface and cultured for 8 days. For skin explants, small skin chips were placed on the DE and cultured at the air-medium interface from the onset. On day 9, the Affirm CO2 laser (Cynosure, MA) was used to create 3 parallel burns in the elevated raft cultures. The laser-treated and control raft cultures without laser injury were cultured for another 24h –72h. The organotypic cultures were harvested at different times after laser wounding to follow the time course of wound healing. The raft cultures were formalin fixed and paraffin embedded and cut into 4-µm sections. Hematoxylin and eosin staining of the sections revealed the histology and the speed of horizontal and vertical wound repair. The laser-created gap was measured on digital images to determine the rate of lateral migration of the basal keratinocytes. The quality of wound healing was assessed molecularly by indirect immunofluorescent detection of keratin 10 and involucrin, suprabasal differentiation markers (data not shown)28.
Clinical materials used in this study were approved by the Institutional Review Board of the University of Alabama (Birmingham, AL).
Results
On the basis of reports showing beneficial results using stem cell-derived fibroblasts for wound healing, we initiated a study to determine whether we could employ the organotypic culture system to investigate the effects of ADSCs on skin wound healing. We incorporated ADSCs in the DE, consisting of rat tail collagen and human fibroblasts. ADSCs were recovered from discarded tissue from liposuction. Early passages were incorporated into the collagen along with primary adult human fibroblasts that were harvested from discarded human skin intraoperatively. Adult human skin chips were explanted onto the DE in the presence or absence of ADSCs. The assembly was then cultured at the air-medium interface for 8 days. Keratinocytes migrated from the skin explants, grew across the DE, and stratified into a squamous epithelium. Our preliminary studies revealed that the presence of ADSCs in the DE produced a healthier and thicker squamous epithelium relative to raft cultures on a DE containing the human fibroblasts alone (Fig. 1).
