Abstract
The purpose of this study was to assess the current status of patient radiation exposure tracking internationally, gauge interest and develop recommendations for implementation. A survey questionnaire was distributed to representatives of countries to obtain information, including the existence of a patient exposure tracking program currently available in the country, plans for future programs, perceived needs and goals of future programs, which examinations will be tracked, whether procedure tracking alone or dose tracking is planned, and which dose quantities will be tracked. Responses from 76 countries, including all of the six most populous countries and 16 of the 20 most populous, showed that although no country has yet implemented a patient exposure tracking program at a national level, there is increased interest in this issue. Eight countries (11%) indicated that such a program is actively being planned and 3 (4%) stated that they have a program for tracking procedures only, but not for dose. Twenty-two (29%) feel that such a program will be “extremely useful”, 46 (60%) “very useful” and 8 (11%) “moderately useful”, with no respondents stating “Mildly useful” or “Not useful”. Ninety-nine percent of countries indicated an interest in developing and promoting such a program. In a first global survey covering 76 countries, it is clear that no country has yet achieved exposure tracking at a national level, although there are successful examples at sub-national level. Almost all have indicated interest and some have plans to achieve dose tracking in the near future.
Keywords: Patient radiation exposure tracking, Smart Card, Dose tracking, Global survey of radiation tracking
1. Introduction
Increasing use of diagnostic medical imaging, with over 4 billion studies performed worldwide each year, is exposing populations to increasing doses of ionizing radiation [1]. Never before has there been a time like the present, when so many patients are receiving cumulative radiation doses which can exceed 100 mSv [2–5]. The majority of this exposure is justified, with medical benefits for patients, but reports in a variety of clinical settings have identified nontrivial proportions of radiological examinations that do not meet appropriateness criteria [5]. While creating awareness among referring physicians, utilization of appropriateness criteria, and emphasizing optimization for those performing examinations have been goals, for many years, the current situation demands additional considerations. One such consideration is tracking of radiation exposure history of patients [2,5,6]. However, there are no data available on worldwide existence or usefulness of and interests for such tracking programs.
The International Atomic Energy Agency (IAEA) initiated the Smart Card project in 2006 [2,5,6], with the objective of developing a flexible template for tracking cumulative radiation exposure (number and type of radiological procedures) and wherever possible dose for individual procedure. This program was subsequently named as SmartCard/SmartRadTrack. This was to dispel the myth that the purpose is to develop a card that contains patient dose history and to indicate a mechanism to track procedures and doses. This is somewhat similar to ATM card or credit card where cash or credit is not on the card but card enables access to cash or credit. Under this project, it was decided to assess the current status of national radiation tracking programs. The issues pertaining to “Why to track” and some aspects on “How to track” have already been dealt with in other papers [2,6]. Also in an earlier paper, the authors have indicated that being able to track patient radiation exposure will provide opportunities for enhancing imaging appropriateness and safety [5]. The present study reports on the first survey to assess the current global status of patient exposure tracking, gauge interest in such programs if not in existence, and suggest solutions based on results of this assessment.
2. Materials and Methods
2.1. Survey Participants
The survey was sent to counterparts participating in medical exposure projects in 85 countries out of a total of 151 IAEA Member States in existence at the time. Most of these countries are, or have been, participating in medical radiation protection projects of the IAEA. Wherever contact points for the IAEA projects were not available, such as in developed countries, the request was sent to members of medical exposure bodies such as members of the European Commission’s Article 31 Group Working Party on Medical Exposures, and professional bodies or national authorities in other countries. Help through personal contacts was sought to reach the most appropriate organizations for dealing with this topic.
2.2. Survey Administration
The survey was coordinated by the IAEA. It was primarily planned for online data entry through Google spreadsheets (Google Inc., Mountain View, CA, USA), but Microsoft Word (Microsoft Corporation, Redmond, WA, USA) based copies were also made available to those who had difficulty in online access and data entry.
