Abstract
Excessive fear is a hallmark of several emotional and mental disorders such as phobias and panic disorders. Considerable attention is focused on defining the neurobiological mechanisms of the extinction of conditioned fear memory in an effort to identify mechanisms that may hold clinical significance for remediating aberrant fear memory. Serotonin modulates the acquisition and retention of conditioned emotional memory, and the serotonin 2A receptor (5HT2AR) may be one of the postsynaptic targets mediating such effects. Here we tested the hypothesis that the 5HT2AR regulates the consolidation and extinction of fear memory in male C57BL/6J mice. The influence of 5HT2ARs on memory consolidation was further confirmed with a novel object recognition task. With a trace fear conditioning paradigm, administration of the 5HT2AR agonist TCB-2 (1.0 mg/kg, i.p.) before the extinction test facilitated the acquisition of extinction of fear memory as compared to vehicle treatment. In contrast, administration of the 5HT2AR antagonist MDL 11,939 (0.5 mg/kg, i.p.) delayed the acquisition of extinction of fear memory. Further, the post-conditioning administration of TCB-2 enhanced contextual and cued fear memory, possibly by facilitating the consolidation of fear memory. Administration of TCB-2 also facilitated the acquisition of extinction of fear memory in delay fear conditioned mice. Stimulation or blockade of 5HT2ARs did not affect the encoding or retrieval of conditioned fear memory. Finally, administration of TCB-2 right after training in an object recognition task enhanced the consolidation of object memory. These results suggest that stimulation of 5HT2ARs facilitates the consolidation and extinction of trace and delay cued fear memory and the consolidation of object memory. Blocking the 5HT2AR impairs the acquisition of fear memory extinction. The results support the view that serotonergic activation of the 5HT2AR provides an important modulatory influence on circuits engaged during extinction learning. Taken together these results suggest that the 5HT2AR may be a potential therapeutic target for enhancing hippocampal and amygdala-dependent memory.
Introduction
Brain serotonin (5-HT) originates from the raphe nuclei and modulates behaviors, emotion, mood, and cognition (Jacobs and Azmitia, 1992). Altered serotonergic neurotransmission is observed in the pathophysiology of memory impairments, depression, anxiety and post-traumatic stress disorders (PTSD). 5-HT has been demonstrated to enhance learning and memory in both invertebrate and vertebrate model systems since 1960s. (Dai et al., 2008; Kandel, 2001; Khaliq et al., 2006; Levkovitz et al., 2003; Porter et al., 2003). Fear memory, due to its intimate relation to clinical mental trauma, has been an issue of considerable attention recently. Alleviation of fear memory associated with emotional disorders (e.g., phobia, PTSD) may help patients resume a normal life and decrease medical burden. However, the mechanisms underlying fear memory extinction remain elusive. 5-HT modulates contextual fear memory in mice (Dai et al., 2008). To date, at least 14 5-HT receptors belonging to 7 families have been reported (Nichols and Nichols, 2008) and the 5-HT2A receptor (5HT2AR) may be one of the targets by which 5-HT modulates fear memory.
Even though the 5HT2AR is assumed to be a brain target for hallucinogenic drugs, a number of studies suggest that 5HT2ARs modulate learning and memory. Central 5-HT2ARs are highly enriched in brain areas participating in learning and memory processes, such as the medial prefrontal cortex (mPFC), hippocampus and lateral amygdala (LA) (Cornea-Hebert et al., 1999). Reduced binding capacity of 5HT2ARs has been observed in normal aging subjects (Meltzer et al., 1998b) and AD patients (Marner et al., 2012; Meltzer et al., 1998a). Functionally, activation of 5HT2ARs enhances cortical presynaptic glutamate release (Ciranna, 2006; Hasuo et al., 2002), NMDA receptor sensitivity (Arvanov et al., 1999), and conditioned avoidance memory in rodents (Alhaider et al., 1993). Blockade of 5HT2ARs attenuates cue-elicited and cocaine-primed memory reinstatement (Pockros et al., 2011). Humans that carry a polymorphism in the 5HT2AR gene exhibit impaired novelty-induced activation of the hippocampus and impaired consolidation of explicit memory (Schott et al., 2011; Wagner et al., 2008). Although a number of studies suggest that 5HT2ARs influence learning and memory (Meneses, 2007a, b), there has been little if any effort to specifically examine the influence of 5HT2ARs on fear memory processes.
Pavlovian fear conditioning is one of the most well characterized paradigms to assess fear memory. In it, a neutral stimulus (e.g., tone, context, light) becomes a conditional stimulus (CS) after it is repeatedly paired with an aversive unconditional stimulus (US, i.e., foot shock) (Phillips and LeDoux, 1992). Defensive freezing, all movement ceasing except for respiration, is a conditioned response to a CS during test for recall and has been used as a reliable measure for rodent fear memory (Blanchard et al., 2001; Blanchard and Blanchard, 1969). In a contextual and delay cued fear conditioning paradigm, the rodent is presented with repeated pairings of a tone CS that co-terminates with a brief foot shock US in a distinct context. The LA is critical for associating the tone CS with the foot shock, and the hippocampal-dependent context memory with the aversive foot shock US stimulus (Kim and Fanselow, 1992; Phillips and LeDoux, 1992). In trace fear conditioning, a temporal gap is imposed between the termination of a tone CS and the onset of an aversive stimulus (US). An appropriately timed conditioned freezing response, which develops progressively over the course of the repeated CS-US pairings during the conditioning session, is a declarative memory dependent on hippocampal function in rodents and humans (Clark and Squire, 1998; McEchron et al., 1998). The present study examined the influence of 5HT2AR-sensitive drugs on fear memory processes, especially extinction, with delay and trace fear conditioning paradigms.
Here, we hypothesized that 5HT2ARs influence fear memory consolidation and extinction. The influence of 5HT2ARs on memory consolidation was further confirmed with a novel object recognition task. Our results indicate that activation of 5HT2ARs facilitates the extinction of cued fear memory after delay and trace fear conditioning paradigms, and the consolidation of contextual fear memory and object memory.
2. Materials and Methods
2.1. Animals
Adult male C57BL/6J mice (8–12 weeks) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in groups of 4 per standard polycarbonate cage with ad libitum access to food and water. Mouse cages were maintained in a temperature- and humidity-controlled colony room with a 12-h/12-h light/dark cycle with lights on at 7:00 AM. Bedding changes occurred 1/week on a day that was convenient to experimental protocol (i.e., bedding was changed before behavioral training was initiated or was delayed until completion of the experiment). All behavioral testing took place during the light phase from 12:00 PM to 5:00 PM. All procedures were conducted in accordance with the guidelines as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Florida Atlantic University.
2.2. Apparatus
Fear conditioning was performed with the MED Associates Near-Infrared Video Fear Conditioning System (Georgia, VT) comprised of four identical rectangular chambers (30.5 cm by 24.1 cm by 21 cm) constructed of brushed aluminum side walls and clear Plexiglas front, back and top walls. An overhead white house light and an infrared light illuminated each chamber, and a speaker attached to the right side wall of each chamber delivered the tone stimuli. The chamber floor was constructed of parallel stainless steel rods (36 rods, 3.2 mm dia, 7.9 mm apart) designed for mice, and connected to a scrambled shock generator. Each chamber was housed inside a larger sound-attenuating cabinet which had a ventilation fan in the right side wall used to provide background noise. A near-infrared FireWire video camera was mounted on the left front door of each noise-attenuating cabinet to acquire mouse behavior. Before each trial, the chamber floors were cleaned thoroughly with a 10% ethanol solution then with 1% LiquiNox (White Plains, New York) to remove olfactory cues. Freezing, a rodent’s natural response to fear (LeDoux, 1993), was recorded and automatically scored to assess the strength of fear memory. The automatic software program operationally defined freezing episodes as the duration of time, greater than 0.6 s, during which less than 20 pixels of each video frame were detected to have changed. The video capture rate was set at 30 fps which resulted in freezing being recorded computationally after less than 20 pixels of motion per frame over the time course of 18 frames.
