Abstract
Lysophosphatidic acid (LPA) is an endogenous lipid growth factor that is thought to play important roles in cell proliferation and antiapoptosis and therefore may have roles in the development and progression of benign prostatic hyperplasia (BPH). CYR61 (CCN1), on the other hand, is a growth factor-inducible immediate early gene that functions in cell proliferation, differentiation, and extracellular matrix synthesis. Here we show the close relationship between LPA-induced expression of CYR61 and prostate enlargement. CYR61 mRNA and protein were dramatically up-regulated by 18:1 LPA (oleoyl-LPA) within 1 and 2 h, respectively, in both stromal and epithelial prostatic cells. G protein-coupled receptors, i.e. Edg-2, Edg-4, and Edg-7, for LPA were also expressed in both stromal and epithelial prostatic cells. Furthermore, on DNA microarray analysis for normal and BPH patients, CYR61 was found to be related to the development and progression of BPH, regardless of symptoms. Although CYR61 mRNA was synthesized in hyperplastic epithelial cells, in many cases of BPH, CYR61 protein was detected in both the epithelial and stromal regions of BPH patient tissues. The functional contribution of CYR61 to prostatic cell growth was demonstrated by recombinant CYR61 protein and anti-CYR61 neutralizing antibodies, which inhibited CYR61-dependent cell spreading and significantly diminished cell proliferation, respectively. In conclusion, these data support the hypothesis that LPAs induce the expression of CYR61 by activating G protein-coupled receptors and that CYR61 acts as a secreted autocrine and/or paracrine mediator in stromal and epithelial hyperplasia, demonstrating the potential importance of this signaling mechanism in the disease. (Endocrinology 145: 2929–2940, 2004)
Prostatic enlargement (benign prostatic hyperplasia; BPH) is the most common benign neoplasm in men. BPH is a hyperplastic proliferative process, which involves both prostatic epithelial elements and stromal cells, i.e. smooth muscle cells and fibroblasts. Progressive enlargement of the prostate is classified into two types. In the first type, cells multiply around the urethra and constrict it. In the second one, middle-lobe prostate cells grow into the urethra and bladder outlet area. In both cases, the result is urinary obstruction accompanied by symptoms of various degrees. BPH occurs in about 50% of men over age 60 yr, and its prevalence increases with age (reviewed in Ref. 1). In the United States alone, the market for BPH pharmacotherapies is currently in excess of $550 million annually (as reported by Intercontinental Marketing Services). With respect to etiology, although the involvement of steroid androgens and/or peptide growth factors has been reported in BPH, the determinants of the pathologic enlargement of the prostate are poorly understood (Ref. 2, and reviewed in Ref. 3). For example, whereas testosterone regulates prostate growth, BPH occurs mostly in elderly men, who have decreased serum levels of testosterone. In this regard, previously we showed that the message encoding CYR61 was significantly up-regulated in BPH (4, 5).
CYR61 (CCN1) is a heparin-binding, extracellular matrix protein of the CCN family. Over the past several years it has become clear that members of this family comprise immediate early genes. Currently this family comprises connective tissue growth factor (CCN2), nephroblastoma overexpressed (CCN3), and Wnt-induced secreted proteins-1 (CCN4), -2 (CCN5), and -3 (CCN6) (reviewed in Refs. 6 and 7). The translational products of most CCN family members are secreted proteins of 343–381 amino acid residues that comprise four distinct structural modules, each having 38 conserved cysteine residues. These proteins have a variety of properties, which may influence cellular behavior such as growth, differentiation, adhesion, and locomotion. Indeed, CYR61 regulates cell adhesion, proliferation, and protection from apoptosis, chemotaxis, and angiogenesis, at least in part through integrin-mediated mechanisms (8–10). Moreover, recent research has indicated that aberrant expression of CYR61 is associated with several diseases, including arteriosclerosis (11, 12), vascular restenosis (13), thrombosis (14), and especially breast cancer (15–17). To the best of our knowledge, however, to date, CYR61 expression in the prostate and its role in the prostatic diseases remain unclear.
Recently, with respect to causality, evidence of the involvement of lysophosphatidic acid (LPA) in the proliferation of prostatic cells has been increasing (Refs. 18–21, and reviewed in Ref. 22). LPA, which has been identified as a hormone- and growth factor-like lipid in body fluids, e.g. serum, plasma, and ascites, is now recognized as an important bioactive lipid mediator with diverse biological activities. LPA elicits a variety of cellular responses, which include mitogenic effects on the cell cycle, induction of cancer cell invasion, regulation of actin stress fiber formation and focal adhesion assembly, cell motility, and mobilization of intracellular calcium (reviewed in Refs. 23–25). Most of these responses are mediated through cell surface-specific receptors, which belong to the endothelial differentiation gene (Edg) family of G protein-coupled receptors. Cognate LPA-specific receptors Edg-2, Edg-4, and Edg-7 are now called LPA1, LPA2, and LPA3, respectively (reviewed in Ref. 26). Among the diverse physiological actions of LPA, its growth-promoting activity has been detected in many cell types (reviewed in Ref. 27), despite the fact that LPA was first shown to promote fibroblast proliferation (28). The ability of serum to induce mitosis appears to be mediated, in large part, by lipids, such as LPA and sphingosine-1-phosphate. Based on these diverse cellular effects, it is tempting to hypothesize that LPA may play an important role in the initiation and/or progression of certain human diseases. In fact, LPA has been reported to play roles in ovarian cancer, airway disease, and neoplasia (reviewed in Refs. 29–31). Although many of these studies dealt with cancer, it has been reported that endogenous serum component LPA also promotes the growth of smooth muscle cells derived from human BPH tissue (32). In addition, supportive studies have shown that LPA receptors are expressed on prostatic cells and tissues (33, 34). These data provide new and intriguing information regarding the involvement of LPA in BPH.
To understand the mechanism(s) underlying prostate enlargement, we have undertaken the present study to determine whether there is a causal link between CYR61 and LPA in BPH. Consequently, we hypothesize that LPA serves as a potent regulator in the initiation and/or progression of BPH. In the this report, we present for the first time experimental evidence that indicates that CYR61 elicited by LPA is a potential mediator of epithelial and stromal hyperplasia. We show: 1) LPA induces CYR61 mRNA and protein in prostatic epithelial and stromal cells, 2) LPA receptors are expressed in prostatic cells, 3) CYR61 is up-regulated in BPH tissues, 4) CYR61 mRNA and protein are localized in BPH tissues, 5) CYR61 acts as a growth mediator for prostatic epithelial cells, and 6) CYR61 promotes prostatic stromal cell spreading and potentiates platelet-derived growth factor (PDGF)-stimulated cell proliferation.
Materials and Methods
Sources of materials
LPA with unsaturated [oleoyl (18:1)] acyl chains was purchased from Avanti Polar Lipids (Alabaster, AL). PDGF was obtained from R&D Systems (Minneapolis, MN). QuantiGene kits were purchased from Bayer Corp. (Berkeley, CA). Kits for quantitative RT-PCR, i.e. a TaqMan PCR core reagent kit and SYBR Green I, were obtained from Applied Biosystems (Foster City, CA) and Molecular Probes (Eugene, OR), respectively. Microtiter plates for cell spreading assays were purchased from Asahi Techno-Glass (Tokyo, Japan). Multiple 96-well plates for cell proliferation assays were purchased from Costar (Corning, NY). Recombinant human CYR61 protein was generously provided by Dr. M. Aoki of our laboratory. Biotinylated rabbit antigoat IgG antibodies (Ab) (Dako, Glostrup, Denmark) and horseradish peroxidase-conjugated streptavidin (Amersham Pharmacia, Uppsala, Sweden) were used for the detection of goat anti-CYR61 polyclonal Ab (CYRO69–2, generated in our laboratory) (5) on Western blotting analysis. A goat anti-CYR61 polyclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and horse-radish peroxidase-conjugated antigoat IgG polyclonal Ab (Amersham Pharmacia) were used for the detection of CYR61 protein on immunohistochemical staining.
Cell culture and cell treatment
Human prostatic epithelial cell line BRF-55T (BRFF, Ijamsville, MD), which was derived from a BPH patient, was propagated in RPMI 1640 medium containing 5% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma, St. Louis, MO). Human prostatic stromal cells (PrSCs, lot no. 9F0951; BioWhittaker, Walkersville, MD), which were derived from a healthy donor, were propagated in stromal cell growth medium containing 5% FBS, 500 μl GA-1000, 5 μg/ml bovine insulin, and 1 ng/ml human basic fibroblast growth factor (BioWhittaker). For the LPA-stimulation assay, 1 × 104 cells were plated on 96-well culture plates (Costar) in RPMI 1640 medium containing 0.01% BSA (Sigma) and penicillin/streptomycin for BRF-55T, or stromal cell basal medium containing 0.01% BSA and penicillin/streptomycin for PrSC, and LPA was added after incubation overnight at 37 C. The cells were incubated at 37 C, lysed with an appropriate lysis buffer, and then examined with branched-chain DNA (bDNA) for mRNA and by Western blotting analysis for protein.