The survey was initially distributed in March 2010. While a majority (>70%) of responses were received within one month, repeated (up to three) reminders and alternate contacts were employed to obtain the widest possible coverage. From some countries, more than one response was received, as shown in results, which required time to coordinate and integrate the multiple responses. There was also a need to obtain clarification from approximately a dozen respondents in order to clarify responses to each question. In the situation of multiple responses, questions were directed back to the respondents to obtain a collective understanding and consensus. Unique unambiguous responses were finally obtained for each question. In addition, a meeting was held at the IAEA in Vienna in October 2010 [7] which was attended by 46 participants from 31 countries: Africa (6), Asia (11), Europe (18), Latin America (4), USA (3), Canada (2) and WHO by teleconference (2). This provided another opportunity to obtain definitive responses from those present and incorporate the responses into the survey results, more so when there were more than one response from a country.
2.3. Survey Contents
The survey consisted of three forms. Form 1, shown in Table 1, was addressed to all survey participants, and addressed prior radiation tracking programs, the potential usefulness of such a program, and familiarity with IAEA efforts in this area. Response options were provided in all questions with free text options to provide more information where applicable. The first question addressed the presence of any current or planned radiation tracking program. A respondent answering that such a program is actively being planned was directed to Form 2 (Table 2) and the respondent stating that such a program is in place was directed to Form 3 (Table 3).
Table 1.
Survey questionnaire for IAEA Smart Card/SmartRadTrack Project, Form 1. Questions addressing prior radiation tracking programs, the potential usefulness of such a program, and familiarity with IAEA efforts in this area.
1. 1. Do you currently have a program to track medical radiation procedures and/or doses to your population?
|
2. Are you aware of the IAEA’s Smart Card/SmartRadTrack program?
|
3. Are you interested in joining and promoting such a program in your country?
|
4. Did you previously have such a program?
|
| If yes, please describe your discontinued program (s) to track medical radiation exposures/doses, and why they were discontinued: |
5. How useful do you think a radiation exposure tracking program would be (assuming practicalities are attended to)?
|
6. Would you be interested in learning more about the IAEA’s Smart Card/SmartRadTrack program?
|
7. Do you need IAEA resources to establish such a program in your country?
|
| 8. Please identify others in your country that would be candidates for a pilot study to participate in a worldwide radiation tracking program (Name, email ID, organization) |
Table 2.
Survey questionnaire for IAEA Smart Card/SmartRadTrack Project, Form 2. Questions for Country Planning a Radiation Tracking Program.
1. When do you plan to establish a program to track medical radiation exposures/doses?
|
2. What type of program is it?
|
3. Will the program(s) track examinations for an individual patient? (e.g. multiple CT scans to the same patient)
|
4. What are the goals of your planned program(s) to track medical radiation exposures/doses (please select all that apply)?
|
5. How do you plan to record data in this program?
|
6. Will data collected be transmitted to a centralized database?
|
7. Which types of examinations (modalities) will be tracked? Please select all that apply.
|
8. Which of the following will be tracked for an individual patient? Please select all that apply.
|
9. Once collected, for what purposes will the data be used? Please select all applicable.
|
| 10. What quantities will be tracked for? Please select all applicable. |
Projection radiography (x-ray, computed/digital/dental radiography)
|
Fluoroscopy
|
Computed Tomography
|
Mammography
|
Nuclear Medicine
|
| List additional measurements and associated modalities here: |
Table 3.
Survey questionnaire for IAEA Smart Card/SmartRadTrack Project, Form 3. Questions for Country with Current Tracking Program.
1. What type of program(s) to track medical radiation exposures/doses exist for your country? (Please select all that apply)
|
2. How widely are the program(s) to track medical radiation procedures and/or doses to your population implemented? (Please complete (a), (b), and (c) below.)