2.3. Habituation
Procedures began at least 1 week after arrival of mice into the vivarium. On the first day of behavioral testing (day 1) each mouse was habituated to the holding room for at least 1 h; on days 2 and 3, each mouse was placed individually in a clean, empty polycarbonate holding cage for at least a 10-min period then returned to its home cage. On days 4 and 5, each mouse received a sham injection in which a 27-gauge needle penetrated the abdominal skin but no fluid was injected (day 4) and then an i.p. injection of 0.9% saline (day 5) before being placed in the polycarbonate cage for 20 min.
2.4. Fear conditioning and extinction
2.4.1. Trace fear conditioning and extinction
The present procedure was modified from that of Weitemier and Ryabinin (2003). On the first training day (day 6), mice were placed individually into clean empty polycarbonate holding cages. Twenty min later each mouse was allowed to freely explore a conditioning chamber during a 10 min Context A pre-exposure session. On the following day, after 20 min in the individual holding cages, each mouse was returned to Context A. After a 60-s exploration interval in Context A to establish baseline freezing, a tone (90 dB, 5000 Hz, CS) was presented for 15 s followed by a 30 s stimulus-free interval, and then a 0.5-s, 0.75 mA foot shock (US) occurred. The CS-US pairing was repeated eight times with a 210-s inter trial interval (ITI). Mice were removed from the conditioning chamber and returned to their home cages 60 s after the final CS-US pairing. Twenty-four h later each mouse was tested for freezing to the CS tone in a modified chamber (Context B). The Context B chamber consisted of a white Plexiglas floor, a black Plexiglas triangular insert and several drops of 10% acetic acid on the tray under the floor. Thus, Context B provided an altered floor texture, light intensity, chamber geometry, and odor. Before each trial, the floors and inserts were cleaned with 10% ethanol to remove olfactory cues. Sixty seconds after the introduction of a mouse into Context B, 8 unpaired 15-s tone CS were presented with a ITI of 210 s (see Figure 1C). Forty-eight h+ after the conditioning session, each mouse was returned to Context A chamber for a 5-min context test, but no foot shock or tone stimuli were presented. Freezing, a rodent’s natural response to fear (LeDoux, 1993), was recorded and automatically scored by the Video Freezing software (MED Associates, Georgia, VT). Mice received saline or drug injection right after the trace fear conditioning session or before the tone test to examine 5HT2AR’s effects on memory consolidation and extinction, respectively.
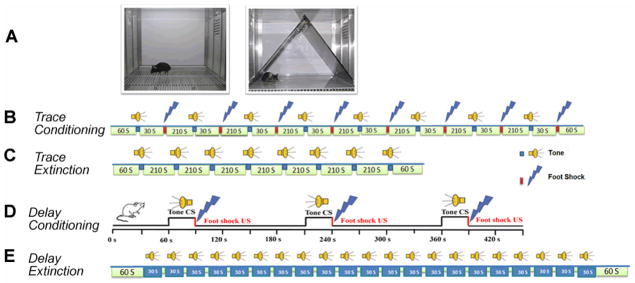
Figure 1.
Experimental design for trace and delay fear conditioning. A. Left, a chamber for fear conditioning (Context A) and contextual fear memory test; right, Context B, a modified chamber with different color, context, lighting, and odor for cued fear memory test. B. Trace fear conditioning training procedure. After 60 s in Context A, a tone was presented for 15 s followed by 30 s empty interval, and then a 0.5-s, 0.75 mA foot shock (US) occurred. The CS-US pairing was repeated eight times with an ITI of 210 s. Mice were removed from the conditioning chamber and returned to their home cages 60 s after the final CS-US pairing. C. Trace fear conditioning extinction procedure. After 60 s in Context B, 8 unpaired 15-s tone CS’s were presented with an ITI of 120 s. D. Delay fear conditioning training procedure. After 60 s in Context A, a tone was presented for 30 s (75 dB, 5000 Hz), which co-terminated with a 1-s, 0.5 mA foot shock (US). The CS-US pairing was repeated twice more. Mice were removed from the conditioning chamber and returned to their home cages 60 s after the final CS-US pairing. E. Delay fear conditioning extinction procedure. Mice were placed into Context B and after 60-s habituation, mice received 20 unpaired tone (30 s) stimuli at an ITI of 5 s and then were returned to their home cages 60 s after the last tone.
2.4.2. Delay fear conditioning and extinction
One day after habituation, naïve mice were placed individually into clean empty polycarbonate holding cages. Twenty min later each mouse was allowed to freely explore the Context A chamber during a 5-min context pre-exposure session. On the following day, after 20 min in the individual holding cages, each mouse was returned to Context A for a 3 CS-US conditioning session. Briefly, after a 60-s exploration interval to establish baseline freezing, a tone (90 dB, 5000 Hz, CS) was presented for 30 s, which co-terminated with a 1-s, 0.5 mA foot shock (US). The CS-US pairing was repeated twice more. Mice were removed from the conditioning chamber and returned to their home cages 60 s after the last CS-US pairing. During the extinction test presented 24 h later, mice were placed into Context B with distinct light density, texture, color and odor. After 60 s in Context B, each mouse received 20 unpaired tone (30 s) CS stimuli with ITI of 5 s and then were returned to their home cages 60 s after the last CS (see Figure 1E).
A second cohort of naïve mice received the same pre exposure and fear conditioning in Context A. Twenty-four h after the conditioning session, each mouse was returned to Context A for a 5-min contextual test, but no foot shock or tone stimuli were presented. At least 2 h later each mouse was placed in Context B (as described above), and 60 s later was presented with a 30-s tone CS and freezing behavior was measured. Data from mice that showed robust (greater than 20%) freezing during the first 60 s of the tone test (pre-tone) were excluded from the analysis due to generalized freezing rather than cued freezing. For conditioning and the tone test sessions, tone-elicited freezing was defined as freezing episodes that occurred during the 30 s of the tone and the 30 s interval following the tone. To determine whether contextual and cued fear conditioning were sensitive to pharmacological manipulation of 5HT2ARs, mice received TCB-2 or MDL 11,939 treatment before the 3 CS-US conditioning session, or before the context test session to investigate fear memory encoding and retrieval, respectively.