Branched DNA assay
The levels of CYR61 mRNA were determined by means of a CYR61 bDNA assay (5). Briefly, cells were lysed with a lysis buffer (Bayer) and then transferred to QuantiGene capture plates (Bayer), and the bDNA assay was carried out according to the manufacturer’s instructions with minor modifications. The luminescence counts of samples were determined with a micro-β-plate counter (Wallac, Turku, Finland).
Western blotting analysis
For the detection of intracellular CYR61 protein by Western blotting, cell protein extracts were prepared by lysis with Tris-SDS-BME sample loading buffer (Owl, Woburn, MA). Cell lysates were directly subjected to SDS-PAGE under reducing conditions and then electrophoretically transferred to polyvinyl difluoride membranes (Immobilon, Daiichi Pure Chemicals, Tokyo, Japan). The membranes were blocked with Block Ace (Dainippon Pharmaceutical Co., Osaka, Japan) by incubation for 2 h at room temperature. After washing with a PBS solution containing 0.1% Tween 20 (PBS/T), 2 μg/ml of anti-CYR61 polyclonal Ab (CYRO69–2) (5) in 0.5% BSA-PBS/T was added. After 1 h incubation, the membranes were washed with PBS/T, and then biotinylated rabbit antigoat Ig polyclonal Ab (Dako) was added. After 1 h incubation, the membranes were washed with PBS/T, and then horseradish peroxidase-conjugated streptavidin (Amersham Pharmacia) was added. After washing with PBS/T after 1 h incubation, the ECL-plus substrate (Amersham Pharmacia Biotech, Buckinghamshire, UK) was added, and then chemiluminescence was detected with hyperfilm ECL (Amersham Pharmacia Biotech).
Quantitative RT-PCRs
RNA for quantitative RT-PCR was prepared with RNA Sepasol I (Nacalai Tesque, Kyoto, Japan) and DNase I (Gibco BRL, Rockville, MD) by the standard method. The RNA samples were stored at −80 C before analysis. Quantitative RT-PCRs were conducted in an ABI Prism 7700 sequence detection system (Applied Biosystems) using 100 ng total RNA as the template, the TaqMan PCR core reagent kit, SYBR Green I, and human specific primers Edg-2, Edg-4, and Edg-7. The oligonucleotide primers for amplifying Edg-2 were forward 5′-cggagactgactgttagcacatg and reverse 5′-ccgtaatgtgcctctcgattg, those for Edg-4 forward 5′-aggct-gtgagtcctgcaatgt and reverse 5′-tctcagcatctcggcaagagt, and those for Edg-7 forward 5′-ccaacctcatggccttcct and reverse 5′-tcctccggcggctgat, respectively. Accumulation of the PCR product was measured in real time as the increase in SYBR Green fluorescence. The thermal cycler conditions were 2 min at 50 C hold, 10 min at 94 C hold, and 40 cycles of denaturation at 94 C for 20 sec and annealing/extension at 60 C for 1 min. Data were analyzed with sequence detection software (Applied Biosystems). LPA receptor expression levels were determined using corresponding standard curves obtained with the respective cDNAs. All quantitative RT-PCR assays were performed in triplicate.
DNA microarray analysis
Analysis of the gene expression profiles in BPH patients and normal individuals using oligonucleotide microarrays was performed as described previously (4, 5). All prostate samples were obtained from prostate transition zone tissue. With respect to CYR61, which was identified as the up-regulated gene (4, 5), we added new data and analyzed the expression levels in two groups, i.e. normal and disease samples. The first group (normal) of prostate samples was from 10 organ donors aged 13–50 yr old. The second group (BPH) comprised asymptomatic BPH samples (eight donors, 51–65 yr old), symptomatic BPH samples (eight donors, 42–77 yr old), and samples of BPH with cancer (eight donors, 60–70 yr old).
Tissue collection and sample preparation
Tissue samples (n = 10) from the prostate transition zone were obtained from nine BPH patients who had undergone transurethral resection of the prostate, and one bladder cancer patient who had undergone radical cystectomy and prostatectomy at Kagawa Medical University Hospital. The patients’ profiles are presented in Table 1. Briefly, the patients were aged between 55 and 82 yr (mean 73.1). The freshly collected tissue samples were fixed in 4% paraformaldehyde for 24–36 h and then embedded in paraffin. The paraffin-embedded tissue specimens were cut into 5-μm-thick sections and then placed on poly-L-lysine-treated glass slides for subsequent in situ hybridization and immunohistochemical staining. These tissue samples were acquired according to the stringent Kagawa University Institutional Review Board guidelines, with appropriate informed consent.
TABLE 1.
Expression of CYR61 in human BPH tissues
| Case | Age | Prostate volume | IPSS score | Operation |
In situ hybridization
|
Immunohistochemistry
|
||
|---|---|---|---|---|---|---|---|---|
| Epithelial cells | Stromal cells | Epithelial cells | Stromal cells | |||||
| 1 | 73 | 41 | 24 | TUR-p | ++ | − | ++ | + |
| 2 | 55 | N.R. | N.R. | Total cystectomy | ++ | − | ++ | + |
| 3 | 80 | 67 | 16 | TUR-p | ++ | + | ++ | + |
| 4 | 78 | 44 | 31 | TUR-p | + | + | − | + |
| 5 | 60 | 106 | N.R. | TUR-p | − | − | − | − |
| 6 | 82 | 91 | N.R. | TUR-p | − | − | + | + |
| 7 | 79 | 82 | 10 | TUR-p | + | − | − | − |
| 8 | 76 | 49 | 25 | TUR-p | ++ | ++ | + | ++ |
| 9 | 78 | 56 | 35 | TUR-p | ++ | + | + | + |
| 10 | 70 | 43 | 21 | TUR-p | ++ | − | + | ++ |
− No signal; the number of plus signs indicates the increase in expression.
N.R., Not recorded.
In situ hybridization and immunohistochemical staining
To determine the localization of CYR61 expression in BPH patient tissues, we examined the expression and localization of CYR61 by in situ hybridization and immunohistochemical staining. Briefly, for in situ hybridization study, an approximately 2.0-kb fragment containing full-length human CYR61 cDNA was inserted into vector pcDNA3 (Invitrogen, Frederick, MD) according to the manufacturer’s instructions. Purified plasmids were linearized with either EcoRI (for transcription using SP6 RNA polymerase) or NotI (for transcription using T7 RNA polymerase) to obtain antisense or sense RNA probes. The labeled full-length antisense-strand of CYR61 mRNA was shortened by alkaline hydrolysis for better tissue penetration. After deparaffinization and hydration, each slide was covered with hybridization buffer containing 50% deionidized formamide (Roche Diagnostics, Basel, Switzerland), 10% dextran sulfate (Sigma), 1× Denhardt’s solution, 10 mM Tris-HCl (pH 7.6), 600 mM NaCl, 0.25% sodium dodecylsulfate (Sigma), 1 mM EDTA (Sigma), and 200 μg/ml tRNA (Sigma) for 30 min at room temperature. The slides were then incubated with digoxygenin-11-uridine 5-triphosphate-labeled CYR61 antisense probe or sense probe, as a negative control, in a humidified chamber for 16 h at 42 C. After hybridization, the slides were rinsed in 5× saline sodium citrate, 50% formamide in 2× saline sodium citrate for 30 min at 42 C, followed by rinsing in TNE buffer [10 mM Tris-HCl (pH 7.6), 500 mM NaCl, 1 mM EDTA] for 10 min at 37 C. Hybridized probes were detected using a nucleic acid detection kit (Roche Diagnostics) according to the manufacturer’s instructions. The hybridized probes were visualized using an alkaline phosphatase-labeled digoxigenin antibody and nitro-blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate as the substrate. Color was developed in the dark for 12 h at room temperature. The slides were counterstained for 30 sec with 0.2% methyl green (Wako Chemicals, Osaka, Japan).
Meanwhile, for immunohistochemical staining, after deparaffinization, hydration, and blocking with 2.5% H2O2 in methanol, the sections were boiled in 0.01 M citrate buffer for 10 min and then incubated with 5% normal blocking serum in Tris-buffered saline for 20 min. The sections were incubated with anti-CYR61 polyclonal Ab (1:100 dilution; Santa Cruz Biotechnology) for 2 h at room temperature. The sections were incubated with biotinylated antigoat IgG (1:200 dilution; Vector Laboratories, Burlingame, CA) for 30 min. After incubation with an avidin-biotin peroxidase complex kit (Vector Laboratories) for 30 min, the samples were exposed to a diaminobenzidine tetrahydrochloride solution and then counterstained with hematoxylin. As specificity controls, the primary antibody was substituted with normal goat IgG, all other steps remaining unchanged.