Figure 1.
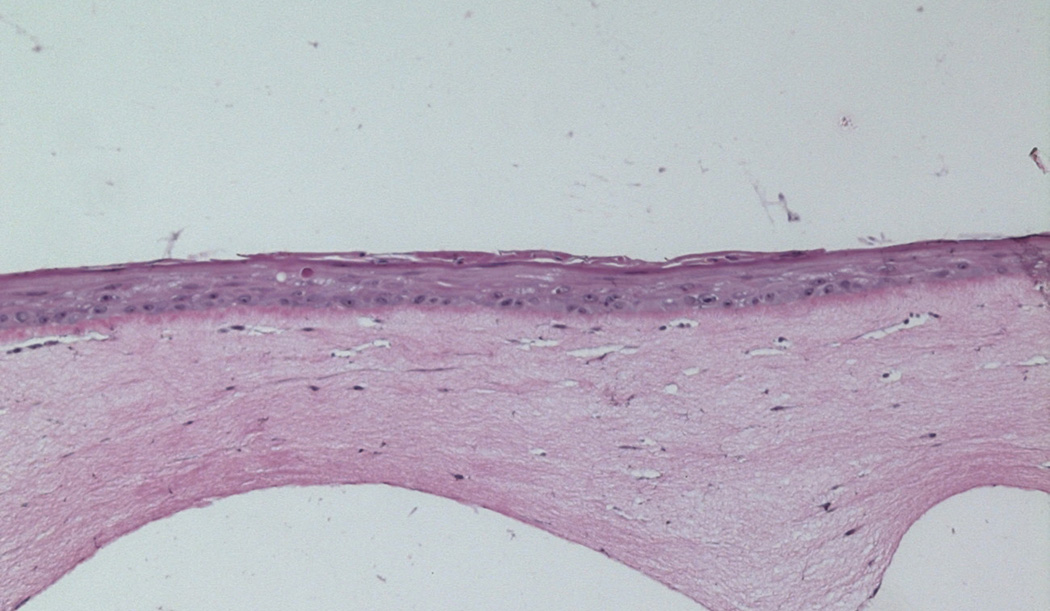
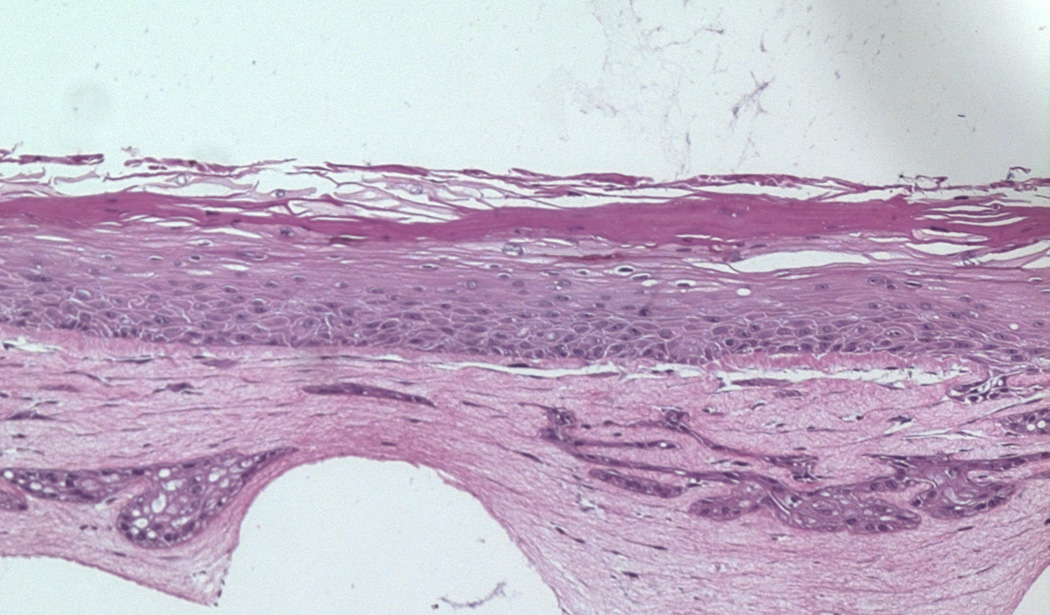
Upper panel: Control raft culture containing only human fibroblasts. Lower panel: Raft cultures containing human fibroblasts and ADSCs 9 days after elevation to the air-medium interface show a thicker multilayer epidermis. Magnification 10×.
Laser injuries down to the dermis were then inflicted on the skin explant raft cultures on day 9. We repeatedly saw accelerated healing in the cultures containing the ADSCs compared to cultures without ADSCs (Fig. 2). However, the limitation in using skin explants is the variability with which the migrating keratinocytes established a new epithelium, possibly associated with the sizes of the explants and elapsed time between the operation and the experimentation. It would be difficult to use this system to optimize the wound healing process. Therefore, we repeated these experiments using primary human keratinocytes recovered from adult skin. Two days after laser injury the raft cultures containing ADSCs in the collagen bed were healing (Fig. 3). Keratinocytes migrated over the wound bed and the size of the defect decreased. In contrast, the control raft cultures containing only human fibroblasts in the DE had no keratinocytes on the wound surface and the defect size was not decreased (Fig. 3). Measurements of wound closure were defined by the limits of reepithelialization and were made using a metric ruler on the two-dimensional digital images taken with the Olympus AX70 microscope using Axiovision image capture software and processed with Adobe Photoshop CS2. The results are shown in Table 1. The P value was 0.01212 with the Mann-Whitney-Wilcoxon rank sum test. These results demonstrated that, in agreement with the skin explant raft cultures, wound healing was accelerated when ADSCs were included in the collagen bed.
Figure 2.