2.5. Novel object memory
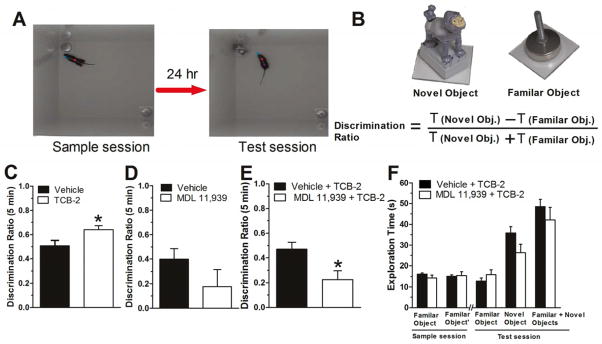
A spontaneous non-spatial novel object recognition (NOR) paradigm was used to test the influence of 5HT2ARs on the consolidation of object memory. The NOR procedure used here was modified from that used in our recent publication (Vick et al., 2010). Specifically, the amount of sample session object exploration or training was limited to 15 s for each object or 19 s for either objects within 5 min, compared to the standard training of 30 s for each object or 38 s for either objects within 10 min in Vick et al. (2010). Mice permitted 30 s of sample object exploration exhibit strong or near ceiling level of novel object preference, leaving little opportunity to identify memory-enhancing effects of TCB-2. Limited sample object exploration for control-treated mice results in weak novel object preference during the test session presented 24 h later (Stackman et al., 2002). Thus, this limited sample session exploration protocol provides an opportunity to examine promnestic effects of drugs on object memory. Briefly, each mouse was habituated to the high-walled white arena (37.5 cm × 37.5 cm × 50.8 cm high) 10 min/day for 2 days. Twenty-four h after the last habituation session, each mouse was returned to the arena, which now contained two identical objects (metal leveling feet) positioned in opposite corners. During this sample session, each mouse was permitted a maximum of 5 min to accumulate 19 s of exploration of either sample object or total of 15 s exploration of both sample objects. Once the mouse reached the object exploration criteria, the trial ended and the mouse was immediately removed from the arena. During the test session presented 24 h later, the mouse was re introduced into the same arena in which one of the familiar objects was replaced with a novel one (plastic toy gorilla, see Figure 6). The mouse was removed from the arena after 5 min had elapsed. Object memory was inferred from the discrimination ratio, calculated for each mouse by subtracting the time spent exploring the familiar object from the time spent exploring the novel object and dividing by the total time spent on exploring both objects. Discrimination ratio scores can range from -1 to 1, with 0 indicating equal or chance exploration levels. Positive discrimination ratio scores demonstrate novel object preference and indicate a successful recognition of the familiar object. 5HT2AR drugs were injected right after the sample session to test effects on the consolidation of object memory.
Figure 6.
Activation of 5HT2ARs enhances the consolidation of object memory. A. Experimental strategy. Left, during the sample session, mice were allowed to explore two identical objects each for at least 15 s or either for 19 s within 5 min. Middle, during the test session, one of the objects was replaced with a novel one and mice were reintroduced to the arena. B. The objects used in this study and the method for calculating the discrimination ratio. These objects elicit equivalent degrees of object exploration by naïve mice (Stackman et al., 2002). C. Mice that received TCB-2 right after the sample session showed a significantly higher novel object discrimination ratio as compared to saline group (t(21) = −2.125, P = 0.046). D. Mice that received the selective 5HT2AR blocker MDL 11,939 alone exhibited a slightly decreased discrimination ratio compared to vehicle-treated mice. E. In another cohort of naïve mice, MDL 11,939 was administered right after the sample session and TCB-2 was injected 10 min later (n = 9). Compared to the control group (n = 9), MDL 11,939 inhibited the enhancement in memory consolidation induced by TCB-2 (t(16) = 2.684, P = 0.016). F. The object exploration times of mice during sample session and test session for novel and familiar objects. Data are expressed as mean ± SEM. *, P < 0.05.
2.6. Data analysis
Data were expressed as mean ± SEM. One-way ANOVA, Student’s t-tests, and post-hoc LSD multiple comparisons tests were used to compare the total percent freezing during the pre-exposure and context tests after fear conditioning, and the discrimination ratio of object memory. Two-way repeated measures ANOVA and post-hoc LSD test were used to compare percent freezing scores during the fear conditioning sessions, cued fear memory tone tests and during the tests of fear memory extinction. Significance was set at P < 0.05.
3. Results
3.1. Systemic activation and blockade of 5HT2ARs on the consolidation and extinction of trace fear memory
The modulation of 5HT2ARs on the consolidation and extinction of fear memory was first examined with a trace fear conditioning paradigm. Each mouse received two injections, one immediately after the fear conditioning session and one before the extinction test, to examine fear memory consolidation and extinction, respectively. There was no difference on Day 1 in total percent freezing during the Context A pre-exposure session across the three groups of mice of the TCB-2 study (Vehicle + Vehicle, TCB-2 + Vehicle, and Vehicle + TCB-2), one-way ANOVA F(2,27)=0.010, P = 0.990. For the trace conditioning session 24 h later, mice were placed into Context A and after 60 s received eight pairings of the CS (white noise, 15 s) followed by a trace period (30 s) and then the US (foot shock, 0.5 s) with an intertrial interval (ITI) of 210 s (see Figure 1B). The freezing scores during the eight ITI ((ITI 1, 107.5–315.5 s; ITI 2, 362–571s; ITI 3, 617.5–826.5 s; ITI 4, 873–1082 s; ITI 5, 1128.5–1337.5 s; ITI 6, 1384–1593 s; ITI 7, 1639.5–1848.5 s; ITI 8, 1896–1954s) were compared with a two-way repeated ANOVA. The analysis yielded a significant effect of ITI (F(7,189) = 20.969, P < 0.001), but a non significant main effect of treatment and a non-significant treatment x ITI interaction effect.
There was no difference in total percent freezing during context pre exposure in the three groups of mice in the MDL 11,939 study (Vehicle + Vehicle, MDL 11,939 + Vehicle, and Vehicle + MDL 11,939) F(2,19) = 1.104, P = 0.352. During the trace conditioning session 24 h later, mice received 8 CS-US paired trace fear conditioning training as described above. The two-way repeated ANOVA on freezing scores across the 8 ITIs yielded a significant ITI effect (F(7,175) = 23.282, P < 0.001), but no significant effect of treatment or treatment x ITI interaction.
3.1.1. Systemic activation and blockade of 5HT2ARs on the consolidation of trace fear memory
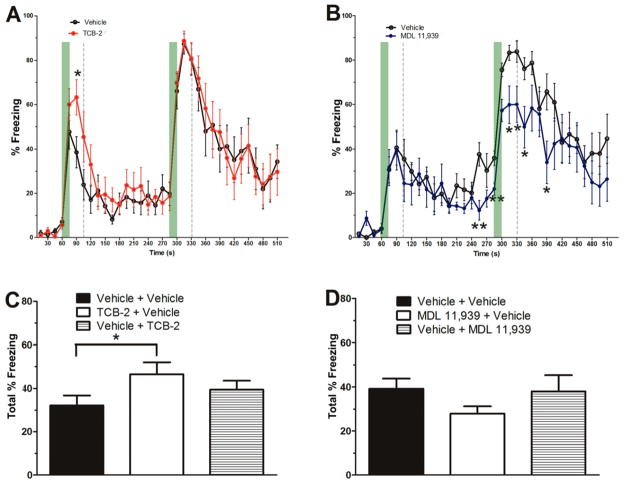
The influence of activation or blockade of 5HT2ARs on the consolidation of trace cued fear memory and of contextual fear memory was examined by administering the 5HT2AR drugs right after trace fear conditioning (see Figure 2). Percent freezing scores in response to the 1st CS stimulus (ITI 1: 79–90 s) were analyzed to measure the strength of the tone fear memory. TCB-2-treated mice had a higher percent freezing score compared to vehicle-treated mice (t(18) = −2.336, P = 0.031; see Figure 2A). In contrast, MDL 11,939 mice exhibited a lower percent freezing score compared to vehicle-treated mice (see Figure 2B) in several epochs during the first 2 CS presentations (241–255 s, t(17)=3.637, P = 0.002; 271–285 s, t(17) = 3.184, P = 0.005; 301–315 s, t(17) = 2.676, P = 0.016; 316–330 s, t(17) = 2.580, P = 0.019; 331–345 s, t(17) = 2.555, P = 0.020; 376–390, t(17) = 3.552, P = 0.021). These data indicate that the 5HT2AR modulates the consolidation of cued fear memory.
Figure 2.