|
3. Do the program(s) track examinations for an individual patient? (e.g. multiple CT scans to the same patient)
|
4. What are the goals of your program(s) to track medical radiation exposures/doses? Please select all that apply.
|
5. How do you plan to record data in this program?
|
6. Will data collected be transmitted to a centralized database?
|
7. Which types of examinations (modalities) are tracked? Please select all that apply.
|
8. Which of the following is tracked for an individual patient? Please select all that apply.
|
9. How useful is the program?
|
10. For what purposes are collected data used? Please select all applicable.
|
| 11. What quantities are tracked for? Please select all applicable. |
Projection radiography (x-ray, computed/digital/dental radiography)
|
Fluoroscopy
|
Computed Tomography
|
Mammography
|
Nuclear Medicine
|
| List additional measurements and associated modalities here: |
| 12. Please describe your current program(s) to track cumulative medical radiation doses, including data collection and storage strategies, organization, number and types of participating sites, budget, etc.: |
| In addition, please attach a data collection form, if available. |
3. Results
3.1. Respondents
A total of 100 responses were received, representing 76 countries. Table 4 lists the countries responding and the number of responses for the 20 countries providing more than one response. Table 4 also lists affiliations of respondents. As discussed above, there was one common response from each country after deliberations with the respondents. Thus, all results have a common denominator of 76 countries.
Table 4.
Survey results from 76 countries that participated in the survey.
| Afghanistan (RPB) | Czech Republic (2) (PS-RG, RPB) | Kyrgyzstan (RD) | Peru (RD) |
| Armenia (2) (RD, RPB) | Dem Rep of Congo (RPB) | Lebanon (2) (RPB) | Philippines (RPB) |
| Australia (RPB) | Egypt (RPB) | Lithuania (RPB) | Poland (RPB) |
| Azerbaijan (2) (MP, RD) | El Salvador (RPB | Macedonia (MP) | Portugal (RPB) |
| Bangladesh (RPB) | Estonia (2) (PS-RG, RPB) | Malaysia (4) (RPB (1), PS-RG (3)) | Qatar (MP) |
| Belgium (2) (RPB) | Ethiopia (RG) | Malta (RPB) | Russian Federation (MP) |
| Bhutan (RG) | Finland (RD) | Mauritius (RD) | Serbia (RPB) |
| Bosnia and Herzegovina (2) (MP) | Gabon (MP) | Moldova (MP) | Slovakia (MP) |
| Brazil (RG) | Georgia (MP) | Mongolia (RPB) | South Africa (PS-RG) |
| Bulgaria (RPB) | Greece (RPB) | Montenegro (MP) | Sri Lanka (RD) |
| Canada (4) (PS-RG, MP, PS-RG, RPB) | Hong Kong, China (2) (PS-RG, RD) | Nepal (MP) | Sweden (MP) |
| Chile (RD) | Hungary (2) (RPB) | Netherlands (2) (MP, PS-RG) | Syria (RPB) |
| China (2) (RD, RPB) | India (2) (PS-RG, MP) | New Zealand (2) (RG, PS-RG) | Taiwan (MP) |
| Colombia (PS-RD) | Indonesia (2) (RPB) | Nicaragua (MP) | Tajikistan (2) (RPB, RD) |
| Costa Rica (MP) | Iran (RPB) | Niger (RD) | Thailand (RPB) |
| Cote d’Ivoire (RPB) | Israel (RPB) | Norway (PS-RG) | Trinidad & Tobago (PS-RG) |
| Croatia (2) (MP, RPB) | Italy (2) (MP) | Pakistan (RPB) | Uganda (PS-RG) |
| Cuba (RPB) | Japan (MP) | Panama (RPB) | USA (PS-RD) |
| Cyprus (RPB) | Kazakhstan (2) (RPB, MP) | Paraguay (RPB) | Vietnam (RPB) |
Affiliation of the organization/person filling in the form is given in parenthesis. PS: Professional Society, RPB: Radiation Protection Body, MP: Medical Physicist, RD: Radiologist, RG: Radiographer. The numbers in parentheses wherever provided represent the number of responses by the country when it was more than one response.
