Abstract
Background
Mifepristone is a glucocorticoid receptor inhibitor shown in vitro to have anti-HIV activity and anti-simian immunodeficiency virus activity in a macaque model. A phase I/II trial was performed to assess the drug’s safety and anti-HIV activity.
Methods
A 28-day double-blind, placebo-controlled trial of mifepristone at doses of 75 mg, 150 mg, and 225 mg given daily was conducted in HIV+ persons with CD4+ lymphocyte counts ≥350 cells per cubic millimeter who had no recent antiretroviral therapy.
Results
Fifty-six male and 1 female subjects with a median entry CD4+ lymphocyte count of 555 cells per cubic millimeter and plasma HIV-1 RNA of 15,623 copies per milliliter were accrued. Forty-five subjects (78.9%) were available for endpoint analysis. In each arm, changes from baseline to day 28 in plasma HIV-1 RNA and CD4+ lymphocyte count were not significantly different from zero (no change). There was no relationship between mifepristone trough concentrations and plasma HIV-1 RNA. Day 28 morning plasma cortisol levels were significantly higher in the 150 mg and 225 mg arms compared with placebo, confirming biologic activity, and returned to baseline by day 56. Serum lipids did not change during the trial. Fasting blood sugar was 2.5 mg/dL higher on day 28 in the mifepristone arms, but the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) did not change. Three subjects (7.3%) receiving mifepristone developed a grade 2 rash.
Conclusions
Mifepristone at doses of 75–225 mg daily was safe and well-tolerated, but did not show significant anti-HIV activity.
Keywords: antiretroviral, clinical trial, mifepristone
An essential step in HIV replication is integration of its genome into the host cell chromosomes within the nucleus.1 HIV transports its genetic material as a large, nucleoprotein preintegration complex (PIC) into the nucleus through the intact nuclear envelope.
Viral protein R (Vpr) was originally classified as an accessory protein because it was thought to be dispensable for virus replication in T-lymphocyte lines.2 More recently Vpr and its receptor (hVIP/mov34) have been reported to form an activated receptor complex with the glucocorticoid receptor II (GR-II).3–5 Vpr induces nuclear translocation of the PIC. Because inhibiting PIC nuclear import should block HIV replication in nondividing cells, this critical step of HIV replication is of interest as a drug target. Vpr shares many characteristics with glucocorticoids, including the inhibition of its activities by the potent antiglucocorticoid compound mifepristone.3,4,6 Mifepristone inhibits the Vpr-mediated nuclear translocation of the Vpr receptor and blocks Vpr-induced apoptosis, cytokine production, and T-cell proliferation.4 In addition, by blocking the nuclear translocation of PIC, mifepristone has been shown to effectively inhibit replication of laboratory and clinical HIV-1 isolates, including drug-resistant strains, divergent clades, and those that use CCR5 and CXCR4 coreceptors. Furthermore, studies of intravenous mifepristone in an simian immunodeficiency virus (SIV)–infected macaque model have shown antiretroviral activity.7
In vitro, mifepristone inhibits HIV replication at concentrations ranging from 40 nM to 1000 nM (17–430 ng/mL), with an IC50 of 8 nM.3 These concentrations are readily achievable in vivo. Clinical studies using daily doses of 1–200 mg of mifepristone achieved steady-state drug concentrations of 85–5400 nM.8–11 Chronic administration of up to 200 mg/day of mifepristone for the experimental treatment of a variety of malignant and nonmalignant conditions has been well tolerated in non–HIV-infected subjects for up to 1 year.12–15 These data supported investigations of the in vivo antiviral effects of mifepristone on HIV replication and CD4+ T-cell counts in humans. The primary goal of this trial was to determine whether mifepristone in doses up to 225 mg/day showed antiretroviral activity in vivo in patients not receiving other antiretroviral therapy (ART).
METHODS
To evaluate the antiviral efficacy and safety of mifepristone, a double-blind, randomized, placebo-controlled phase I/II study was undertaken of 3 dose levels (75, 150, and 225 mg/day) of orally administered mifepristone for 28 days. This trial was sponsored by the AIDS Clinical Trials Group and given the number A5200.