Activation or blockade of 5HT2AR modulates the consolidation of cued trace fear memory and contextual fear memory. A. Mice that received TCB-2 (1.0 mg/kg, i.p., n = 10) right after the trace fear conditioning exhibited significantly higher percent freezing scores as compared to vehicle-treated mice (n = 10). B. Mice that received MDL 11,939 (0.5 mg/kg, i.p., n = 9) right after the trace fear conditioning exhibited significantly lower percent freezing scores as compared to vehicle-treated mice (n = 10). C. Mice that received TCB-2 (n = 10) right after the trace fear conditioning session exhibited significantly higher total percent freezing scores as compared to vehicle-treated mice in the contextual fear memory test. D. Post-conditioning MDL 11,939 did not significantly influence contextual fear memory consolidation. However, mice that received MDL 11,939 (n = 9) right after the trace fear conditioning session exhibited a trend toward lower total percent freezing scores as compared to vehicle-treated mice in the contextual fear memory test. Data are expressed as mean ± SEM. *, P < 0.05.
The strength of the contextual fear memory was tested by returning the mice to Context A for a 5-min test session 48 h after trace fear conditioning. Mice that received post-conditioning TCB-2 displayed significant higher total percent freezing in Context A as compared to the postconditioning vehicle mice (see Figure 2C, TCB-2 + Vehicle vs. Vehicle + Vehicle, P = 0.044). Pre-test TCB-2-treated mice exhibited freezing during the context test that was comparable to that of pre-test vehicle-treated mice (Vehicle + TCB-2 vs. Vehicle + Vehicle, P = 0.295). Mice that received post-conditioning MDL 11,939 exhibited a tendency towards decreased total percent freezing during the context test compared to post-conditioning vehicle-treated mice (Vehicle + MDL 11,939 vs. Vehicle + Vehicle, P = 0.077, one-way ANOVA; Figure 2D). These results suggest that stimulation of 5HT2ARs enhances the consolidation of contextual fear memory.
3.1.2. Systemic activation and blockade of 5HT2ARs on the extinction of trace fear memory
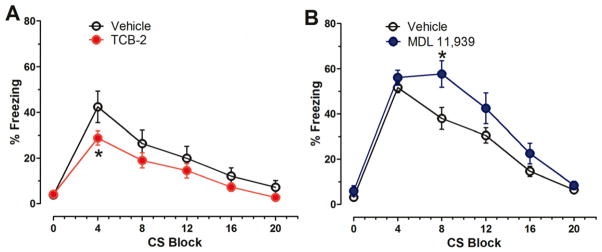
The influence of pharmacological manipulation of 5HT2ARs on the acquisition of extinction of trace fear memory was tested by administering TCB-2 or MDL 11,939 before the extinction test and then examining percent freezing during the 1st through the 8th presentation of the nonreinforced CS stimuli in Context B. Mice that received pre-test TCB-2 exhibited significantly decreased percent freezing in response to the 2nd through 8th CS stimuli compared to the vehicle-treated control mice (see Figure 3A). A two-way repeated measures ANOVA on percent freezing scores yielded a significant effect of treatment (F(1,18) = 29.449, P < 0.001), of ITI (F(7,126) = 3.847, P = 0.001) and the treatment × time interaction (F(7,126) = 3.217, P = 0.004). Given the significant interaction, further analysis of each ITI revealed significant treatment effects at ITI 2, 301–510 s, treatment, F(1,18) = 26.565, P < 0.001; ITI 3, 526–735 s, treatment, F(1,18) = 24.254, P < 0.001; ITI 4, 751–960 s, treatment, F(1,18) = 8.110, P = 0.011, ITI 5, 976–1185 s, treatment, F(1,18) = 13.623, P = 0.002; ITI 6, 1201–1410 s, treatment, F(1,18) = 13.062, P = 0.002; ITI 7,1426–1635 s, treatment, F(1,18) = 18.319, P < 0.001; and ITI 8,1651–1710 s, treatment, F(1,18) = 13.855, P = 0.002). These results indicate that stimulation of 5HT2ARs facilitates the acquisition of extinction of cued fear memory.
Figure 3.
Stimulation of 5HT2ARs enhances the acquisition of extinction of cued trace fear memory. A. Mice that received TCB-2 (1.0 mg/kg, i.p., n = 10) before the trace fear memory extinction test exhibited accelerated acquisition of extinction as indicated significantly lower freezing scores earlier in the course of extinction as compared to vehicle-treated mice (n = 10). TCB-2 significantly decreased percent freezing from 2nd to eighth ITIs (treatment P < 0.05, two-way repeated measures). B. In contrast, the MDL 11,939-treated mice (n = 9) exhibited significantly higher percent freezing during a 15 s epoch during ITI 1 (181–195 s, t(17) = −2.758, P = 0.013) as compared to vehicle-treated mice (n = 10), but the 5HT2AR antagonist did not affect the acquisition of extinction of trace fear memory. Data are expressed as mean ± SEM. *, P < 0.05.
Mice that received pre-test MDL 11,938 exhibited significantly higher percent freezing scores during the ITI after the first CS stimulus, but the percent freezing scores of MDL 11,939-treated mice during the 2nd through 8th CS stimuli were not significantly different from the pre-test vehicle-treated mice (see Figure 3B). Taken together these results indicate that activation of 5HT2ARs by TCB-2 facilitates the acquisition of extinction of trace cued fear memory, but the antagonism of 5HT2ARs did not influence extinction.
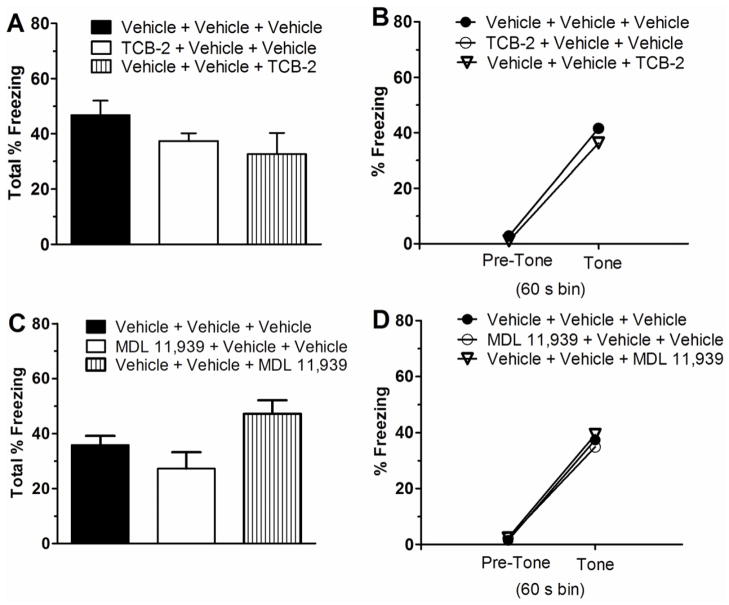
3.2. Systemic activation and blockade of 5HT2ARs on the encoding and retrieval of contextual and cued fear memory
Influence of pharmacological manipulation of 5HT2ARs on the encoding and retrieval of the fear memory was also tested. The effects of systemic TCB-2 (1.0 mg/kg) on contextual and cued fear memory were examined with a 3 CS-US pairings delay fear conditioning protocol in a separate cohort of naïve C57BL/6J mice. All mice received a 5-min context pre-exposure session in Context A on the first day. There was no significant effect of treatment group on total percent freezing scores during pre-exposure (F(2, 28) = 0.875, P = 0.429, one-way ANOVA). Twenty-four h later, each mouse was returned to Context A where it received 3 CS-US pairings during the conditioning session. TCB-2 or vehicle was administered 20 min before fear conditioning or 20 min before the context test session to investigate fear memory encoding and retrieval, respectively. A two-way ANOVA on percent freezing scores, with treatment as a between-subjects variable and CS-US pairing as a repeated-measures variable revealed a significant effect of CS-US pairing (F(3, 78) = 105.004, P < 0.001), but non-significant main effect of treatment (F(2, 26) = 0.153, P = 0.859 < 1, n.s.), or treatment × CS-US pairing interaction (F(6, 78) = 1.027, P = 0.414, n.s., figure not shown). Overall, these data indicate that there was no significant influence of systemic TCB-2 on the development of conditioned freezing over the course of the 3 CS-US delay conditioning session. All mice displayed conditioned fear to the context when tested 24 h after conditioning, expressed as an increase in total percent freezing relative to the pre-exposure session (see Figure 4A), and there was no difference between the vehicle- and TCB-2-treated groups (F(2, 28) = 1.622, P = 0.217). There were also no treatment differences in tone-elicited freezing during the tone test (see Figure 4B). A two-way repeated ANOVA indicated a significant effect of time bin (F(1, 26) = 147.179, P < 0.001), but non-significant effects of treatment and treatment × time bin interaction (both F’s (3, 36) < 1, n.s.). These findings indicate that TCB-2 did not affect contextual or cued fear memory encoding and retrieval after a 3 CS-US pairings protocol.