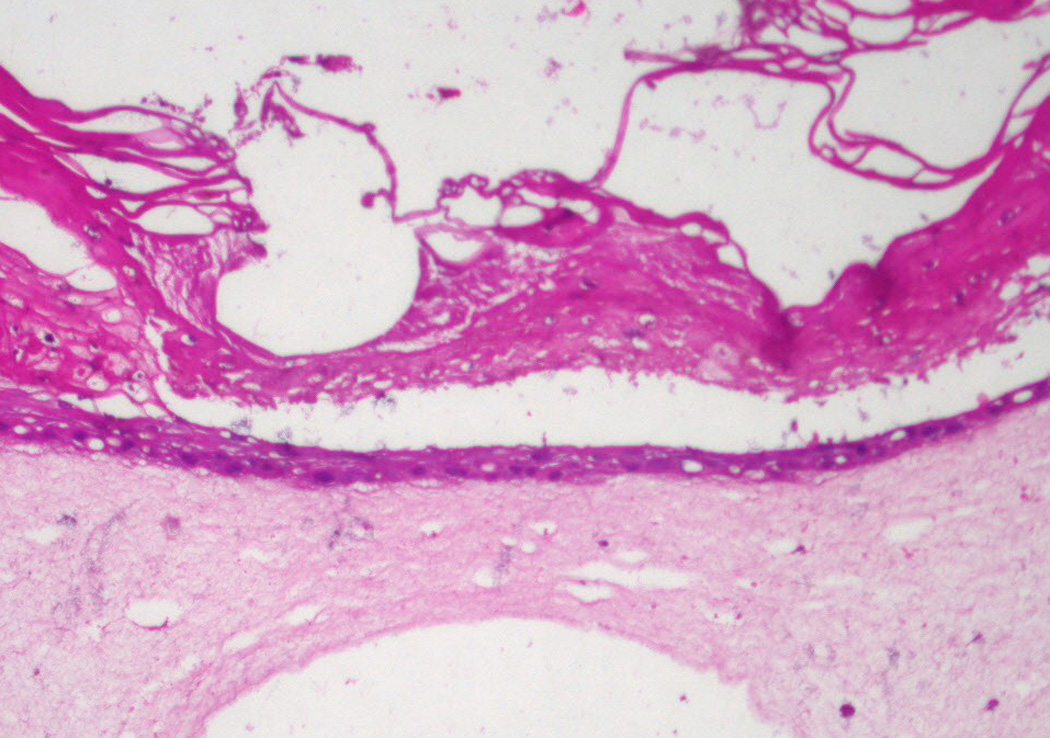
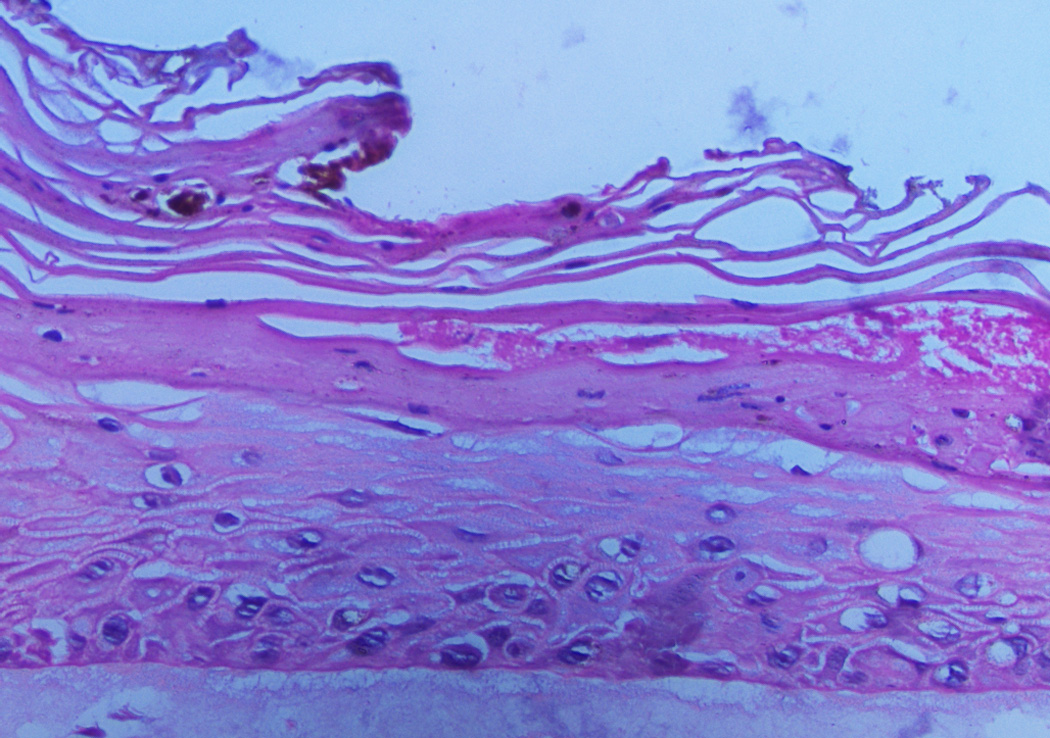
Upper panel: Laser site in control raft cultures containing only human fibroblasts 2 days after injury. The site of injury was not completely healed. Lower panel: Raft cultures containing human fibroblasts and ADSCs 2 days after injury show a completely healed multilayer epidermis. Magnification 10×.
Figure 3.