3.2. Radiation Exposure Tracking Program
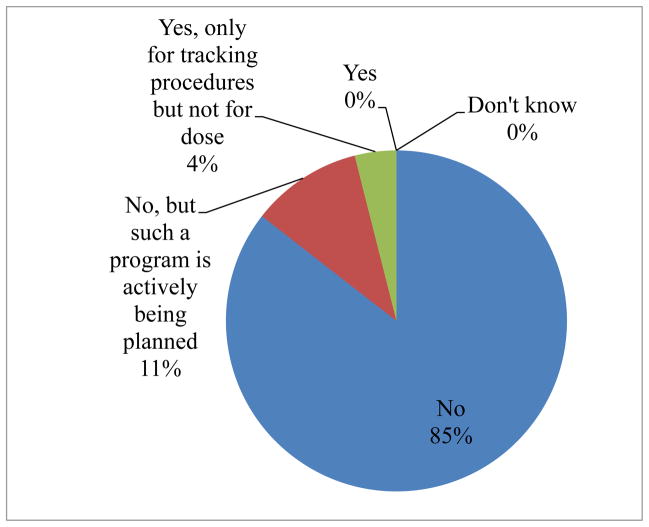
No country replied that it currently has a radiation tracking program at the national level to track medical radiation procedures. Fig. 1 shows the summary of responses in the question “Do you currently have a program to track medical radiation procedures and/or doses to your population?”. Eight respondents, one from each of 8 countries, 8/76 (10.5%) indicated that such a program is actively being planned. For 3/76 (4%) countries (Czech Republic, Israel, Malta) the answer was “Yes, only for tracking procedures but not for dose”. No country reported previously having such a program: 73/76 (96%) stating “No”, 2/76 being unsure and 1/76 had no answer. Fifty six out of 76 (74%) countries reported being aware of the IAEA’s Smart Card/SmartRadTrack project, 19/76 (25%) were unaware, 1/76 (1%) did not answer this question. Seventy five out of 76 (99%) reported being interested in learning more about the IAEA’s Smart Card/SmartRadTrack project, and the same number reported being interested in joining and developing and promoting such a program in their country.
Fig. 1.
Summary of responses to the question: “Do you currently have a program to track medical radiation procedures and/or doses to your population?”.
3.3. Usefulness of and interest in Radiation Exposure Tracking Programs
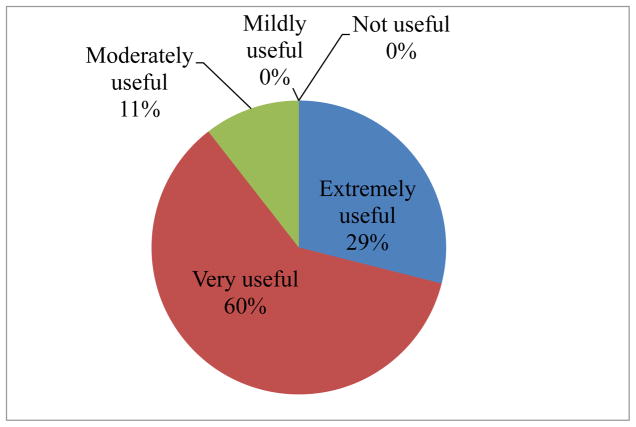
Fig. 2 shows responses about the usefulness of radiation exposure tracking programs. Twenty two out of 76 countries (29%) responded that a radiation tracking program would be “extremely useful”, 46/76 (60%) stated “very useful” and 8/76 (11%) stated “moderately useful” with none stating “mildly useful” or “not useful”. Thus, a total of 89% of countries were covered by “extremely useful” and “very useful”. Fifty nine out of 76 countries (77.6%) are interested in obtaining IAEA resources to establish such a program if eligible under IAEA norms, 12/76 (16%) reported needing no funds but desiring technical collaboration with the IAEA, and 5/76 (7%) reported no interest in IAEA resources.
Fig. 2.
Summary of responses to the question: “How useful do you think a radiation exposure tracking program would be (assuming practicalities are attended to)?”.
In the meeting held at the IAEA, Vienna, from 18 to 21 October 2010, as referred to earlier above, the following outcomes were arrived at [7]:
There was a unanimous feeling that the project is of utmost importance at this juncture;
Nationwide: Some countries have plans for a nationwide Picture Archiving and Communication System (PACS) and, thus, it appears that a number of countries shall have future capacity for nationwide access to patients’ radiological examinations at the click of a mouse.
A presentation by Finland indicated radiation exposure tracking is now a reality within 33 hospitals of the Helsinki county region, and also possible through some steps in data transfer from other parts of the country. The same is true for Sweden.