Documented HIV-1–infected subjects ≥18 years of age with CD4+ cell count ≥350 cells per cubic millimeter and plasma HIV-1 RNA of ≥2000 copies per milliliter (within 90 days before study entry) were recruited for enrollment. Subjects had not received antiretroviral treatment within the 16 weeks before study entry nor intended to initiate ART within 60 days after entry. This population was selected because ART was not routinely recommended for them according to the then current United States Public Health Service (USPHS) guidelines.16 The subjects also needed to have stable hepatic, renal, endocrine (TSH between 0.5–6.0 mIU/L and a stimulated plasma cortisol ≥20 µg/dL) and hematological indices.17 Subjects were excluded if they were pregnant or breast feeding; had any chronic medical conditions such as adrenal insufficiency, active hepatitis, porphyria, cirrhosis, hemorrhagic disorders; prior pituitary tumor, surgery, or failure; diabetes requiring medical treatment; dysfunctional uterine bleeding within the past 12 months; currently had an intrauterine device; or weighed less than 40 kg. Subjects who were using potent inhibitors or inducers of cytochrome P450 3A4, anticoagulants, hormonal contraception, and any use in the past 90 days of corticosteroids, other hormones, immunomodulators, HIV vaccines, or investigational therapy were also excluded.
Subjects were enrolled at 8 ACTG sites. At entry, they were randomized, in permuted blocks without institutional balancing, with equal probability to 1 of 4 arms. Mifepristone was administered at the following doses in the 3 active arms: arm 1—75 mg (1 tablet); arm 2—150 mg, (2 tablets); and arm 3–225 mg (3 tablets). Subjects administered placebo were randomized to receive 1, 2 or 3 placebo tablets. Thus, the subjects and investigators were blinded as to whether subjects received active drug or placebo but not to dose group. Subjects self-administered the assigned number of study tablets once daily in the morning with a light meal for 28 days. Mifepristone was supplied as 75 mg tablets from Viral Genomics Pharmaceuticals with a matching placebo made with the excipients only.
At each study visit, subjects were asked about drug adherence, screened for any grade ≥1 adverse events (AEs), and had vital signs, including an assessment of orthostasis. Targeted physical examination driven by any previously identified signs or symptoms were performed. Safety blood studies were obtained weekly while on study drug (28 days) and 4 weeks afterwards. These included routine hematology, chemistries, liver and pancreas function tests, and CD4+/CD8+ lymphocyte counts. Lipids, insulin, and free fatty acids, morning cortisol, and a urinalysis were obtained on the first and last day of mifepristone. Plasma HIV-1 RNA determinations were performed on days 0, 3, 7, 14, 21, 28, and 56. Predose mifepristone levels were obtained on days 14 and 28.
Any subject who experienced a grade ≥3 AE, or a grade ≥2 rash, as defined in the Division of AIDS Table for Grading Adult Adverse Experiences,17 considered to be possibly or probably related to study drug was permanently discontinued from further treatment. All other grade ≥3 toxicities were reviewed by the safety monitoring committee and team. The study included a mechanism for early stopping in the case of prohibitively high toxicity/intolerance rates defined as ≥3 adverse event or ≥2 rash. Subjects who missed 3 or more doses of study medication during the first 14 days on study or who failed to provide a day 28 plasma HIV-1 RNA were excluded from primary analyses and were therefore replaced in accrual but were asked to attend remaining study visits.
Statistical Considerations
The primary endpoint was the within-subject change in log10 plasma HIV-1 RNA from baseline to days 14 and 28. For each arm, these changes were compared with the fixed hypothesized value of zero (no change), using the one-sided, one-sample t test with significance level 0.05. The study was powered to detect clinically meaningful short-term decreases in HIV-1 RNA within each active dose level, not to detect differences in antiretroviral activity between the active doses. Assuming standard deviations of changes to be about 0.6 log10, 12 evaluable subjects in each arm gave 85% power to detect mean changes in log10 HIV-1 RNA, in any one arm, at days 14 and 28 of 0.5 log10 copies per milliliter or greater. The placebo group provided comparison data for the safety and tolerability objective.