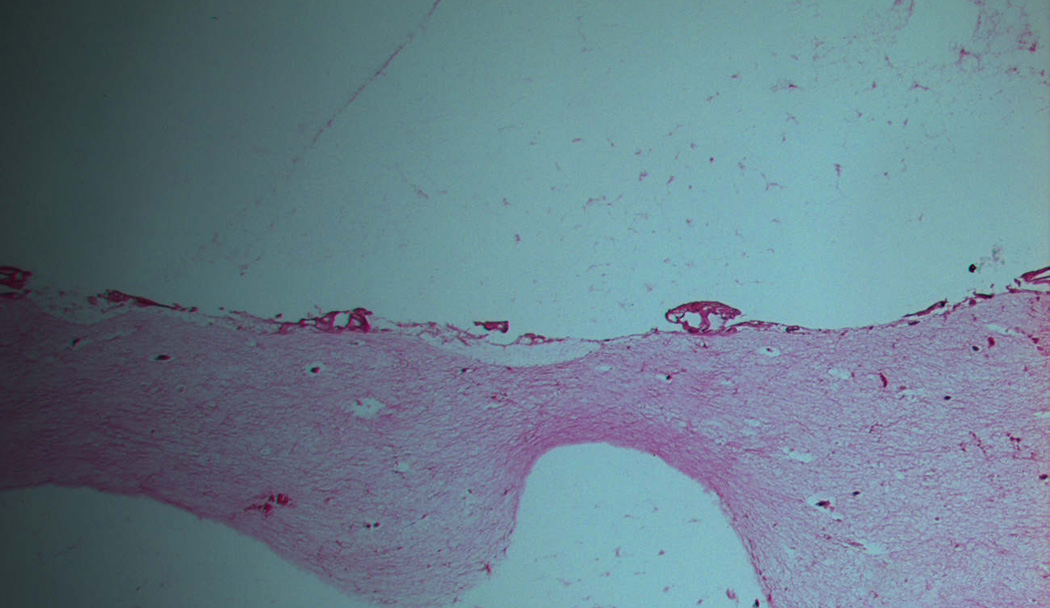
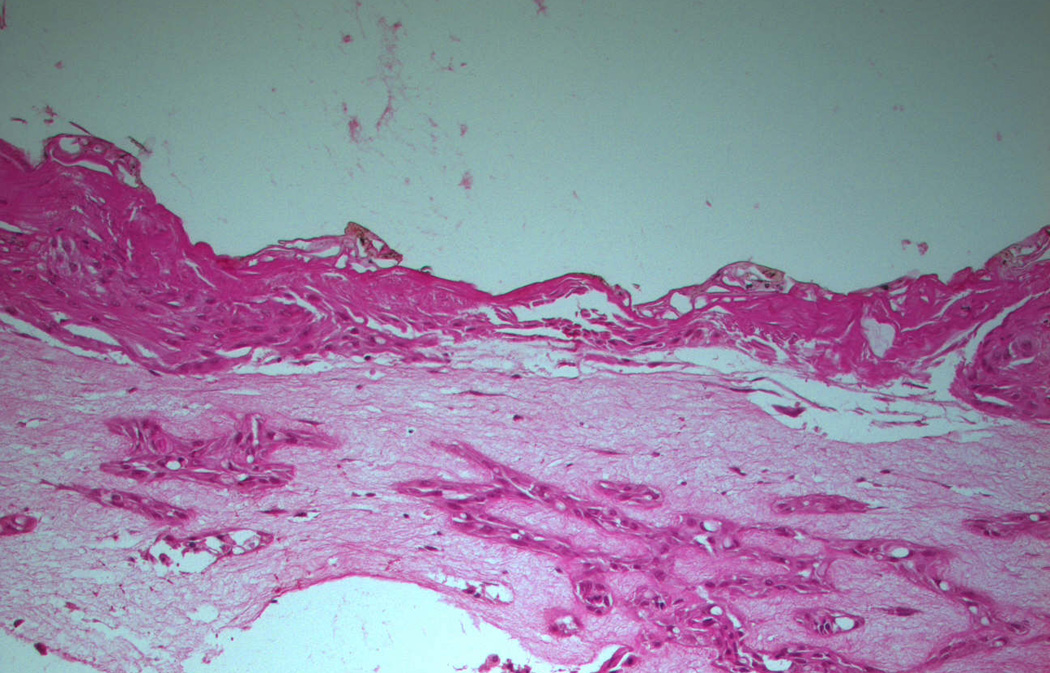
Upper panel: Laser site in control raft cultures containing only human fibroblasts 2 days after injury. The site of injury was not healed. Lower panel: Raft cultures containing human fibroblasts and ADSCs two days after injury show a healing epidermis. Magnification 10×.