Cell adhesion, spreading, and proliferation assay
For cell adhesion assay of epithelial cell line BRF-55T, 96-well microtiter plates (Asahi Techno-Glass) were coated with 50 μl BSA (Sigma), CYR61, or fibronectin in PBS for 2 h at 37 C. After washing with 0.1% BSA-PBS, a 50-μl aliquot of BRF-55T cells, 1 × 104 cells, was plated into each well in RPMI 1640 medium containing 0.01% BSA and penicillin/ streptomycin (0.01% BSA-RPMI 1640). In the case of examination of the effect of EDTA (Wako Chemicals), 50 μl of 5 mM EDTA in 0.01% BSA-RPMI 1640 was added before cell plating. The cells were incubated with a Cell Counting Kit-F (Calcein-AM; Dojindo, Kumamoto, Japan) for 2–3 h at 37 C. After washing four times with 100 μl of 0.01% BSA-RPMI 1640 to remove nonadherent cells, adherent cells were subjected to fluorescence counting at 490 nm (ref. 515 nm) with the calcein reagent. The effect of CYR61 on the cell proliferation of epithelial cell line BRF-55T was examined as follows. Briefly, multiple 96-well plates (Costar) were coated with 50 μl of 10 μg/ml CYR61 in PBS overnight at 4 C. After removing the coating solution, the wells were washed with 0.1% BSA-PBS. Fifty microliters of BRF-55T cells, 8 × 103 cells, was plated into each well in 0.01% BSA-RPMI 1640 supplemented with penicillin/streptomycin. After 2 h incubation at 37 C, 40 μl of FBS, 0.5% (final concentration), was added to each well, 10 μl of normal rabbit IgG, 1 mg/ml, or anti-CYR61 polyclonal Ab (CYRO70–1; 5), 1 mg/ml, in 0.1% BSA-RPMI 1640 being added simultaneously. Cellular growth was assessed 6 d later. Ten microliters of Cell Count Reagent SF (Nacalai Tesque) were added to 100 μl culture for 3–4 h at the end of the culture. The absorbance at 450 nm (ref. 650 nm) of each well was measured with a microplate reader (Molecular Devices, Sunnyvale, CA).
For cell spreading assay of stromal cell PrSC, 96-well microtiter plates (Asahi Techno-Glass) were coated with 50 μl of 10 μg/ml BSA, 3 or 10 μg/ml CYR61 (generous gift from Dr. M. Aoki of our laboratory), or 10 μg/ml fibronectin in PBS for 2 h at 37 C. After washing with 0.1% BSA-PBS, 50 μl of normal rabbit IgG (Chemicon International, Temecula, CA) or anti-CYR61 polyclonal Ab (CYRO70–1; generated in our laboratory) (5), 100 μg/ml in 0.1% BSA-PBS, was added, followed by incubation for 2 h at room temperature. After washing two times with 0.1% BSA-PBS, 50 μl PrSC cells, 1 × 104 cells, were plated into each well in stromal cell basal medium containing 0.01% BSA and penicillin/streptomycin. Cell spreading was examined by microscopy, after incubation for 1 h. Proliferation of PrSC was examined in a similar experimental system. Multiple 96-well plates (Costar) were coated with 50 μl of 10 μg/ml CYR61 or 10 μg/ml fibronectin in PBS overnight at 4 C. After removing the coating solution, unbound sites were blocked with 0.5% BSA-PBS by incubation for 2 h at room temperature. The wells were washed with 0.1% BSA-PBS, and 100 μl of normal rabbit IgG, 100 μg/ml, or anti-CYR61 polyclonal Ab (CYRO70–1; 5), 100 μg/ml in 0.1% BSA-PBS, was added, followed by incubation for 2 h at room temperature. After washing with 0.1% BSA-PBS, 50 μl of PrSC cells, 8 × 103 cells, were plated into each well in stromal cell basal medium containing 0.01% BSA and penicillin/streptomycin. After 2 h incubation at 37 C, 50 μl PDGF, 5 ng/ml (final concentration) were added to each well, and cellular growth was assessed 5 d later. Ten microliters of Cell Count Reagent SF (Nacalai Tesque) were added to 100 μl culture for 3–4 h at the end of the culture. The absorbance at 450 nm (ref. 650 nm) of each well was measured with a microplate reader (Molecular Devices).
Statistical analysis
For the microarray study (see Fig. 4), statistical significance was determined by one-way ANOVA with a Dunnet post hoc test. Differences were considered significant only at P < 0.05. For the in vitro study, data are presented as means ± SD. Statistical significance was determined with Student’s t test. Differences were considered significant only at P < 0.05.
Fig. 4.
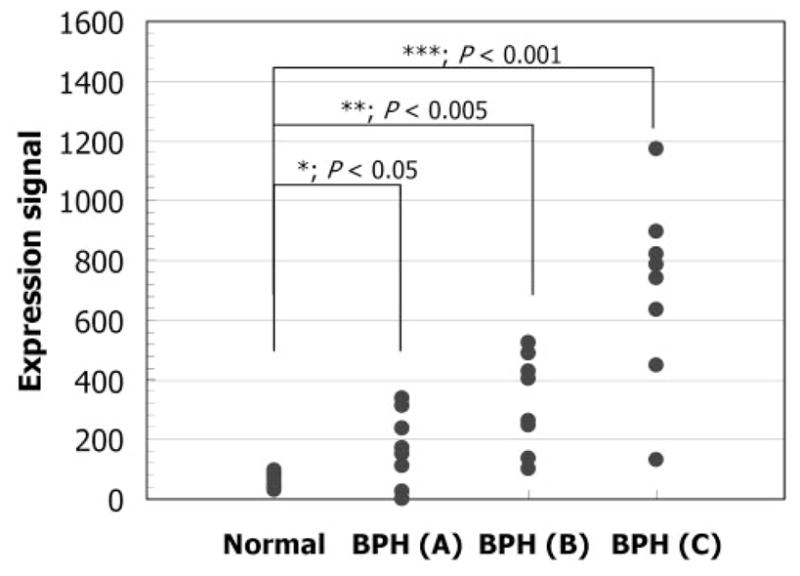
CYR61 gene expression determined in prostate transition zone tissue derived from BPH patients and normal individuals using microarrays. The CYR61 levels given are the mean expression counts based on the signal intensity determined with the microarrays. The first group (normal) of prostate samples was from 10 organ donors. The second group (BPH) comprised asymptomatic BPH samples (A), symptomatic BPH samples (B), and BPH with cancer samples (C). Statistical significance was determined by one-way ANOVA with a Dunnet post hoc test. *, P < 0.05; **, P < 0.005; ***, P < 0.001 vs. normal.
Results
18:1 LPA induces CYR61 mRNA and protein in prostatic epithelial and stromal cells
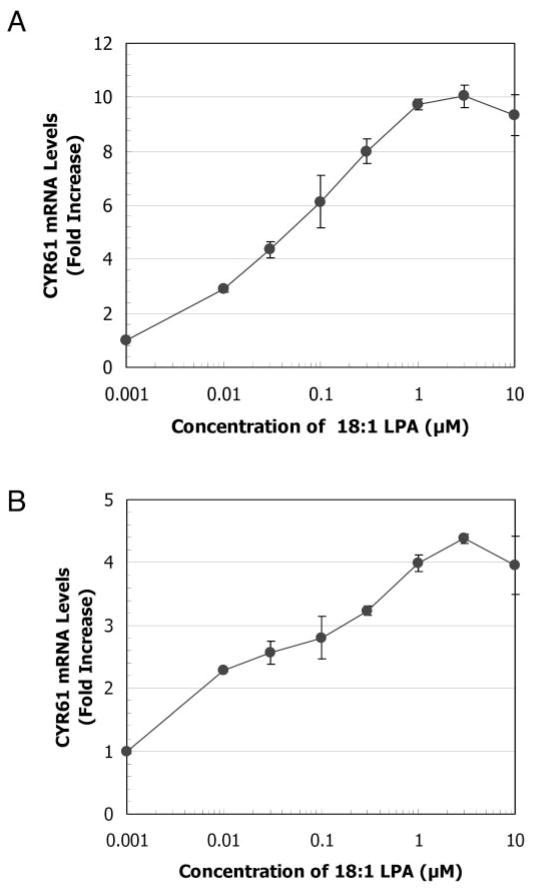
To determine whether transcription of CYR61 in prostatic cells is induced by LPA in a short period of time, we assessed the CYR61 mRNA level in both BRF-55T epithelial and PrSC stromal cells 1 h after treatment with various concentrations of LPA using the bDNA assay (5). LPA with oleic acid (18:1) in an acyl linkage at the 1-position, commonly referred to as oleoyl-LPA or 18:1 LPA, is typically used as an experimental agonist. In this study, 18:1 LPA was used as a general molecular species of LPA instead of specific ones. CYR61 gene expression was dramatically up-regulated by 18:1 LPA in a dose-dependent manner (Fig. 1). The EC50 values for BRF-55T and PrSC were 0.06 μM and 0.04 μM, respectively. After 1 h treatment, increased CYR61 gene expression was achieved with 1–3 μM LPA, maximum stimulation occurring with 3 μM LPA.