RESULTS
Fifty-seven subjects were enrolled and randomized over a 4 month period. Twelve subjects were not evaluable for the primary endpoint analysis. Four had protocol-defined toxicities, 3 with rash, and 1 neutropenia. Six received prohibited drugs (for 3, concurrent administration of prohibited medications was discovered after the trial was complete), 1 was non-adherent to the regimen, and for 1 subject, the plasma HIV-1 RNA assay could not be conducted (poor sample condition). Thus 45 subjects were included in the primary analysis.
Demographic and clinical characteristics by arm are shown in Table 1. Median entry CD4+ lymphocyte count was 555 cells per cubic millimeter and plasma HIV-1 RNA was 4.19 log10 copies per milliliter. Pill counts to assess study drug compliance showed no missed doses at 89% of study visits.
TABLE 1.
Demographics, Clinical Characteristics and Outcomes of ACTG 5200
| Treatment Arm |
All Arms Combined |
||||
|---|---|---|---|---|---|
| Placebo | 75 mg | 150 mg | 225 mg | ||
| Accrual number enrolled | 13 | 14 | 14 | 16 | 57 |
| Demographics | |||||
| % Male | 100% | 100% | 100% | 94% | 98% |
| % White/nonhispanic | 85% | 64% | 79% | 63% | 72% |
| % African-American/nonhispanic | 15% | 14% | 0% | 31% | 16% |
| Age, median (range), yrs | 40 (22–60) | 38 (25–56) | 40 (23–53) | 42 (26–55) | 40 (22–60) |
| % Antiretroviral naive | 72.7% (8/11) | 63.6% (7/11) | 76.9% (10/13) | 90.0% (9/10) | 75.6% (34/45) |
| Baseline clinical, median (range) | |||||
| Viral load, copies/mL | 4.16 (3.51–5.24) | 4.24 (3.20–4.95) | 4.21 (3.01–4.86) | 4.19 (3.57–5.40) | 4.19 (3.01–5.40) |
| CD4+, cells/mm3 | 623.0 (283–888) | 457.0 (249–1237) | 524.5 (277–1043) | 656.5 (279–949) | 555.0 (249–1237) |
| Efficacy, day 28 change from baseline: mean (95% confidence interval), N | |||||
| Viral load, c/mL | −0.15 (−0.3 to −0.01), 11 | 0.09 (−0.09 to −.28), 11 | 0.05 (−0.12 to −.21), 13 | 0.02 (−0.24 to 0.28), 10 | 0.01 (−0.08 to 0.09), 45 |
| CD4+, cells/mm3 | 36.5 (−35.5 to 108.5), 11 | −41.5 (−134 to 51.3), 11 | −4.2 (−68.0 to 59.5), 13 | −12.7 (−97.4 to 72.1), 10 | −5.2 (−40.3 to 29.8), 45 |
| Plasma concentrations, mean (95% confidence interval) | |||||
| Mifepristone, trough, ng/mL* | All ,5.1 ng/mL assay lower limit | 275.5 (174–377) | 790.7 (667–914) | 797.6 (519–1078) | NA |
| Cortisol, day 28, µg/dL | 13.0 (10.0–16.1) | 20.9 (14.5–27.3) | 21.5 (16.2–26.8) | 26.0 (21.5–30.5) | NA |
| Safety/tolerability, percent† | |||||
| Stop any reason | 7.7% | 14.3% | 0.0% | 13.3% | 8.9% |
| Rash‡ | 0.0% | 14.3% | 0.0% | 7.1% | 5.7% |
| Any toxicity grade $3§ | 23.1% | 0.0% | 7.1% | 13.3% | 10.7% |
Arithmetic mean of day 14 and 28 predose concentrations.
Of subjects taking any study drug.
Excludes 1 subject whose rash was consistent in history and presentation with herpes zoster outbreak; all rashes were grade 2.
Consisted of grade 3 neutropenia (3 subjects), grade 4 lipase elevation (1 subject), grade 4 blood glucose elevation (1), and grade 4 creatine kinase (1); no grade 3 or higher sign/ symptoms were reported.