TABLE 1.
Measurements of wound defects 2 days after laser injury.
| Burns | Wound diameter in ADSC rafts |
Wound diameter in fibroblast control rafts |
|---|---|---|
| 4.5 units | 15 units | |
| 7.0 units | 15 units | |
| 7.5 units | 15 units | |
| 3.0 units | ||
| 0.0 units | ||
| 9.0 units | ||
| 7.5 units | ||
| 0.0 units |
Discussion
Wound healing after laser injury was accelerated in our 3D skin cultures containing adipose-derived stromal cells (ADSCs) in the collagen bed. The addition of ADSCs to the raft cultures could result in differentiation of the ADSCs into other cell types, some of which might provide functionality in the raft culture system29. But, it is also known that ADSCs, similar to fibroblasts, express various growth factors25,29,30. Lu et al showed that the levels of hepatocyte growth factor and keratinocyte growth factor were higher in conditioned media containing fibroblasts and ADSCs25. Ebrahimian et al reported that cultured ADSCs in keratinocyte medium produced high levels of keratinocyte growth factor29. In addition, Chen et al reported that bone marrow-derived mesenchymal stem cells (BM-MSCs) secreted different amounts and types of cytokines and chemokines compared with dermal fibroblasts and that BM-MSC-conditioned media enhanced migration and proliferation of keratinocytes compared to fibroblast-conditioned medium31. And, as shown by Gnecchi et al, paracrine factors are thought to provide signaling that promotes healing in wounded cardiac muscle32. In summary, in our 3D cultures, wound healing is influenced positively by the presence of ADSCs, and the factors they produce will also likely play a role.
Conclusion
In these studies, we demonstrated that ADSCs added to the collagen bed in humanized skin raft cultures accelerated wound healing after laser injury compared to a DE which contained only the human fibroblasts. Our future goal is to characterize the effective ADSCs and to optimize culture conditions that accelerate re-epithelialization of skin wounds and result in regeneration of a more normal and functional skin and subcutaneous tissue unit in the area of injury. Our long term goal is to develop new strategies for clinical use in tissue engineering and wound healing. Importantly, this cell-based therapy uses a readily available source (adipose-derived stromal cells from liposuction) from the patient to promote and accelerate the wound healing process without any risk of rejection. Future clinical applications of ADSCs will significantly advance the current treatments of skin and subcutaneous tissue injury.
Footnotes
Presented at the SESPRS meeting Naples, Florida, June 8, 2011.
SESPRS funding to continue this research was presented at this meeting.
Contributor Information
Sherry S. Collawn, Division of Plastic Surgery, University of Alabama, Birmingham, AL.
N. Sanjib Banerjee, Department of Biochemistry and Molecular Genetics, University of Alabama, Birmingham, AL.
Jorge de la Torre, Chairman Division of Plastic Surgery, University of Alabama, Birmingham, AL.
Luis Vasconez, Division of Plastic Surgery, University of Alabama, Birmingham, AL.
Louise T. Chow, Department of Biochemistry and Molecular Genetics, University of Alabama, Birmingham, AL.