Fig. 1.
Effects of various concentrations of 18:1 LPA on CYR61 mRNA expression. 1 × 104 per well of BRF-55T cells (A) and PrSC cells (B) were stimulated with various concentrations of 18:1 LPA and then incubated for 1 h. CYR61 mRNA levels were measured using a bDNA assay. Based on the luminescence counts, numerical values (fold increase) were obtained by dividing the counts in stimulated cells by those in nonstimulated cells after 1 h stimulation. Values represent the means ± SD (n = 3) for single representative experiments. All experiments were performed at least twice.
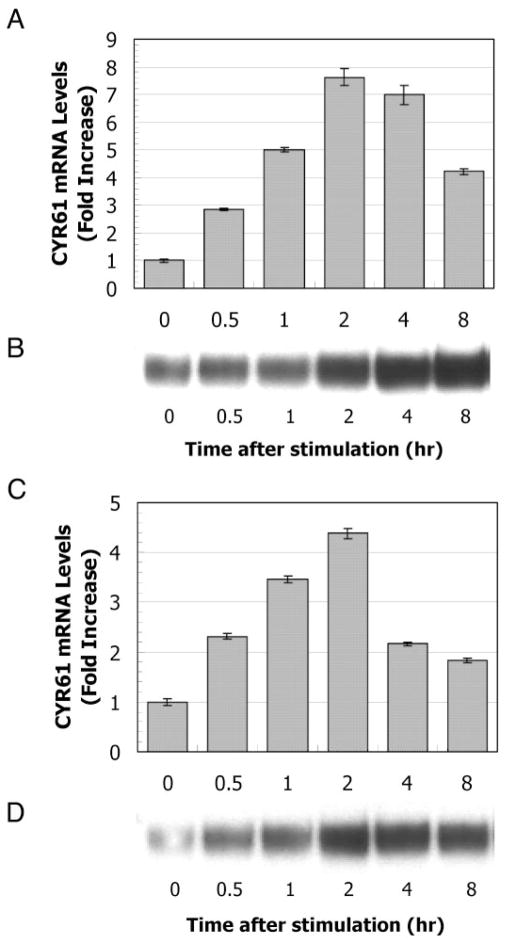
Next, we determined the time course of the stimulation of the cells with LPA. Cells were stimulated with 1 μM 18:1 LPA, and then the CYR61 mRNA and protein levels were measured using a bDNA assay and Western blotting analysis, respectively (Fig. 2, A–D). CYR61 mRNA was rapidly and transiently induced on 1 μM 18:1 LPA stimulation in both BRF-55T and PrSC cells. In BRF-55T cells, the CYR61 mRNA level reached a maximum within 2 h, remained almost constant up to 4 h, and then decreased gradually (Fig. 2A). In PrSC cells, the CYR61 mRNA level also reached a maximum within 2 h and then decreased to the basal level (Fig. 2C). On the other hand, a rapid accumulation of intracellular CYR61 protein was observed on Western blotting analysis (Fig. 2, B and D). These data indicate that CYR61 protein was produced in an immediate-early manner after up-regulation of mRNA expression induced by LPA. Although it was not remarkable, a reduced level of CYR61 protein (Fig. 2D) was observed in PrSC cells but not in BRF-55T cells. The main reason for this difference may be a change in the balance between production and secretion in both epithelial cells and stromal cells because CYR61 is a secreted protein. In other words, given that the secretion rate is similar in the two cell types, mRNA synthesis in epithelial cells, e.g. that at 4 h was higher than that in stromal cells, and an obvious decline in the level of intracellular CYR61 protein thereby might be not observed in BRF-55T cells (Fig. 2B), or an overproduced protein in PrSC cells might have been promptly secreted extracellularly (Fig. 2D).
Fig. 2.
Time course of 18:1 LPA stimulation of the expression of CYR61 mRNA and protein. 1 × 104 per well of BRF-55T cells (A and B) and PrSC cells (C and D) were stimulated with 1 μM 18:1 LPA and then incubated for 0, 0.5, 1, 2, 4, and 8 h. mRNA and protein samples were prepared at the indicated time points. The bar graph indicates the CYR61 mRNA expression levels for LPA-stimulated cells (A and C). CYR61 mRNA levels were measured using a bDNA assay. The values shown are mean fold increases ± SD (n = 3), compared with the counts for unstimulated cells at 0 h in a single representative experiment. Increases in CYR61 protein levels were determined by Western blotting analysis (B and D). The results of one typical experiment of two are shown.
Expression of LPA receptors in prostatic cells
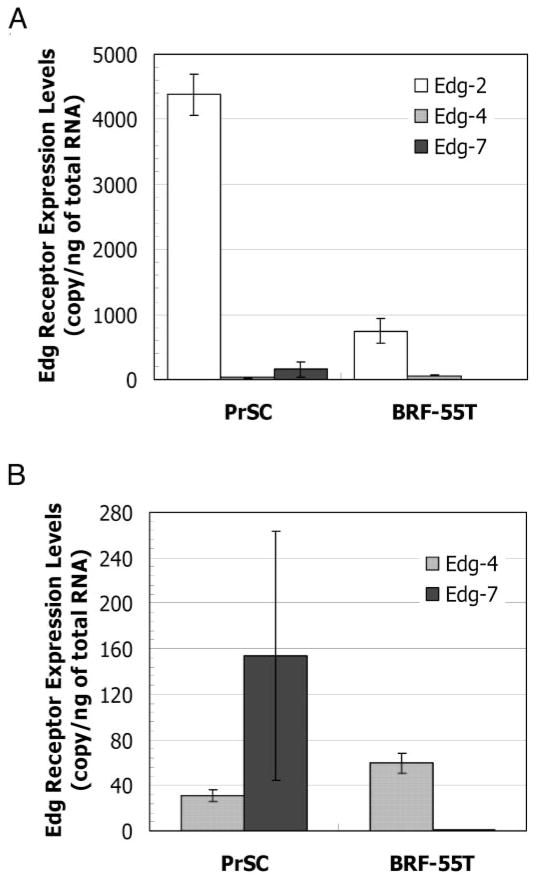
It is well known that LPA induces cellular responses by binding to specific members of the Edg family comprising seven transmembrane receptors. To determine whether cultured prostatic cells express LPA receptors of the Edg family, we assessed the expression level in both epithelial cells BRF-55T and stromal cells PrSC by quantitative RT-PCR (Fig. 3). The expression of Edg-2 (now LPA1) was relatively high on both cell types, especially in stromal cells (Fig. 3A). The expression of Edg-4 (now LPA2) was also observed on both cell types, but its level was lower than that of Edg-2 (now LPA1). Edg-7 (now LPA3) expression on prostatic cells exhibited a biased distribution, and the expression of Edg-7 was observed only on the stromal cells (Fig. 3B). These data indicate that the mRNAs for at least two LPA receptor subtypes were present in these cells (Fig. 3) and therefore that both prostatic cell types could respond to LPA (Figs. 1 and 2).
Fig. 3.
Differential expression of LPA receptors on PrSCs and epithelial cells (BRF-55T). LPA receptor mRNA levels were determined by quantitative RT-PCR using a TaqMan PCR core reagent kit; SYBR Green I; and human specific primers Edg-2, Edg-4, and Edg-7. A total of 100 ng total RNA/sample was analyzed in triplicate, and levels were determined using corresponding standard curves obtained with the respective LPA receptor cDNAs. The expression levels of Edg-2 (open bar), Edg-4 (hatched bar), and Edg-7 (solid bar) (A) and Edg-4 (hatched bar) and Edg-7 (solid bar) (B) are expressed as copy numbers per total RNA (copy per nanogram of total RNA ± SD). The same experiments were performed four times in triplicate, and the average value and SD, which were derived from 12 points, were displayed.