Figure 4.
Activation of 5HT2ARs with systemic TCB-2 (1.0 mg/kg) or blockade of 5HT2ARs with systemic MDL 11,939 (0.5 mg/kg) did not affect the encoding and retrieval of contextual and cued fear memory. Compared to the vehicle group (n = 10), naïve male C57BL/6J mice that received TCB-2 before contextual and cued fear conditioning (n = 9), or before the context test session (n = 10) exhibited equivalent freezing during the context test (A) and during the tone test (B). Mice that received vehicle or MDL 11,939 before conditioning (n = 9) or before the test session (n = 9) also exhibited equivalent freezing to the vehicle mice (n = 9) during the context test (C) and the cue test (D). Data are expressed as mean ± SEM.
The influence of 5HT2AR blockade with MDL 11,939 (0.5 mg/kg) on fear memory was also tested with the same 3 CS-US pairings protocol in 4 groups of mice. There was no significant treatment group effect on pre-exposure total percent freezing scores (F(2, 26) = 0.460, P = 0.637, one-way ANOVA). Twenty-four h later, mice were returned to Context A and received 3 CS-US pairings during the conditioning session. MDL 11,939 or vehicle was administered 20 min before fear conditioning or 20 min before the context test session to investigate memory encoding and retrieval, respectively. A two-way repeated measures ANOVA on percent freezing data revealed a significant effect of CS-US pairing (F(3, 72) = 68.912, P < 0.001), but no significant main effect of treatment (F(2, 24) = 2.879, P = 0.076) or treatment × CS-US pairing interaction effect (F(6, 72) = 0.843, P = 0.541, figure not shown). All mice exhibited conditioned fear to Context A when tested 24 h after conditioning, expressed as an increase in percent freezing relative to the pre-exposure session (see Figure 4C). The one-way ANOVA on total percent freezing scores yielded a significant effect of treatment (F(2, 24) = 4.248, P = 0.026). However, the post hoc LSD multiple comparisons revealed that mice administered MDL 11, 939 before fear conditioning were significantly different from the mice that received MDL before context testing, but neither MDL 11,939 group was significantly different from the control group (P > 0.05). There were also no significant treatment differences in tone-elicited freezing during the tone test (see Figure 4D). A repeated-measures ANOVA revealed a significant effect of time bin (pre-tone vs. tone) on freezing (F(1, 24) = 107.290, P < 0.001), but no significant effect of treatment or treatment × time bin interaction (both F’s(3, 32) < 1, n.s.). These findings indicate that administration of MDL 11,939 to C57BL/6J mice does not affect encoding or retrieval of contextual or cued fear memory with a 3 CS-US pairings protocol.
3.3. Systemic activation and blockade of 5HT2ARs on the extinction of delay cued fear memory
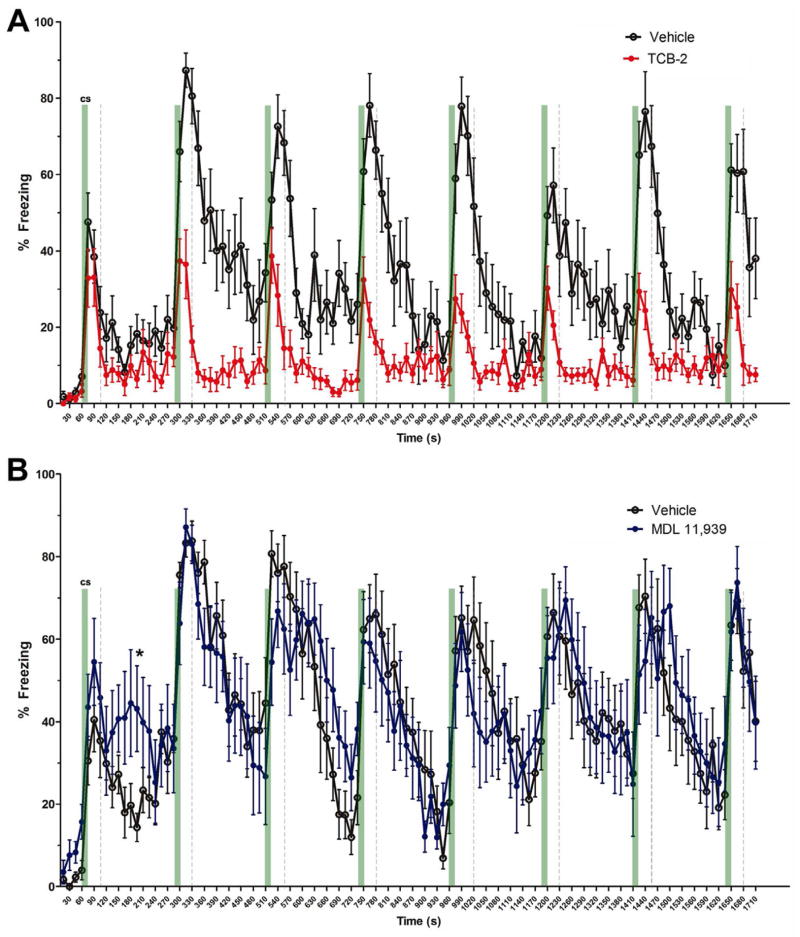
Here we tested the pharmacological activation of 5HT2ARs with pre-test session injections of TCB-2 (1.0 mg/kg, i.p.) on the extinction of cued fear memory after delay fear conditioning. A cohort of naïve male C57BL/6J mice received saline injections and then a 5-min context pre-exposure session to habituate to the conditioning chamber (Context A) on the first day. There was no significant difference in freezing behavior between the mice that were to receive pre-test saline (n=13) and those that were to receive pre-test TCB-2 (n=10), t(21) = −0.796, P = 0.435, Student’s t-test. Twenty-four h later, mice again received a saline injection and then were returned to Context A and received 3 CS-US pairings during a delay fear conditioning session. A two-way ANOVA on percent freezing data, with treatment as a between-subjects variable and CS-US pairing as a repeated-measures variable revealed a significant effect of CS-US pairing (F(3, 108) = 150.074, P < 0.001), but no significant main effect of treatment (F(1, 21) = 2.143, P = 0.158), or treatment × CS-US pairing interaction (F(3, 63) = 0.602, P = 0.616, figure not shown). Overall, these data indicated that there was no significant difference in the development of conditioned freezing over the course of the conditioning session between the saline and TCB-2 groups. The mice received either pre-test saline or pre-test TCB-2 injections 24 h after the conditioning session. After a 20 min delay, all mice were placed into Context B chambers, where they received 20 unpaired CS tone stimuli (see Figure 1D for experimental procedure). TCB-2 facilitated the acquisition of extinction of cued fear memory (see Figure 5A). A two-way repeated measures ANOVA revealed a significant effect of treatment (F(1, 21) = 4.614, P = 0.044) and a significant interaction of treatment × CS presentations (F(5, 105) = 4.648, P = 0.001). This finding indicates that activation of the 5HT2AR facilitates cued fear memory extinction in C57BL/6J mice.