Some countries have acquired technologies to make it possible to track a patient’s exposure within a hospital (Belgium, Bosnia and Herzegovina, Czech Republic, Estonia, Israel, Malta and Poland).
No variance in response was found as compared to one sent through online survey
3.4. Planned Programs
Form 2 was completed by 8 respondents from 8 different countries (Belgium, Bulgaria, Iran, Italy, Lebanon, PR of China, Slovakia and USA), who stated that they do not have a program but such a program is actively being planned. Three of the 8 countries plan to establish a program to track medical radiation exposures/doses within the next 2 years, 3 countries within 3 to 5 years, and, for two countries, the time frame was uncertain. In two countries, the program is planned to be national, in three the planned program will be regional within one country, in two it will be institutional, and one country responded that it will be international. Six of the 8 planned programs will track examinations for an individual patient (e.g. multiple CT scans to the same patient), one will not, and one respondent responded “do not know”. In response to the question “What are the goals of your planned program(s) to track medical radiation exposures/doses?” all 8 respondents included quality assurance/quality improvement. Some also included policy development (2/8), licensure/certification/regulation (2/8), decision support for ordering examinations (3/8) and for population doses (1/8).
Out of 8, one did not answer the question “which types of examination (modalities) will be tracked?”, and one was uncertain. Of the remaining 6, all (6/6) respondents reported that computed radiography (CR), digital radiography (DR), mammography, diagnostic fluoroscopy, interventional fluoroscopy and computed tomography (CT) will be tracked, whereas some also included conventional radiography/x-ray (2/6), planar nuclear medicine (1/6), SPECT (1/7), PET (1/7) and hybrid imaging (SPECT/CT and PET/CT) (2/6). In terms of what information will be tracked for individual patients, most (6/7) respondents reported that numbers of examinations/procedures and type of examination/procedure (e.g. CT), 7/7 stated radiation dosimetric information (e.g. Dose-Length Product in mGy × cm) will be recorded; 2/7 also included information about a specific site performing an examination/procedure (e.g. at National University Hospital) and geographic information about the examination/procedure (e.g. in Northern Province).
3.5. Quantities Tracked
In terms of quantities that will be tracked, for projection radiography (x-ray, computed/digital/dental radiography), the most common choice was dose-area product (DAP)/kerma-area product (KAP) (5/7), while some also chose entrance surface air kerma (2/7), exposure index (2/7), organ absorbed and effective dose. For fluoroscopy, again, the most common choice was dose-area product (DAP)/kerma-area product (KAP) (7/7), but some participants also chose fluoroscopy time (4/7), cine time (4/7), cine runs (2/7), and/or dose/air kerma at the interventional reference point (4/7). For computed tomography, the most common choices were CT dose index (CTDIw) (2/7), volume (CTDIvol) (7/7), and dose-length product (DLP) (6/7); one respondent also chose effective dose. For mammography, three respondents only selected average glandular dose, whereas one also included incident air kerma. For nuclear medicine, most (two) selected administered activity (MBq) and organ absorbed dose; one also included effective dose, and the column was left blank by three participants which may indicate that they did not understand the question.
4. Discussion
An IAEA survey of 76 countries addressing patient radiation exposure tracking found that while no country currently has a nationwide program, that technical capabilities now exist, eight countries are planning a program and there is near-universal interest in patient exposure tracking.
This investigation is the first survey, to the best of our knowledge, of the international status of radiation tracking programs. We believe that the sample is reasonably representative of a major part of the world, since the total population of respondent nations is approximately 5 billion, and responses were received from 6 continents, all of the 6 most populous countries (Brazil, China, India, Indonesia, Pakistan and USA) as well as 16 of the 20 most populous countries.
The survey found that no national radiation tracking programs are presently in place, although technology in some countries exists, that there are 3 countries with programs tracking radiation-associated procedures, and that 8 countries are planning programs to track radiation doses. Nevertheless, the results of the survey found that there is a great desire worldwide for such programs, with 89% of countries responding that such a program would be extremely or very useful.