Up-regulation of CYR61 in BPH tissues
Previously we reported the results obtained for the differential gene expression in BPH using microarrays (4). CYR61, a member of the immediate early gene family, was significantly up-regulated in BPH (5). In the present study, to confirm these results, the gene expression profiles of prostate transition zone tissue derived from BPH patients and normal individuals were further analyzed. As shown in Fig. 4, the CYR61 expression level was elevated 2.7-fold (P < 0.05) in asymptomatic BPH patients (n = 8), compared with normal individuals (n = 10). Moreover, the CYR61 expression level was elevated 5.2-fold (P < 0.005) in symptomatic BPH patients (n = 8) and 11.3-fold (P < 0.001) in BPH patients with cancer (n = 8). Surprisingly, we observed overexpression of CYR61 by more than 2-fold in 19 (79%) of the 24 BPH samples. Because CYR61 was originally reported to be a key growth mediator for the endothelium in a number of cellular processes, based on its elevated levels, we conjecture that CYR61 is a putative mediator in the pathogenesis of prostate enlargement.
Localization of CYR61mRNA and protein in BPH tissues
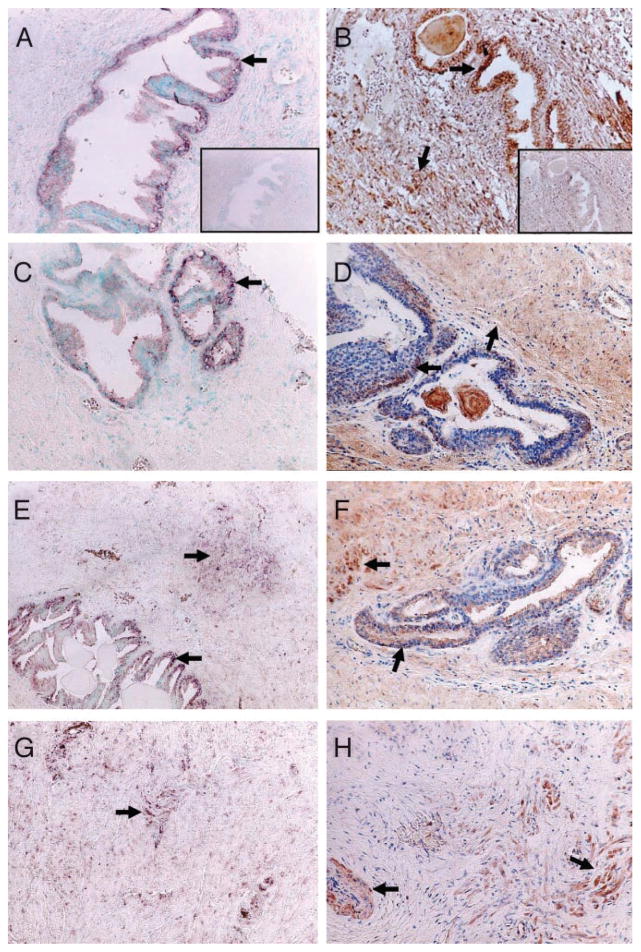
To determine the localization of CYR61 expression in BPH patient tissues, we prepared sections of tissue specimens exhibiting hyperplastic changes, which were obtained from the prostate transition zone, and examined the expression and localization of CYR61 by in situ hybridization and immunohistochemical staining. The patients’ profiles are presented in Table 1. All of the patients from whom tissue samples were obtained for this study had symptomatic BPH but not prostatic cancer. Representative results obtained with an antisense probe are shown in Fig. 5, A, C, E, and G. In Fig. 5, A and C, i.e. cases 1 and 2, respectively, CYR61 mRNA expression was predominantly detected in the cytoplasm of the epithelium but not in stromal tissue. Specifically, CYR61 mRNA was mainly expressed in the basal cells. The sense-strand of CYR61 cDNA, used as a negative control, did not give a detectable signal (Fig. 5A, inset). In tissue sections from other patients, CYR61 mRNA expression was occasionally observed in the stromal region (Fig. 5, E and G, i.e. cases 3 and 4); however, CYR61 mRNA expression in the stromal region seemed to be weak, except in case 8 (Table 1). The intensity in the epithelium was stronger than that in stromal cells in six of the 10 samples.
Fig. 5.
Analysis of CYR61 mRNA and protein expression in a human BPH patient by in situ hybridization and immunohistochemical staining. In situ hybridization was accomplished with a digoxygenin-11-uridine 5-triphosphate-labeled CYR61 antisense probe (A, C, E, and G). A sense probe (A, inset), as a negative control, gave no signal. Arrows indicate representative marked expression of CYR61 transcripts (A, C, E, and G). Original magnification, ×100. Immunohistochemical staining was performed with anti-CYR61 polyclonal Ab (Santa Cruz Biotechnology) (B, D, F, and H) as described in Materials and Methods. Normal goat IgG (B, inset), as a negative control for immunostaining, gave no intense signal. Arrows indicate representative benign epithelium with marked staining and stromal region tissue with significant immunoreactivity (B, D, F, and H). Original magnification, ×100.
On the other hand, immunohistochemical staining was also performed for prostate tissue specimens from the same patients. The observed epithelial immunoreactivity was consistent with the localization of CYR61 mRNA in prostatic epithelial cells, especially in basal cells (Fig. 5, B, D, and F). Interestingly, in Fig. 5, B and D, CYR61 immunoreactivity was observed not only in the epithelium but also in the stromal region. Because CYR61 is a secretory protein, it seems likely that it was produced in the epithelium due to a certain stimulus and then was secreted into the stromal region. In Fig. 5H, i.e. case 4, CYR61 protein expression was observed in the stromal region rather than in epithelial cells. However, there was no case in which strong expression of CYR61 mRNA was mainly detected in stromal tissues, and CYR61 protein was detected in the epithelium. The intensity in the epithelium was stronger than that in stromal cells in three of the 10 samples, whereas that in the stromal cells was more prominent in these three cases. Indeed, samples exhibiting no or only weak immunoreactivity in both epithelial and stromal cells were also seen (cases 5 and 7). Although CYR61 expression appears likely to vary with each BPH patient’s condition, e.g. epithelial cell-driven hyperplasia and/or stromal cell-driven hypertrophy, these observations suggest the possibility that in most, if not all, BPH cases, CYR61 is mainly synthesized in the epithelium, and then the produced CYR61 acts as a signaling molecule for both epithelial and stromal cells. Unfortunately, regarding the relationship of CYR61 expression to prostate volume or International Prostate Symptom Score scores, no remarkable tendency was observed.
CYR61 promotes prostatic cell adhesion and spreading and potentiates cell proliferation
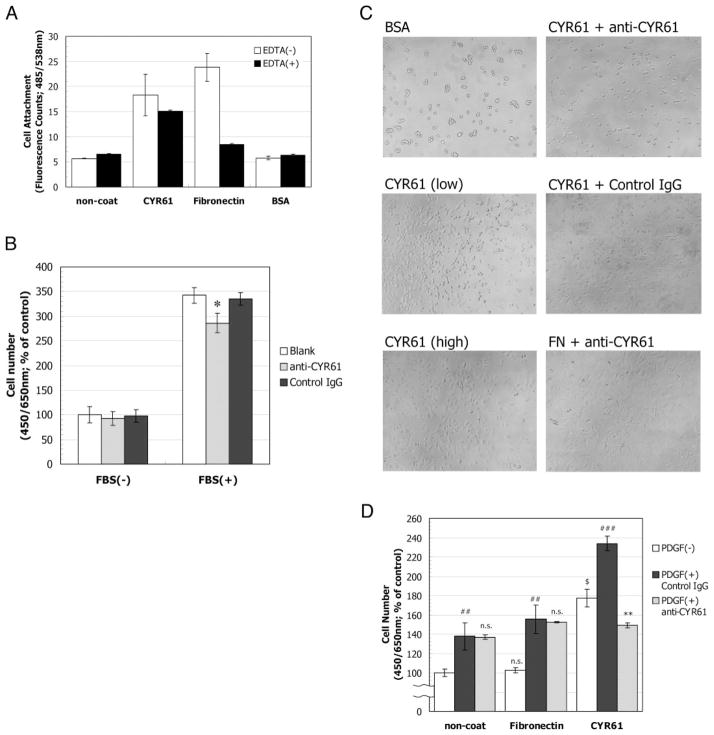
In the present study, we have shown that CYR61 expression was significantly influenced by 18:1 LPA stimulation in both epithelial and stromal cells in vitro (Figs. 1 and 2). Next, we investigated the function of the produced CYR61 protein on cellular responses. Because CYR61 protein has been shown to mediate signal transduction via the interaction of integrins on human umbilical vein endothelial cells and NIH 3T3 cells (8–10), we examined the effect of CYR61 on cellular adhesion using recombinant CYR61 protein. CYR61 protein promoted the adhesion of prostatic epithelial cells (Fig. 6A). Interestingly, this ability was partly inhibited on the addition of EDTA, suggesting interaction through cell surface receptor integrins, which is known to depend on divalent ions, whereas the fibronectin-mediated adhesion was completely abolished on the addition of EDTA. These data suggested that epithelial cells adhere to CYR61 through many kinds of cell surface receptors, i.e. not only integrins. The functional contribution of CYR61 protein to the growth of BRF-55T cells was examined by using anti-CYR61 polyclonal Ab (CYRO70–1) on FBS-induced cell proliferation. BRF-55T cells were plated on CYR61 precoated plates and then stimulated with 0.5% FBS (final concentration) in the presence of anti-CYR61 polyclonal Ab. Here we used FBS as the growth stimulus for inducing cell proliferation because assay conditions for BRF-55T growth with a sole growth factor, e.g. epithelial growth factor or basic fibroblast growth factor, could not be established except for the use of FBS. Cellular growth was assessed after 6 d (Fig. 6B). The increased cell number on FBS stimulation significantly decreased on the addition of anti-CYR61 polyclonal Ab (P < 0.05, Student’s t test, two sided). Because we previously reported that FBS strongly induces CYR61 expression in BRF-55T cells (5), the approximately 25% reduction appeared to result from the functional inhibition of both exogenously coated CYR61 and endogenously FBS-produced CYR61 by anti-CYR61 polyclonal Ab. The results of treatment with normal rabbit IgG were similar to those under blank conditions.