Figure 5.
Activation of 5HT2ARs with systemic TCB-2 (1.0 mg/kg) facilitates the extinction of cued delay fear memory. A. Compared to the vehicle group (n = 13), TCB-2 administered before extinction test decreased the percent freezing of mice (n = 10) to repeated tone test (treatment, F(1,21) = 4.614, P = 0.044). B. In contrast, MDL 11,939 administered before extinction test delayed the recovery of freezing in mice (n = 10) to repeated tone test (treatment, F(1,18) = 4.574, P = 0.046) as compared to the vehicle group (n = 10). Data are expressed as mean ± SEM. *, P < 0.05.
The influence of 5HT2AR blockade with pre-test MDL 11,939 (0.5 mg/kg, i.p.) on the extinction of fear memory was tested with the same protocol in another 2 groups of mice. There was no difference in freezing of the mice during the 5-min Context A pre-exposure (t(18) = −0.211, P = 0.803, Student’s t-test). Twenty-four h later, mice were returned to Context A and received 3 CS-US pairings during the conditioning session. A two-way ANOVA on percent freezing data did not yield a significant effect of treatment (F(1,18) = 0.002, P = 0.968), or treatment × CS-US pairing interaction effect (F(3,54) = 0.530, P = 0.664, figure not shown). On the extinction test presented 24 hr after fear conditioning, MDL 11,939 delayed the acquisition of extinction of freezing (treatment, F(1,18) = 4.574, P = 0.046) (see Figure 5B), indicating that blockade of 5HT2AR inhibits fear memory extinction.
3.4. Influence of 5HT2AR on the consolidation of object memory
The modulation of 5HT2AR on the consolidation of memory was further examined with a modified version of a NOR task as described in section 2.5 in naive mice. The modified NOR task protocol provided an opportunity to observe drug-induced enhancements in object memory. Figures 6A and 6B illustrates the experimental procedure, the objects used and the calculation of discrimination ratio. A cohort of 64 naïve C57BL/6J mice was used in this study and 6 mice were excluded for not meeting the object exploration criterion during the sample session. Mice that received TCB-2 right after the sample session exhibited a significantly higher novel object discrimination ratio during the test session, as compared to the saline group (t(21) = −2.125, P = 0.046, see Figure 6C). Mice that received MDL 11,939 alone exhibited a tendency towards a weaker novel object preference as indicated by lower discrimination ratio scores than vehicle-treated controls. However, this difference was not significant (t(15) = 0.209, P = 0.209, see Figure 6D). In another cohort of mice, the selective 5HT2AR blocker MDL 11,939 was administered right after the sample session and TCB-2 was injected 10 min later. Compared to the control group, MDL 11,939 inhibited the TCB-2-induced enhancement of memory consolidation (t(16) = 2.684, P = 0.016, see Figure 6E). These data indicate that the activation of 5HT2ARs enhances the consolidation of object memory. The latency to reach the sample object exploration criterion was 197.43 ± 18.77 s and 198.87 ± 17.63 s in the vehicle + TCB-2 group and MDL 11,939 +TCB-2 group, respectively (t(16) = 0.056, P = 0.956, Figure not shown). As describe in Figure 6F, both groups of mice spent essentially equal time exploring the two identical objects during the period of sample session. During the test session, even though we did not find statistical differences of the exploration time mice used to exploring familiar object, novel object or both (P > 0.05), mice given TCB-2 showed a tendency towards spending more time on the novel object and less time on the familiar one. Mice that received TCB-2 10 min after the sample session exhibited a lower discrimination ratio score as compared to the mice administered TCB-2 right after the sample session (Figs. 6C and 6E). Memory consolidation likely begins during and right after learning, and may last from several minutes to several hours. Our results suggest that stimulation of 5HT2ARs at the early stage after learning has a more potent effect on consolidation of object memory.
Discussion
In this study we have used fear conditioning and novel object recognition task to assess the role of 5HT2ARs on the consolidation and extinction of fear memory and consolidation of object memory. We found that stimulation of 5HT2ARs with the 5HT2AR agonist TCB-2 enhanced the acquisition of extinction of cued fear memory in mice after trace and delay fear conditioning paradigms. In contrast, blockade of 5HT2ARs with MDL 11,939 exerted the opposite effect. Additionally, activation of 5HT2ARs enhanced the consolidation of fear memory and object memory. To the best of our knowledge, this study is the first to report memory-enhancing effects of the 5HT2AR agonist TCB-2, and to demonstrate a significant impact of 5HT2ARs on extinction of fear memory. The influence of the 5HT2AR on the extinction of fear memory may have significant relevance for the development of therapeutic approaches for patients with fear memory invasion, e.g., phobias and PTSD.
The pharmacological manipulation of serotonergic neurotransmission has been used to alleviate emotional disorders, such as depression and anxiety, for decades. More recently there has been renewed interest in the therapeutic application of hallucinogenic 5HT2 agonists such as psilocybin to treat depression and anxiety and to elevate mood states of chronic cancer patients (Grob et al., 2011). Such results suggest that despite the historical stigma associated with 5HT2 agonists, they may offer important medical potential for remediating affective and cognitive symptoms associated with neuropsychological disorders and other medical problems.
Here we found that TCB-2-induced activation of 5HT2ARs enhanced memory consolidation and facilitated the extinction of fear memory. Less consistent effects on conditioned fear memory were found for MDL 11.939, a selective 5HT2AR antagonist; such results concur with mixed results reported by others, (as reviewed in Harvey, 2003b), or after serotonin depletion (Romano et al., 2006; Welsh et al., 1998). The formation of CS-aversive US associations during Pavlovian fear conditioning requires synaptic plasticity within the LA (Sigurdsson et al., 2007), the CA1 area for context-dependent modulation of these associations, and the infralimbic area of the mPFC for extinction processes (Myers and Davis, 2007). Interestingly, 5HT2ARs are expressed in each of these regions (Cornea-Hebert et al., 1999). It will be of interest to next identify the specific contribution of 5HT2ARs in these discrete brain regions to the modulatory effects on fear memory processes that are described in the present report. Hippocampal neurons discharge to the presentation of the CS tone stimulus presented during early stages of auditory trace conditioning (Green and Arenos, 2007; McEchron and Disterhoft, 1997), and in response to the context and foot shock association (Dai et al., 2008), and are critical for the encoding and consolidation of trace fear memory (Clark and Squire, 1998; Huerta et al., 2000; McEchron et al., 1998). Before conditioning, the CS and US are temporally dissociated and may be encoded by distinct ensembles of hippocampal neurons. During trace fear conditioning, the CS-related domain and US-related domain may become functionally and morphologically incorporated. Hippocampal neurons responding selectively to the CS would encode the association of the CS and the US by sustaining their neuronal activity. For instance, the hippocampal CS-responsive cells maintain a high neuronal firing rate to the US during eye-blink conditioning (McEchron and Disterhoft, 1997). In this case, the onset of the US would overlap with the CS-entrained ensembles, forming a hippocampal-dependent association in downstream substrates, e.g., amygdala. Trace fear conditioning has a very clear timing requirement because it demands that the CS and the US be associated across a stimulus-free interval known as the “trace”. Learning occurs as the mice link the original neutral CS with a US, so that later presentation of the CS alone elicits the anticipatory conditioned freezing response. Besides hippocampal neuronal dynamics, glutamate neurotransmission is also deemed to influence synaptic plasticity and memory consolidation (Wang et al., 2006). We reported that stimulation of 5HT2ARs enhances glutamate efflux from the dorsal hippocampus and the firing rates of hippocampal neurons of male C57BL/6J mice (Zhang and Stackman Jr., 2011). In the present study we found that activation of 5HT2ARs right after conditioning enhanced percent freezing to both cue and context during subsequent test sessions. Considering the dense distribution of 5HT2ARs in the hippocampus, LA and mPFC (Cornea-Hebert et al., 1999), it is conceivable that 5HT2AR activation facilitates the information transmission and retention between each domain, and thereby influences memory consolidation. Additionally, local field potentials, such as theta rhythm, synchronize neural network activity that is considered essential for the consolidation of memory (Lesting et al., 2011; Seidenbecher et al., 2003). 5HT2ARs influence low-frequency field potential oscillations in rat frontal cortex (Celada et al., 2008). Recently 5HT2ARs has been observed to spur spinogenesis (Yoshida et al., 2011). Taken together, the enhanced consolidation of fear memory observed in mice that received TCB-2 post-training treatment may be a reflection of 5HT2AR activation effects on local field potentials, enhanced hippocampal and amygdala neuronal activity and spinogenesis.