The increase in support for radiation tracking programs has been dramatic. Five years ago, there were few professionals willing to support such an initiative. Now, there is, as evidenced by the survey a much more pervasive interest. In part, this may be related to literature addressing multiple imaging procedures received by patients, with some studies recently demonstrating a significant number of patients receiving more than 10 CT scans or nuclear medicine procedures and thus having received a few tens of mSv of effective dose, and some patients exceeding 100 mSv [2–5]. Unfortunately errors and accidental exposures serve as another driving force and the recent escalation of interest in tracking is closely related to overexposures in CT over the last 3 years [2,8].
Thirdly, technology has advanced. Previously, PACS systems of different vendors were not sufficiently developed to cross communicate and to send dose information from the scanning device to the PACS; dose quantities were also not standardized in terms of international agreement; and the electronic health record (EHR) was still in its infancy. Numerous changes have occurred that address these barriers to dose tracking. The international code of practice of dosimetry in diagnostic radiology has been formulated in recent years [9]. This facilitates standardization of dose quantities to be used. Most vendors now provide patient dose information in the form of structured dose reports as standardized by the Integrated Health Enterprise (IHE) as a part of the radiation exposure monitoring (REM) for important imaging modalities such as CT and, to some extent, angiography in interventional imaging. The communication of structured dose reports between different vendors of PACS is currently possible.
With these technological advancements, some countries have recently reached the stage of limited tracking of radiation exposure of patients. Specifically, tracking is possible when a procedure is conducted in several dozen hospitals throughout the country which are connected by PACS, and, through slightly increased effort, when conducted in another part of the country from which data can be transferred through PACS. The adoption of EHR in Europe has advanced for practice areas such as primary care and geriatrics. In selected parts of Europe, the adoption of EHRs is nearly universal, e.g. 98% of primary care physicians in Denmark can manage their patients electronically. The European Union is undertaking further efforts, including legislation to achieve interoperability of health information systems [10–12].
This study has several strengths, including being the first such survey; its worldwide scope; a high response rate from radiation protection authorities representing the majority of the world’s population; and its obtaining of information pertaining to programs, needs, and quantities tracked. Nevertheless, there are several potential limitations. One limitation, as with any such survey, is that responses reflect the views and knowledge of the individual respondent completing the form, and thus reflect the national situation in a country only insofar as the respondent is aware of all activity undergoing in that country. Sending the survey questionnaire only to organizations for such an unfamiliar topic is counterproductive. While the IAEA’s extensive network of contacts was utilized to target individuals likely to serve as the best providers of information on dose tracking in their countries, inevitably some programs can be expected to have been missed, and individuals’ ideas relating to the need for tracking programs may not be generalizable throughout a country’s medical, radiation protection, and regulatory communities. Moreover, no single individual can reflect the entire repository of knowledge related to radiological protection in a country. For example, there were two non-respondents (25 %) to the question “What quantities will be tracked for projection radiography, x-ray, computed/digital/dental radiography?”, suggesting lack of awareness of some respondents on the appropriate dose quantities for a defined modality; nevertheless, other individuals within their countries with more specialized knowledge might be better able to address the quantities to be tracked. National radiation regulatory authorities may not be aware of the extent of coverage of the PACS systems in their country, while radiology department chairs may not be informed what dose quantities are planned to be collected on a national level and what mechanisms are available to track an individual patient’s history.
Nevertheless, the study’s approach was to reach out to knowledgeable and resourceful individuals in each country whom it was thought would be able to best compile and assess information related to their national state of radiation protection. We believe that this approach, together with the use of additional follow-up mechanisms wherever necessary, as described in the materials and methods section above, provides the best means of surveying the worldwide status of radiation dose tracking. Except for items relating to the choice of radiation dose quantity, which was queried of those who are planning to start a tracking program, there were no major problems with survey completion, and the branching response structure of the questionnaire required only nine simple questions to be answered by the vast majority of respondents who do not have a tracking program and who are not planning one.