Fig. 6.
Functional contribution of CYR61 to prostatic cellular responses. A, Effect of recombinant CYR61 protein on prostatic epithelial cell adhesion. CYR61-induced adhesion of BRF-55T cells. BRF-55T cells in 100 μl of 0.01% BSA-RPMI 1640 were plated at 1 × 104 cells into each well of 96-well microtiter plates coated with 1 mg/ml BSA, 5 μg/ml CYR61, or 10 μg/ml fibronectin. The cells were incubated with a Cell Counting Kit-F (Calcein-AM) for 2–3 h at 37 C. After washing four times with 100 μl of 0.01% BSA-RPMI 1640 to remove nonadherent cells, the adherent cells were subjected to fluorescence counting at 490 nm (ref. 515 nm) for the calcein reagent. Values represent the means ± SD (n = 3) for single representative experiments. B, Effect of anti-CYR61 polyclonal Ab (CYRO70–1) on FBS-induced prostatic epithelial cell proliferation. BRF-55T cells were plated at 8 × 103 cells into each well of multiple 96-well plates coated with CYR61. After 2 h incubation at 37 C, 0.5% (final concentration) FBS and normal rabbit IgG (Chemicon International; solid bar) or anti-CYR61 polyclonal Ab (100 μg/ml, hatched bar) were added to each well, and then cellular growth was assessed 6 d later. Cell proliferation, as measured as the number of viable cells, was evaluated at 450 nm (ref. 650 nm) using Cell Count Reagent SF (Nacalai Tesque). Numerical values shown are mean percent ± SD (n = 3), compared with the counts for unstimulated cells (open bar) 6 d later in a single representative experiment. All experiments were performed at least twice. Statistical significance was determined with Student’s t test (two sided). *, P < 0.05 vs. antibody nontreated blank. C, Effect of recombinant CYR61 protein on prostatic stromal cell spreading. PrSC cells were plated at 1 × 104 cells into each well of 96-well microtiter plates coated with BSA (A), CYR61 (B, 3, or C, 10 μg/ml), or fibronectin. Before cell plating, each coated well was treated with normal rabbit IgG (Chemicon International; E) or anti-CYR61 polyclonal Ab (CYRO70–1; D or F) at 100 μg/ml. Photomicrographs were taken after incubation for 1 h. Original magnification, ×40. All experiments were performed at least three times. D, Effect of recombinant CYR61 protein on PDGF-induced prostatic stromal cell proliferation. PrSC cells were plated at 8 × 103 cells into each well of multiple 96-well plates coated with CYR61 or fibronectin. Before cell plating, each coated well was treated with normal rabbit IgG (Chemicon International; solid bar) or anti-CYR61 polyclonal Ab (CYRO70–1; 100 μg/ml, hatched bar). After 2 h incubation at 37 C, PDGF was added at 5 ng/ml (final concentration) to each well, and cellular growth was assessed 5 d later. Cell proliferation, as measured as the number of viable cells, was evaluated at 450 nm (ref. 650 nm) using Cell Count Reagent SF (Nacalai Tesque). Numerical values shown are mean percent ± SD (n = 3), compared with the counts for unstimulated cells (open bar) 5 d later in a single representative experiment. All experiments were performed at least three times. Statistical significance was determined with Student’s t test (two sided). $, P < 0.005 vs. noncoated control. ##, P < 0.005; ###, P < 0.0005 vs. unstimulated control. **, P < 0.005 vs. PDGF stimulation on CYR61. n.s., No significance.
On the other hand, we examined the effect of CYR61 protein on spreading using PrSC cells. Representative results of cell spreading assays are shown in Fig. 6C. CYR61 protein caused the spreading of prostatic stromal cells in a dose-dependent manner. To confirm signal transduction through integrins, we examined whether activation of p42/p44 MAPK, a typical signaling event via integrins, was induced by coated CYR61. The phosphorylation of p42/p44 MAPK in PrSC was rapidly and transiently induced by binding to CYR61 within 1 h as well as to fibronectin (data not shown). The ability of spreading was clearly inhibited by the CYR61-specific polyclonal Ab but not by normal rabbit IgG. Pre-treatment with the CYR61-specific polyclonal Ab had no effect on the spreading of PrSC on fibronectin (Fig. 6C). Next, we plated stromal cells on a plate coated with CYR61 or fibronectin to assess the effect of CYR61 on growth factor-induced cell proliferation and to address the effect of anti-CYR61 polyclonal Ab (CYRO70–1) on stromal cell proliferation (Fig. 6D). After 2 h incubation, cell spreading was observed for most cells plated on CYR61 or fibronectin. PrSC cells were then stimulated with 5 ng/ml PDGF, a potent regulator of BPH, and its effect was assessed 5 d later. Although cell spreading was observed on fibronectin, there were no significant differences between the basal and PDGF-induced cell numbers in non- and fibronectin-coated wells. Additionally, the result of pretreatment with normal rabbit IgG was similar to that with anti-CYR61 polyclonal Ab, and it had no effect on the growth of PrSC cells. In contrast, increased numbers of cells were seen in CYR61-coated wells, compared with non- and fibronectin-coated wells (P < 0.0001 or P < 0.0005). Additionally, the increased cell numbers were reduced by anti-CYR61 polyclonal Ab (P < 0.005). This effect depended on the anti-CYR61 polyclonal Ab dose (data not shown). Pretreatment with anti-CYR61 polyclonal Ab had no effect on the growth of PrSC in non- or fibronectin-coated wells.
Discussion
The etiology and mechanism of BPH progression remain poorly understood despite intense research efforts over the past decades. Although the pathogenesis of BPH is thought to involve alterations in the hormonal balance associated with aging, recent studies suggested that hormonal changes alone may not explain hyperplastic development of the prostate gland (reviewed in Ref. 2).
Our study outlined here indicates a novel causal relationship between LPA and CYR61. Furthermore, we also propose that this causal link between these two molecules is key to BPH progression (Fig. 7). In the first series of experiments, we demonstrated for the first time that CYR61 gene expression was dramatically up-regulated by 18:1 LPA. This effect occurred in a short period in a dose-dependent manner in both prostatic epithelial cells, BRF-55T, and stromal cells, PrSC. CYR61 protein was produced in an immediate-early manner after up-regulation of mRNA expression. Interestingly, other LPAs (14:0, 16:0, and 18:0) had also drastically up-regulated CYR61 mRNA at 1 h in both epithelial and stromal cells, and specificity and/or dependency of the LPA species was not observed in a one-dose (10 μM; serum levels of LPA) experiment (data not shown). In addition, the LPA-mediated induction of CYR61 expression was similar to that caused by FBS, as a positive control (data not shown), and seems to be fairly strong, compared with that by other inducers reported earlier (Ref. 5, reviewed in Ref. 35). LPA is produced by activated cells, increased levels of LPA are observed in tissue injury and neoplasia (reviewed in Ref. 36), and LPA activity has been detected in seminal fluid (37). These data suggest that endogenous LPA may induce CYR61 expression in vivo, especially in the human prostate gland. Because the induction of CYR61 gene expression by LPA is dose and time dependent, it is plausible that such an effect is mediated by specific receptors. To date, the cDNAs for three structurally distinct receptors for LPA have been reported (26). In this study, we investigated, for the first time, their expression patterns in human prostatic cells, which are unlike cancer cells (33, 34), by quantitative RT-PCR. Our study demonstrated that both prostatic cell types contain mRNAs for at least two LPA receptor subtypes, and respond to LPA, as assessed by as the increases in CYR61 mRNA and protein. CYR61 gene expression in prostatic cells appears to be induced directly by LPA.