The 5HT2AR is a member of the G-protein coupled membrane receptor family. Activation of 5-HT2ARs enhances phospholipase C activity, elevates intracellular Ca2+ and elicits excitatory transduction (Hoyer et al., 1994), and may enhance information acquisition and filtering. Frontal cortex, amygdala and hippocampus comprise components of brain circuits for memory extinction, and histological studies have revealed the dense expression of 5HT2ARs in each of these brain areas (Cornea-Hebert et al., 1999). During the cued fear memory extinction test, the presentation of the tone or CS in a novel environment re-activates the CS-entrained amygdala and hippocampal ensembles (established during conditioning), which trigger downstream circuits responsible for the conditional freezing response. Support for this idea arises from the observation that transient cue exposure can reactivate the hippocampal ensembles established through the preceding association with the cue (Quirk et al., 1990), suggesting that the hippocampus is capable of reestablishing the original pattern of activity, perhaps through a process of pattern completion. During the first CS exposure of the extinction test, there was no difference in percent freezing between the TCB-2-treated mice and the vehicle-treated mice, suggesting equivalent fear memory strength in the two groups. However, from the 2nd to 8th trials, the percent freezing of the TCB-2-treated mice was significantly lower than that of the control mice, suggesting that activation of 5HT2ARs facilitates the acquisition of extinction. Thus, 5HT2ARs may enhance the development of synaptic plasticity amongst the basolateral amygdala projection neurons to the infralimbic cortex; plasticity which is essential for extinction of cued fear memory (Herry et al., 2008). Even so, the rapid development of significant extinction of fear memory by TCB-2-treated mice compared to vehicle-treated mice is intriguing. Consistent with this result, the non-specific 5HT2AR agonist d-lysergic acid diethylamide (LSD) significantly enhanced associative learning in rabbits (Harvey, 2003a). Identifying the precise mechanisms by which 5HT2AR activation enhances extinction of sefear memory will be a challenge. During fear conditioning, CS+ presentation is correlated with theta oscillations occurring at each region of a network comprising the CA1, LA, and the mPFC (Lesting et al., 2011). Theta coupling between CA1 and the LA is increased during the consolidation of conditioned fear (Seidenbecher et al., 2003). Theta coupling in CA1-LA, LA-mPFC and in CA1-mPFC increases during the retrieval of conditioned fear, but then coupling in all these regions decreases when non-reinforced CS+’s are presented during the acquisition of extinction (Herry et al., 2010; Lesting et al., 2011). The mPFC, particularly the infralimbic cortex, is involved in fear extinction, and increased CS-related unit activity and induction of synaptic plasticity following extinction training were observed in the infralimbic cortex (Maren and Quirk, 2004; Milad and Quirk, 2002). Stimulation of the mPFC inhibits amygdala central nucleus unit responses to afferent stimulation (Quirk et al., 2003). Interestingly, 5HT2ARs influence neuronal dynamics and cortical local field potential (Celada et al., 2008) suggesting that stimulation of 5HT2ARs facilitates the extinction of cued fear memory via cortical mechanisms. It will be of interest to examine the influence of serotonin and the 5HT2ARs on the coupling of local field potentials and neuronal spiking across the CA1-LA-mPFC network during distinct phases of fear memory.
With the trace fear conditioning paradigm, we found that post-conditioning activation of 5HT2ARs enhanced the consolidation of contextual fear memory. A similar enhancement of the consolidation of object memory was found in mice given post-training TCB-2 in an NOR task. The encoding and consolidation of object memory acquired in NOR tasks is dependent upon the rodent hippocampus (Clark et al., 2000; Hammond et al., 2004) and perirhinal cortex (Winters et al., 2008). Our lab has previously established that limiting object exploration time during the sample session impairs the strength of the novel object preference exhibited 24 h later during the test session, as one might predict (Stackman et al., 2002). Here, mice that completed the limited sample session protocol and then were administered TCB-2 exhibited significantly stronger novel object preference than post-sample saline-treated mice. This result suggests that activation of the 5HT2AR after the sample session may have strengthened the nascent object memory encoded during the sample session and sufficiently enhanced its consolidation. In a separate cohort of naïve mice we also found that the co-administration of MDL 11, 939 blocked the memory-enhancing effect of post-sample TCB-2. This result supports the view that the memory enhancing effect of TCB-2 is a consequence of the activation of 5HT2ARs. Post-sample administration of MDL 11, 939 alone decreased object memory albeit not to a significant degree. Hippocampal neurons, especially place cells, assemble spatial and non-spatial information (Ego-Stengel and Wilson, 2010; Muller and Kubie, 1987; Muller et al., 1987; O’Keefe et al., 1998; O’Keefe and Conway, 1978). With in vivo single-unit recording, Eichenbaum (2001) proposed that the hippocampal neuronal firing array contributes to declarative memory through the constructing a “memory space” composed of a network of linked episodic representations. Previously, we demonstrated that systemic TCB-2 increased hippocampal neuronal firing rate and extracellular glutamate efflux, suggesting that stimulation of 5HT2ARs may facilitate neuronal dynamics and glutamate transmission, and thereby enhance neural mechanisms that support memory consolidation (Zhang and Stackman Jr., 2011).
The glutamatergic neurons in the amygdala, cortex and hippocampus play a vital role in memory consolidation and extinction. NMDAR antagonists block the extinction of fear memory and fear-elicited startle when administered either systemically or directly into the basolateral amygdala or CA1 region of hippocampus before extinction training (Baker and Azorlosa, 1996; Szapiro et al., 2003). The NMDA receptor partial agonist D-cycloserine facilitates fear memory extinction (Ledgerwood et al., 2003; Walker et al., 2002). Knockout of NMDARs in hippocampal CA1 pyramidal cells exclusively impairs the temporal memory process between the CS and the US as demonstrated by trace fear conditioning, suggesting that the CS representation is entrained within hippocampus cell ensembles, probably via NMDAR-dependent synaptic plasticity (Huerta et al., 2000; McHugh et al., 1996). 5HT2ARs are present in the dendrites and dendritic spines of dentate gyrus neurons where glutamate NMDAR and AMPAR are assumed to be distributed (Peddie et al., 2008). 5HT2ARs have been found to directly interact with the PSD-95 and regulate receptor trafficking and signal transduction (Xia et al., 2003). The 5HT2AR inverse agonist pimavanserin reversed object memory impairments induced by NMDA receptor antagonism (Snigdha et al., 2010), suggesting a modulatory influence of 5HT2ARs on NMDAR-dependent memory mechanisms. It is conceivable that 5HT2AR activation, leading to a direct elevation of intracellular Ca2+, combined with the recent or coincident elevation in intracellular Ca2+ due to NMDA receptor activation, would facilitate the induction of behaviorally triggered synaptic plasticity. Activation of 5HT2ARs produced an increase in the frequency and amplitude of cortical neuronal spontaneous excitatory postsynaptic potentials/currents (Aghajanian and Marek, 1999), and facilitated NMDA receptor activity and synaptic plasticity in the cortex (Arvanov et al., 1999) and basolateral amygdala (Chen et al., 2003). It will be of interest to examine the degree to which NMDARs contribute to the 5HT2AR activation-induced enhancement of memory consolidation and extinction.