5. Conclusions
This paper represents the first survey covering 76 countries on the international status of patient exposure tracking, and demonstrates great interest in a relatively underdeveloped program. The current situation makes this possible within a few dozen hospitals covered by PACS systems; and that there are nascent efforts to extend coverage to nationwide. These findings can serve as a benchmark and stimulus for future radiation exposure and dose recording efforts, which offer the potential to create a data-rich environment enabling maximal global implementation of the radiation protection principles of justification, optimization, and dose reference levels.
Acknowledgments
The authors acknowledge the extra budgetary assistance provided by the United States Government for the IAEA Smart Card project. A number of participants in the meeting organized by the IAEA for this project have contributed in finalizing the survey forms and their contribution is gratefully acknowledged.
Role of the Funding Source: The funding source had no influence in planning, conduct and reporting of the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Madan M. Rehani, Email: madan.rehani@gmail.com, International Atomic Energy Agency, Radiation Protection of Patients Unit, Vienna International Centre, PO Box 200, A-1400 Vienna, Austria, Tel: +43-1-2600-22733, Fax: +43-1-26007-22733
Donald P. Frush, Email: donald.frush@duke.edu, Department of Radiology, Duke University Medical Center, Children’s Health Center, PO Box 3808 DUMC, Durham, NC 27710, USA, Tel: +1-919-6847343, Fax: +1-919-6847151
Theocharis Berris, Email: theocharisberris@yahoo.com, International Atomic Energy Agency, Radiation Protection of Patients Unit, Vienna International Centre, PO Box 200, A-1400 Vienna, Austria, Tel: +43-1-2600-21268, Fax: +43-1-26007-21268.
Andrew J. Einstein, Email: andrew.einstein@columbia.edu, Columbia University Medical Center, 622W 168th St, PH 10-408, New York, NY 10032, USA Tel: +1-212-3056812 and +1-212-3054275, Fax: +1-212-3054648
References
- 1.UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) UNSCEAR 2008 Report. I. New York: United Nations; 2010. Report to the General Assembly Scientific Annexes A and B. [Google Scholar]
- 2.Rehani M, Frush D. Patient exposure tracking: the IAEA smart card project. Radiat Prot Dosim. 2011 doi: 10.1093/rpd/ncr300. Advance Access published July 20, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175–84. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 4.Einstein AJ, Weiner SD, Bernheim A, Kulon M, Bokhari S, Johnson LL, Moses JW, Balter S. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA. 2010;304:2137–44. doi: 10.1001/jama.2010.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehani M, Frush D. Tracking radiation exposure of patients. Lancet. 2010;376(9743):754–755. doi: 10.1016/S0140-6736(10)60657-5. [DOI] [PubMed] [Google Scholar]
- 6.Rehani MM. Smart protection. [Accessed March 31, 2012];IAEA Bulletin. 2009 50(2):31–3. http://www.iaea.org/Publications/Magazines/Bulletin/Bull502/50205813137.html. [Google Scholar]
- 7.Report of the Technical Meeting of the IAEA Smart Card/SmartRadTrack Project; 18–21 October 2010; VIC, Vienna, Austria. [Accessed 20 October 2011]. https://rpop.iaea.org/RPOP/RPoP/Content/News/report-SmartRadTrack-project.htm. [Google Scholar]
- 8.New era in CT scanning. [Accessed March 31, 2012];IAEA Radiation Protection of Patients. website. http://rpop.iaea.org/RPOP/RPoP/Content/News/new-era-ct-scanning.htm.
- 9.International Atomic Energy Agency (IAEA) Technical Reports Series. Vol. 457. Vienna, Austria: IAEA; 2007. Dosimetry in Diagnostic Radiology: An International Code of Practice. [Google Scholar]
- 10.Buddrus U. [Accessed March 31, 2012];EMR Adoption in Europe. http://www.worldofhealthit.org/education/documents/UWE_Buddrus.pdf.
- 11.DIRECTIVE 2011/24/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 9 March 2011 on the application of patients’ rights in cross-border healthcare. [Accessed March 31, 2012]; http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:088:0045:0065:EN:PDF.
- 12.Kierkegaard P. Electronic health record: Wiring Europe’s healthcare. Computer Law & Security Review. 2011;27(5):503–15. [Google Scholar]