Fig. 7.
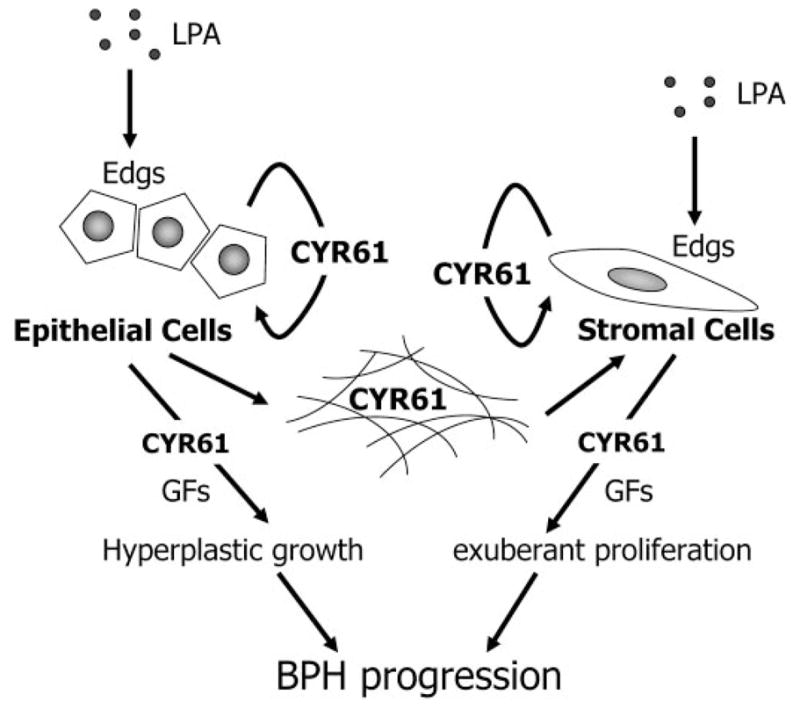
A hypothetical model for the behavior of CYR61 in the progression of benign prostatic enlargement. Given that LPA serves as a potent regulator in the initiation and/or progression of BPH, we propose the following schema. LPA induces CYR61 mRNA and protein in prostatic tissues by interacting with cell surface-specific LPA receptors (Edgs), probably mainly in the epithelium, in vivo. CYR61 protein triggers a growth-promoting signal, e.g. the activation of p42/ p44 MAPK, via corresponding integrins in both epithelial and stromal cells in an autocrine manner. Some of the CYR61 protein is secreted into the stromal region and affects stromal cells in a paracrine manner. CYR61 coordinates with a certain growth factor (GF), e.g. FBS or PDGF, and plays a functional role in prostatic cell growth, or potentiates GF-stimulated prostatic cell proliferation. As a consequence, epithelial and stromal hyperplasia occurs. Arrows represent relationships that are supported by our data. Edgs, Edg receptors.
CYR61 expression has been reported to be up-regulated in several diseases and cell lines, especially in breast cancer that metastasizes to bone, and the mechanism is thought to be similar to that of prostate cancer (11–17). Aberrant expression of CYR61 was associated with clinical and pathological parameters (15, 38), RNA and protein were induced by 17β-estradiol (16), and CYR61 played critical roles in tumorigenesis and metastasis (15–17). On microarray analysis for BPH patients, CYR61 gene expression was found to be markedly up-regulated as well (Fig. 4). In fact, the CYR61 expression level was elevated 5.2-fold (P < 0.005) in samples obtained from prostate transition zone tissue from symptomatic BPH patients, compared with normal individuals, and 11.3-fold (P < 0.001), compared with BPH patients with cancer. In contrast, interestingly, down-regulation of CYR61 has been documented in human prostate cancer (39) and uterine leiomyomas as well (40). The expression of CYR61 in prostate cancer was not evaluated in our microarray study, and the presumable role of CYR61 down-regulation has not been studied (39). Although the significance of such down-regulation of CYR61 in prostate cancer is unclear at the moment, the seemingly opposite expression in the same tissue is very intriguing. Part of this down-regulation may be due to the fact that in these study comparisons were made with either benign adjacent tissue or BPH but not with normal individuals. We have shown that these tissues exhibit high levels of CYR61 expression, and therefore the down-regulation observed on cancer staining may be related to these high levels.
We then attempted to determine the localization of CYR61 expression in BPH patient tissues. In situ hybridization studies indicated that CYR61 was mainly synthesized in the epithelium exhibiting hyperplastic changes, especially in the basal cells. The intensity in the epithelium was stronger than that in stromal cells in six of the 10 samples. Of course, in other specimens, CYR61 mRNA expression was infrequently observed in the stromal region. The epithelial immunoreactivity observed on immunohistochemical analyses was consistent with the localization of CYR61 mRNA in prostatic epithelial cells, but the immunoreactivity of CYR61 was not confined to the epithelial region, being also found in the stromal region. Because CYR61 is a secretory protein, it seems likely that CYR61 is mainly synthesized in the epithelium, and then the produced CYR61 acts as a signaling molecule for both epithelial and stromal cells in BPH tissues. In general, prostatic enlargement progresses as a result of both hyperplastic growth of the glandular cells and excessive growth of stromal cells in the transition zone of the prostate. With respect to CYR61, therefore, whether CYR61 is the autocrine or paracrine mediator in the prostate appeared to differ with each patient’s condition, i.e. epithelial cell-driven hyperplasia or stromal cell-driven hypertrophy. The higher expression of CYR61 mRNA in epithelial cells may result from the LPA production by epithelial cells in an autocrine manner (21).
The rapid accumulation of CYR61-related data have occurred over the past decade (reviewed in Refs. 6 and 7). Here we show that CYR61 protein promotes not only the adhesion of BRF-55T cells but also the spreading of PrSC cells (Fig. 6, A and C). The partial inhibition of CYR61-mediated adhesion by EDTA suggests that integrins maybe involved (Fig. 6A). In addition, the CYR61-mediated spreading was completely abolished on the addition of EDTA (data not shown). In fact, various integrins were present on the cell surface, such as α2, α3, α6, β1, and β4 for BRF-55T, and α1, α2, α5, αv, β1, and β3 for PrSC. These data indicate that CYR61 could act as an extracellular matrix protein that interacts with a growth-promoting signal through integrins (41). In Fig. 6B, we demonstrate the functional contribution of CYR61 to BRF-55T cell growth using recombinant CYR61 protein and anti-CYR61 neutralizing antibodies, which partially suppressed FBS-induced cell proliferation. These data indicated the possibility that CYR61 has a potential role in prostatic epithelial cellular growth. Moreover, an enhancing effect of CYR61 on prostatic stromal cell growth was demonstrated in the PDGF-induced cell proliferation assay. Recombinant CYR61 protein enhanced the growth of PrSC cells and this effect was completely suppressed by CYR61-specific polyclonal Ab (Fig. 6D), which also inhibited CYR61-dependent cell spreading (Fig. 6C). Taken together, these data indicated that the produced CYR61 plays an important role in growth factor-stimulated prostatic cellular growth; therefore, these findings permit us to speculate that CYR61 is involved the BPH and its progression.
In conclusion, CYR61 that is elicited by LPA appears to be a potential mediator in prostatic epithelial and stromal hyperplasia via both autocrine and/or paracrine roles (Fig. 7). Earlier research led to the elucidation of the functions of CYR61 in cell adhesion, cell proliferation, and the regulation of gene expression in vitro (reviewed in Refs. 6–10). But a series of recent papers suggested new paradigms. Aberration in a potent regulator, which underlies the disorder, causes the overexpression of CYR61, and the overproduced CYR61 evokes dysfunction, especially in vivo (11, 12, 14–17). Hereafter, there will be much interest in the results of in vivo administration studies involving suppressive or inhibitory compounds for CYR61. To date, research on BPH progression has been centered on steroid hormones, α1-adrenergic receptors, 5-α-reductase, and some growth factors. However, based on the present results and those of our ongoing studies (5), CYR61 appears to be an attractive target for therapeutic research.
Acknowledgments
We thank Dr. Michiko Aoki for generously providing the recombinant CYR61 protein and Mr. Junichiro Tsuruha for his excellent technical assistance. We are also grateful to Mr. Masahiro Furuno for generously providing the cDNA and specific PCR primers for each LPA receptor and Dr. Yutaka Saito for the helpful discussions and expert advice.