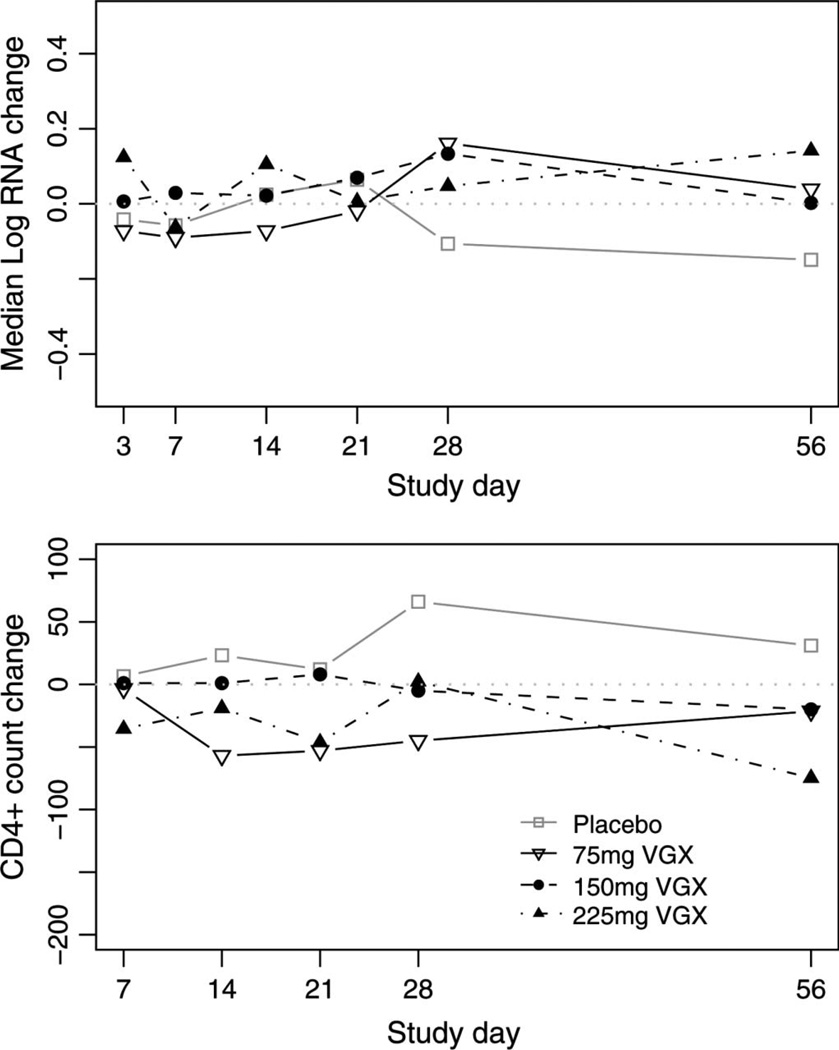
Figure 1 shows the anti-HIV response to mifepristone. For all arms combined, subject-specific changes in plasma HIV-1 RNA were small, ranging from −0.65 to +0.67 log10 copies per milliliter, and the null hypothesis of no change could not be rejected. Pooling active arms, changes were also not different from zero (P = 0.855). Day 28 antiviral efficacy is also represented in Table 1; for all arms, the 95% confidence intervals include the value zero. In a secondary analysis using all available day 28 viral loads, this finding holds for the active arms. Figure 1 and Table 1 also show the CD4+ lymphocyte response over the time to the study drugs. Again, no statistically significant increases in CD4+ cell count were seen in the active arms.
FIGURE 1.
Arm-specific change in plasma log10 HIV-1 RNA (copies/mL, upper panel) and in CD4+ lymphocyte count (cell/mm3, lower panel) by scheduled study day. Dotted horizontal lines marks zero (no change).
Mifepristone was generally well tolerated. There were similar rates of protocol-defined toxicities in the placebo (6.3%) and the mifepristone arms (7.3%). Rash has been reported in prior studies of mifepristone. In this study, 3 subjects receiving mifepristone developed a grade 2 rash by week 2, none of which were dose related. Because mifepristone blocks glucocorticoid receptors, there was concern for the development of symptoms of hypoadrenalism, but no grade 3 symptoms consistent with adrenal insufficiency were noted. The incidence of all grade 3 or higher toxicities is shown in Table 1. There was overlap around the 95% confidence interval for the rates of all AEs, and all grade 3 toxicities were related to laboratory abnormalities.