In summary, this study indicates that stimulation of 5HT2ARs enhances the extinction of cued fear memory and the consolidation of fear memory and object memory in mice. The present data support the view that hippocampal 5HT2ARs play an important role in the regulation of cellular mechanisms of explicit memory. It will be interesting to delineate the intracellular cascades as well as the interaction of hippocampus, LA and mPFC that support 5HT2AR-mediated memory enhancement. A number of neuropsychological disorders such as panic disorders, PTSD and affective disorders, present with fear memory symptoms, and may involve brain areas expressing 5HT2ARs, such as the amygdala and hippocampus. Activation of 5HT2ARs may facilitate the desensitization for these patients by accelerating the building of new circuits and/or reorganization of existing pathways to alleviate fear memory. In this regard, the 5HT2AR may be a valuable target for therapeutic intervention.
Highlights.
We investigated the role of 5-HT2A receptors in modulation of memory processes in mice.
The agonist TCB-2 enhanced the consolidation of both fear memory and object memory.
Fear memory extinction was facilitated by TCB-2 and impaired by the antagonist MDL 11,939.
MDL 11,939 blocked the TCB-2-induced enhancement of object memory.
The results provide new insight into the modulation of memory processes by 5-HT
Acknowledgments
This work was supported by funds from NIH NIMH award (R01-MH08965 to RWS) and from a FAU Researcher of the Year award from the FAU Division of Research to RWS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Alhaider AA, Ageel AM, Ginawi OT. The quipazine- and TFMPP-increased conditioned avoidance response in rats: role of 5HT1C/5-HT2 receptors. Neuropharmacology. 1993;32:1427–1432. doi: 10.1016/0028-3908(93)90040-a. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999;11:2917–2934. doi: 10.1046/j.1460-9568.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behavioral neuroscience. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Diaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience. 2003;119:53–63. doi: 10.1016/s0306-4522(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4:101–114. doi: 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, Huang Y, Xu TL, Ding YQ, Xu L. Enhanced contextual fear memory in central serotonin-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behavioural brain research. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiology of learning and memory. 2007;87:269–284. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of general psychiatry. 2011;68:71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of learning and memory. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem. 2003a;10:355–362. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learning & memory. 2003b;10:355–362. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuo H, Matsuoka T, Akasu T. Activation of presynaptic 5-hydroxytryptamine 2A receptors facilitates excitatory synaptic transmission via protein kinase C in the dorsolateral septal nucleus. J Neurosci. 2002;22:7509–7517. doi: 10.1523/JNEUROSCI.22-17-07509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. The European journal of neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Khaliq S, Haider S, Ahmed SP, Perveen T, Haleem DJ. Relationship of brain tryptophan and serotonin in improving cognitive performance in rats. Pak J Pharm Sci. 2006;19:11–15. [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behavioural brain research. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PloS one. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitz Y, Ophir-Shaham O, Bloch Y, Treves I, Fennig S, Grauer E. Effect of L-tryptophan on memory in patients with schizophrenia. J Nerv Ment Dis. 2003;191:568–573. doi: 10.1097/01.nmd.0000087182.29781.e0. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature reviews. Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marner L, Frokjaer VG, Kalbitzer J, Lehel S, Madsen K, Baare WF, Knudsen GM, Hasselbalch SG. Loss of serotonin 2A receptors exceeds loss of serotonergic projections in early Alzheimer’s disease: a combined [11C]DASB and [18F]altanserin-PET study. Neurobiol Aging. 2012;33:479–487. doi: 10.1016/j.neurobiolaging.2010.03.023. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. Journal of neurophysiology. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF., 3rd Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology. 1998a;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, Price JC, Reynolds CF, 3rd, Mathis CA, Greer P, Lopresti B, Mintun MA, Pollock BG, Ben-Eliezer D, Cantwell MN, Kaye W, DeKosky ST. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Res. 1998b;813:167–171. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- Meneses A. Do serotonin(1–7) receptors modulate short and long-term memory? Neurobiol Learn Mem. 2007a;87:561–572. doi: 10.1016/j.nlm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Meneses A. Stimulation of 5-HT1A, 5-HT1B, 5-HT2A/2C, 5-HT3 and 5-HT4 receptors or 5-HT uptake inhibition: short- and long-term memory. Behav Brain Res. 2007b;184:81–90. doi: 10.1016/j.bbr.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1998;353:1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- Peddie CJ, Davies HA, Colyer FM, Stewart MG, Rodriguez JJ. Colocalisation of serotonin2A receptors with the glutamate receptor subunits NR1 and GluR2 in the dentate gyrus: an ultrastructural study of a modulatory role. Exp Neurol. 2008;211:561–573. doi: 10.1016/j.expneurol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Lunn BS, O’Brien JT. Effects of acute tryptophan depletion on cognitive function in Alzheimer’s disease and in the healthy elderly. Psychol Med. 2003;33:41–49. doi: 10.1017/s0033291702006906. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rat’s recent experience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Liu R, Dave KD, Schwab D, Alexander G, Aloyo VJ, Harvey JA. Effect of serotonin depletion on 5-HT2A-mediated learning in the rabbit: evidence for constitutive activity of the 5-HT2A receptor in vivo. Psychopharmacology. 2006;184:173–181. doi: 10.1007/s00213-005-0245-7. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Richter S, Wustenberg T, Debska-Vielhaber G, Schubert H, Heinze HJ, Richardson-Klavehn A, Duzel E. Genetic variation of the serotonin 2a receptor affects hippocampal novelty processing in humans. PloS one. 2011;6:e15984. doi: 10.1371/journal.pone.0015984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Horiguchi M, Huang M, Li Z, Shahid M, Neill JC, Meltzer HY. Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2010;332:622–631. doi: 10.1124/jpet.109.156349. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Vick KA, Guidi M, Stackman RW., Jr In vivo pharmacological manipulation of small conductance Ca(2+)-activated K(+) channels influences motor behavior, object memory and fear conditioning. Neuropharmacology. 2010;58:650–659. doi: 10.1016/j.neuropharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Schuhmacher A, Schwab S, Zobel A, Maier W. The His452Tyr variant of the gene encoding the 5-HT2A receptor is specifically associated with consolidation of episodic memory in humans. Int J Neuropsychopharmacol. 2008;11:1163–1167. doi: 10.1017/S146114570800905X. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu Y, Tsien JZ. Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- Welsh SE, Romano AG, Harvey JA. Effects of serotonin 5-HT(2A/2C) antagonists on associative learning in the rabbit. Psychopharmacology. 1998;137:157–163. doi: 10.1007/s002130050605. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience and biobehavioral reviews. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kanamaru C, Ohtani A, Li F, Senzaki K, Shiga T. Subtype specific roles of serotonin receptors in the spine formation of cortical neurons in vitro. Neurosci Res. 2011;71:311–314. doi: 10.1016/j.neures.2011.07.1824. [DOI] [PubMed] [Google Scholar]
- Zhang G, Stackman RW., Jr . Neuroscience Meeting Planner. Society for Neuroscience; Washington DC: 2011. Activation of serotonin 2A receptors enhances non-spatial memory consolidation and extracellular glutamate in the hippocampus of C57BL/6J mice. Program No. 295.210. [Google Scholar]