Abbreviations
- Ab
Antibodies
- bDNA
branched-chain DNA
- BPH
benign prostatic hyperplasia
- Edg
endothelial cell differentiation gene
- FBS
fetal bovine serum
- LPA
lysophosphatidic acid
- PBS/T
PBS solution containing 0.1% Tween 20
- PDGF
platelet-derived growth factor-BB
- PrSC
prostatic stromal cell
- WISP
Wnt-induced secreted protein
Footnotes
Contributors
S. Sakamoto was involved in all aspects of this study. S. Sakamoto was responsible for analysis of the CYR61 expression profiles induced by LPA, assessment of the CYR61 function in vitro assay, and preparation of this manuscript. S. Sakamoto and M. Yokoyama cooperated in the project coordination, study design, and discussion on the concept. X. Zhang determined the localization of CYR61 mRNA and protein in BPH patient tissues. K. Prakash carried out the microarray analyses and analyzed the gene expression profiles in normal and BPH patient tissues. K. Nagao examined the LPA induction of CYR61 gene expression. T. Hatanaka analyzed the presence of mRNAs of LPA receptors in prostatic cells by means of quantitative RT-PCR. R. H. Getzenberg provided patient tissue samples and supervised the microarray studies. Y. Kakehi provided patient tissue samples and supervised the in situ hybridization and immunohistochemical staining analyses.
References
- 1.Jonler M, Riehmann M, Bruskewitz RC. Benign prostatic hyperplasia. Current pharmacological treatment. Drugs. 1994;47:66–81. doi: 10.2165/00003495-199447010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Brown TR, Lee C. Conference summary on prostate growth and aging, September 13–15, 2000. Prostate. 2001;48:54–65. doi: 10.1002/pros.1081. [DOI] [PubMed] [Google Scholar]
- 3.Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate. 2000;42:150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Prakash K, Pirozzi G, Elashoff M, Munger W, Waga I, Dhir R, Kakehi Y, Getzenberg RH. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci USA. 2002;99:7598–7603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto S, Yokoyama M, Prakash K, Tsuruha J, Masamoto S, Getzenberg RH, Kakehi Y. Development of quantitative detection assays for CYR61 as a new marker for benign prostatic hyperplasia. J Biomol Screen. 2003;8:701–711. doi: 10.1177/1087057103259159. [DOI] [PubMed] [Google Scholar]
- 6.Perbal B, Brigstock DR, Lau LF. Report on the second international workshop on the CCN family of genes. Mol Pathol. 2003;56:80–85. doi: 10.1136/mp.56.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 8.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolesnikova TV, Lau LF. Human CYR61-mediated enhancement of bFGF-induced DNA synthesis in human umbilical vein endothelial cells. Oncogene. 1998;16:747–754. doi: 10.1038/sj.onc.1201572. [DOI] [PubMed] [Google Scholar]
- 11.Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Roth-kotter HJ, Schieffer B, Drexler H. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002;106:254–260. doi: 10.1161/01.cir.0000021426.87274.62. [DOI] [PubMed] [Google Scholar]
- 12.Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC. Identification of integrin αMβ2 as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- 13.Wu KJ, Yee A, Zhu NL, Gordon EM, Hall FL. Characterization of differential gene expression in monkey arterial neointima following balloon catheter injury. Int J Mol Med. 2000;6:433–440. [PubMed] [Google Scholar]
- 14.Pendurthi UR, Allen KE, Ezban M, Rao LV. Factor VIIa and thrombin induce the expression of Cyr61 and connective tissue growth factor, extracellular matrix signaling proteins that could act as possible downstream mediators in factor VIIa x tissue factor-induced signal transduction. J Biol Chem. 2000;275:14632–14641. doi: 10.1074/jbc.275.19.14632. [DOI] [PubMed] [Google Scholar]
- 15.Xie D, Miller CW, O’Kelly J, Nakachi K, Sakashita A, Said JW, Gornbein J, Koeffler HP. Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease. J Biol Chem. 2001;276:14187–14194. doi: 10.1074/jbc.M009755200. [DOI] [PubMed] [Google Scholar]
- 16.Sampath D, Winneker RC, Zhang Z. Cyr61, a member of the CCN family, is required for MCF-7 cell proliferation: regulation by 17β-estradiol and over-expression in human breast cancer. Endocrinology. 2001;142:2540–2548. doi: 10.1210/endo.142.6.8186. [DOI] [PubMed] [Google Scholar]
- 17.Menendez JA, Mehmi I, Griggs DW, Lupu R. International Congress on Hormonal Steroids and Hormones and Cancer: the angiogenic factor CYR61 in breast cancer: molecular pathology and therapeutic perspectives. Endocr Relat Cancer. 2003;10:141–152. doi: 10.1677/erc.0.0100141. [DOI] [PubMed] [Google Scholar]
- 18.Qi C, Park JH, Gibbs TC, Shirley DW, Bradshaw CD, Ella KM, Meier KE. Lysophosphatidic acid stimulates phospholipase D activity and cell proliferation in PC-3 human prostate cancer cells. J Cell Physiol. 1998;174:261–272. doi: 10.1002/(SICI)1097-4652(199802)174:2<261::AID-JCP13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Guo C, Luttrell LM, Price DT. Mitogenic signaling in androgen sensitive and insensitive prostate cancer cell lines. J Urol. 2000;163:1027–1032. [PubMed] [Google Scholar]
- 20.Kue PF, Daaka Y. Essential role for G proteins in prostate cancer cell growth and signaling. J Urol. 2000;164:2162–2167. [PubMed] [Google Scholar]
- 21.Xie Y, Gibbs TC, Mukhin YV, Meier KE. Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J Biol Chem. 2002;277:32516–32526. doi: 10.1074/jbc.M203864200. [DOI] [PubMed] [Google Scholar]
- 22.Daaka Y. Mitogenic action of LPA in prostate. Biochim Biophys Acta. 2002;1582:265–269. doi: 10.1016/s1388-1981(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 23.Jalink K, Hordijk PL, Moolenaar WH. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta. 1994;1198:185–196. doi: 10.1016/0304-419x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 24.Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 25.Tigyi G. Physiological responses to lysophosphatidic acid and related glycerophospholipids. Prostaglandins. 2001;64:47–62. doi: 10.1016/s0090-6980(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 26.Lynch KR. Lysophospholipid receptor nomenclature. Biochim Biophys Acta. 2002;1582:70–71. doi: 10.1016/s1388-1981(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 27.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, Jaffe RB, Wiener JR, Erickson JR, Mills GB. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann NY Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 28.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 29.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 30.Toews ML, Ediger TL, Romberger DJ, Rennard SI. Lysophosphatidic acid in airway function and disease. Biochim Biophys Acta. 2002;1582:240–250. doi: 10.1016/s1388-1981(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 31.Huang MC, Graeler M, Shankar G, Spencer J, Goetzl EJ. Lysophospholipid mediators of immunity and neoplasia. Biochim Biophys Acta. 2002;1582:161–167. doi: 10.1016/s1388-1981(02)00151-8. [DOI] [PubMed] [Google Scholar]
- 32.Adolfsson PI, Ahlstrand C, Varenhorst E, Svensson SP. Lysophosphatidic acid stimulates proliferation of cultured smooth muscle cells from human BPH tissue: sildenafil and papaverin generate inhibition. Prostate. 2002;51:50–58. doi: 10.1002/pros.10077. [DOI] [PubMed] [Google Scholar]
- 33.Im DS, Heise CE, Harding MA, George SR, O’Dowd BF, Theodorescu D, Lynch KR. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol. 2000;57:753–759. [PubMed] [Google Scholar]
- 34.Gibbs TC, Xie Y, Meier KE. Regulation of expression of EDG family receptors in human prostate cancer cell lines. Ann NY Acad Sci. 2000;905:290–293. doi: 10.1111/j.1749-6632.2000.tb06563.x. [DOI] [PubMed] [Google Scholar]
- 35.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/ nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 36.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- 37.Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H. Lysophosphatidic acid (LPA) receptors are activated differentially by biological fluids: possible role of LPA-binding proteins in activation of LPA receptors. FEBS Lett. 2002;523:187–192. doi: 10.1016/s0014-5793(02)02976-9. [DOI] [PubMed] [Google Scholar]
- 38.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–8923. [PubMed] [Google Scholar]
- 39.Pilarsky CP, Schmidt U, Eissrich C, Stade J, Froschermaier SE, Haase M, Faller G, Kirchner TW, Wirth MP. Expression of the extracellular matrix signaling molecule Cyr61 is downregulated in prostate cancer. Prostate. 1998;36:85–91. doi: 10.1002/(sici)1097-0045(19980701)36:2<85::aid-pros3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Sampath D, Zhu Y, Winneker RC, Zhang Z. Aberrant expression of Cyr61, a member of the CCN (CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17 β-estradiol and basic fibroblast growth factor in human uterine leiomyomas. J Clin Endocrinol Metab 2001. 2001;86:1707–1715. doi: 10.1210/jcem.86.4.7423. [DOI] [PubMed] [Google Scholar]
- 41.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]