Plasma and serum mifepristone trough values were obtained with good correlation observed between the day 14 and 28 values (Pearson correlation coefficient r = 0.96, P < 0.001) and a strong linear association (r > 0.99, P < 0.001) between the serum and plasma concentrations (data not shown). Plasma concentrations of mifepristone, shown in Table 1, increased with dose but not linearly. The difference between the 75 mg and 150 mg does was much larger than between the 150 mg and 225 mg dose. The mean concentrations of the 75 mg, 150 mg, and 225 mg doses of mifepristone were 271.9, 788.2, and 792.8 ng/mL, respectively. There was considerable overlap between the 150 and 225 mg arms with no significant difference in levels. This is consistent with prior studies of mifepristone that doses above 150 mg did not yield increases in serum drug levels. Higher mifepristone concentrations were not associated with greater decreases in plasma HIV-1 RNA levels at day 28.
To assess whether mifepristone was biologically active, morning cortisol levels were measured on day 28. As shown in Table 1, cortisol levels increased with mifepristone dose and were significantly different from the zero on the 3 active arms (paired t test P values = 0.023, 0.017, 0.0020, respectively), but not the placebo arm (P = 0.165). By day 56, cortisol levels had returned to baseline, and changes were not significantly different from zero on any arm.
Plasma lipids and measures of insulin sensitivity were performed on day 0 and 28. Changes in cholesterol, triglycerides, fatty acids, fasting blood sugar, and insulin did not seem to increase or decrease with dose level and were not different between active and placebo arms.
CONCLUSIONS
In this randomized partially blinded placebo-controlled phase I/II trial of mifepristone at doses up to 225 mg/dL, there was no evidence of antiretroviral activity over a 28-day period. This was true despite the fact that there was a measured increase in serum cortisol levels at the 150 and 225 mg daily doses that confirmed the in vivo biologic activity of the mifepristone. The drug at these doses was safe and well tolerated.
The discordance of the results of the in vitro systems and macaque model and those in our trial may have been from inadequate drug action or levels at the target site. In the macaque, the mifepristone was given intravenously and thus avoided gut and hepatic metabolism that could have occurred with the oral dosing in the trial. The IV administration in macaques (3.2 mg/kg) may have obtained higher free drug levels than achieved in subjects receiving 225 mg of mifepristone orally, although preclinical studies of orally dosed cynomolgus monkeys suggested the serum drug levels were 6-fold higher in man. Another possibility for lack of correlation in efficacy may have resulted from differential binding of the mifepristone to serum proteins or its cellular receptors in the SIV model compared with those in an HIV-infected human. A difference in the affinity or concentration of VIP/mov34 receptor on T-lymphocytes between the SIV model and man could also explain the lack of activity. Alternatively, incomplete mifepristone interference of the HIV PIC and glucocorticoid receptor II interaction in vivo might have prevented more complete viral inhibition. No studies were conducted to assess the affinity of these cellular receptors in primates or in the patients tested. Although this trial did not demonstrate antiviral activity as expected, a trial evaluating higher doses of mifepristone in a similar HIV-1–infected population is underway with the hope of identifying another HIV therapeutic agent.
ACKNOWLEDGMENTS
The ACTG acknowledges the following in performance of this trial: Diane Gochnour, RN—The Ohio State University (A2301) CTU Grant #AI069474; Sharon Riddler, MD, MPH, and Barbara Rutecki, MSN, MPH, CRNP—University of Pittsburgh (A1001) CTU Grant # 1 U01 A1 69494-01; Princy Kumar, MD and Ioulia Vvedenskaya—Georgetown University (A1008); Beck A. Royer, PA-C and N. Jeanne Conley, RN, BSN—University of Washington, Harborview Med Center (A1401) CTU Grant #AI 069434; Angela Grbic–Harbor-UCLA Med Center (A0603) CTU Grant #AI069424-01; Mark Rodriguez, RN, BSN and Michael Royal, RPh - Washington University at St. Louis (A2101) CTU Grant # AI69495-01; Kathryn Maffei, RN, BSN—University of Pennsylvania (A6201) CTU Grant # UOI-AI 032783-13, CFAR Grant #5-P30-AI-045008-07; W. Keith Henry, MD, and Kathy Fox, RN—University of Minnesota (A1501) CTU Grant #AI27661; and Susan Pedersen, RN—University of North Carolina (A3201) CTU Grant # AI69423-01, University of North Carolina General Clinical Research Center Grant #RR00046.
Supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (AI68636), SDAC (AI68634) and VGX Pharmaceuticals. This trial was also supported in part by the General Clinical Research Center Units funded by the National Center for Research Resources.
Footnotes
Presented in part at the 46th ICAAC, September 27–30, 2006, San Francisco, CA.
REFERENCES
- 1.Levy J. Pathogenesis of human immunodeficiency virus infection. Microbial Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connor RI, Chen BK, Choe S, et al. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 3.Refaeli Y, Levy DN, Weiner DB. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci U S A. 1995;92:3621. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayyavoo V, Mahboubi A, Mahalingam S, et al. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan MP, Curley E, Su M, et al. Carboxyl terminus of hVIP/ mov34 is critical for HIV-1-Vpr interaction and glucocorticoid-mediated signaling. J Biol Chem. 2002;277:47854–47860. doi: 10.1074/jbc.M203905200. [DOI] [PubMed] [Google Scholar]
- 6.Kino T, Gragerov A, Kopp JB, et al. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthumari K, Boyer J, Choo A, et al. Presented at: 12th CROI; February 22–25, 2005. Boston, MA: Therapeutic inhibition of SIV replication in macaques by GR blockade and implications for therapy of HIV 1. Abstract 158. [Google Scholar]
- 8.Heikinheimo O, Kekkonen R. Dose response relationship of RU486. Ann Med. 1993;25:71–76. doi: 10.3109/07853899309147861. [DOI] [PubMed] [Google Scholar]
- 9.Foldesi I, Falkay G, Kovacs L. Determination of RU486 (mifepristone) in blood by radioreceptor assay: a pharmacokinetic study. Contraception. 1996;54:27–32. doi: 10.1016/0010-7824(96)00116-3. [DOI] [PubMed] [Google Scholar]
- 10.Kekkonen R, Heikinheimo O, Mandelin E, et al. Pharmacokinetics of mifepristone after low oral doses. Contraception. 1996;54:229–234. doi: 10.1016/s0010-7824(96)00193-x. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar N. Mifepristone: bioavailability, pharmacokinetics and useeffectiveness. Euro J Obst Gynecol Repro Bio. 2002;101:113–120. doi: 10.1016/s0301-2115(01)00522-x. [DOI] [PubMed] [Google Scholar]
- 12.Liu IH, Carzo VO, Ven SSC. Pharmacokinetics of antiprogesterone RU486 in women after oral administration. Fertil Steril. 1988;50:245–249. doi: 10.1016/s0015-0282(16)60067-5. [DOI] [PubMed] [Google Scholar]
- 13.Sartos O, Figg W. Mifepristone: antineoplastic studies. Clin Obstet Gynecol. 1996;39:498–505. doi: 10.1097/00003081-199606000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Heikinheimo O. Clinical pharmacokinetics of mifepristone. Clin Pharmacokinet. 1997;33:7–17. doi: 10.2165/00003088-199733010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Yong-en S, Zhi-hou Y, Chang-hai H, et al. Pharmacokinetic study of RU486 and its metabolites after oral administration of single doses to pregnant and non-pregnant women. Contraception. 1993;48:133–149. doi: 10.1016/0010-7824(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed February 1, 2010];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Developed by the Panel on Clinical Practices for Treatment of HIV Infection convened by the Department of Health and Human Services. 2004 Mar 23;:1–97. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 17.Regulatory Compliance Center. Division of AIDS (DAIDS) Table for Grading Severity of Adult Adverse Experiences. Bethesda, MD: NAIDD; 1992. Aug, Available at: http://rcc.tech-res.com/tox_tables.htm. [Google Scholar]