References
- 1.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 2.Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993 Nov 15;268(32):23906–23914. [PubMed] [Google Scholar]
- 3.Loo BM, Salmivirta M. Heparin/Heparan sulfate domains in binding and signaling of fibroblast growth factor 8b. J Biol Chem. 2002 Sep 6;277(36):32616–32623. doi: 10.1074/jbc.M204961200. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda M, Kamimura K, Nakato H, et al. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 1999 Jul 15;400(6741):276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- 5.Alexander CM, Reichsman F, Hinkes MT, et al. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 2000 Jul;25(3):329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 6.Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001 Jan;128(1):87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Sun XY, Liu HL, Fu XB. Dedifferentiation of epidermal cells into transit amplifying cells induced by bFGF. Nan Fang Yi Ke Da Xue Xue Bao. 2010 Sep;30(9):2041–2046. [PubMed] [Google Scholar]
- 8.Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997 Jul 18;272(29):18000–18006. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- 9.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans. 2006 Jun;34(Pt 3):458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 10.Monslow J, Sato N, Mack JA, Maytin EV. Wounding-induced synthesis of hyaluronic acid in organotypic epidermal cultures requires the release of heparin-binding egf and activation of the EGFR. J Invest Dermatol. 2009 Aug;129(8):2046–2058. doi: 10.1038/jid.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Steigelman MB, Walker JA, et al. In vitro osteogenic differentiation of adipose stem cells after lentiviral transduction with green fluorescent protein. J Craniofac Surg. 2009 Nov;20(6):2193–2199. doi: 10.1097/SCS.0b013e3181bf04af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 13.Lu F, Li J, Gao J, et al. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg. 2009 Nov;124(5):1437–1446. doi: 10.1097/PRS.0b013e3181babbb6. [DOI] [PubMed] [Google Scholar]
- 14.Ripoll CB, Bunnell BA. Comparative characterization of mesenchymal stem cells from eGFP transgenic and non-transgenic mice. BMC Cell Biol. 2009;10:3. doi: 10.1186/1471-2121-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu F, Mizuno H, Uysal CA, Cai X, Ogawa R, Hyakusoku H. Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg. 2008 Jan;121(1):50–58. doi: 10.1097/01.prs.0000293876.10700.b8. [DOI] [PubMed] [Google Scholar]
- 16.Stoff A, Rivera AA, Sanjib Banerjee N, et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp Dermatol. 2009 Apr;18(4):362–369. doi: 10.1111/j.1600-0625.2008.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garlick JA, Taichman LB. Effect of TGF-beta 1 on re-epithelialization of human keratinocytes in vitro: an organotypic model. J Invest Dermatol. 1994 Oct;103(4):554–559. doi: 10.1111/1523-1747.ep12396847. [DOI] [PubMed] [Google Scholar]
- 18.Yeh AT, Kao B, Jung WG, Chen Z, Nelson JS, Tromberg BJ. Imaging wound healing using optical coherence tomography and multiphoton microscopy in an in vitro skin-equivalent tissue model. J Biomed Opt. 2004 Mar-Apr;9(2):248–253. doi: 10.1117/1.1648646. [DOI] [PubMed] [Google Scholar]
- 19.Wilmink GJ, Opalenik SR, Beckham JT, Davidson JM, Jansen ED. Assessing laser-tissue damage with bioluminescent imaging. J Biomed Opt. 2006 Jul-Aug;11(4) doi: 10.1117/1.2339012. 041114. [DOI] [PubMed] [Google Scholar]
- 20.Shamis Y, Hewitt KJ, Carlson MW, et al. Fibroblasts derived from human embryonic stem cells direct development and repair of 3D human skin equivalents. Stem Cell Res Ther. 2011 Feb 21;2(1):10. doi: 10.1186/scrt51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JL, Dollard SC, Chow LT, Broker TR. Epithelial-specific gene expression during differentiation of stratified primary human keratinocyte cultures. Cell Growth Differ. 1992 Aug;3(8):471–483. [PubMed] [Google Scholar]
- 22.Chow LT, Broker TR. In vitro experimental systems for HPV: epithelial raft cultures for investigations of viral reproduction and pathogenesis and for genetic analyses of viral proteins and regulatory sequences. Clin Dermatol. 1997 Mar-Apr;15(2):217–227. doi: 10.1016/s0738-081x(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995 Oct 1;9(19):2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 24.Chien WM, Parker JN, Schmidt-Grimminger DC, Broker TR, Chow LT. Casein kinase II phosphorylation of the human papillomavirus-18 E7 protein is critical for promoting S-phase entry. Cell Growth Differ. 2000 Aug;11(8):425–435. [PubMed] [Google Scholar]
- 25.Lu W, Yu J, Zhang Y, et al. Mixture of Fibroblasts and Adipose Tissue-Derived Stem Cells Can Improve Epidermal Morphogenesis of Tissue-Engineered Skin. Cells Tissues Organs. 2011 Apr 15; doi: 10.1159/000324921. [DOI] [PubMed] [Google Scholar]
- 26.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008 Jan 4;102(1):77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee NS, Rivera AA, Wang M, et al. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol Cancer Ther. 2004 Apr;3(4):437–449. [PubMed] [Google Scholar]
- 28.Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994 Oct;4(5):725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 29.Ebrahimian TG, Pouzoulet F, Squiban C, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009 Apr;29(4):503–510. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 30.Rehman J, Li J, Parvathaneni L, et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004 Jun 16;43(12):2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008 Nov 21;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]


