Abstract
Objectives
To address the value of surgery in sporadic Zollinger-Ellison syndrome (ZES) patients with negative imaging studies.
Background
Medical control of acid hypersecretion in patients with sporadic Zollinger-Ellison syndrome (ZES) is highly effective. This has led to these patients frequently not sent to surgery, especially if preoperative imaging studies are negative, due in large part because almost no data exists on the success of surgery in this group.
Methods
58 prospectively studied sporadic ZES patients (17% of total studied) had negative imaging studies and their surgical outcome was compared to 117 patients with positive imaging results.
Results
35 patients had negative imaging in the pre-somatostatin receptor scintigraphy era (SRS) and 23 in the post-SRS era. The image negative patients had long disease histories prior to surgery (mean±SEM, from onset=7.9±1[range −0.25-35 yrs]) and 25% were followed ≥2yrs from diagnosis. At surgery, gastrinoma was found in 57/58 patients (98%). Tumors were small (mean=0.8cm, 60% < 1 cm). The most common primary sites were: duodenal 64%, pancreatic 17%, and lymph node (LN)(10%). 50% had a primary only, 41% primary + LN, and 7% had liver metastases. 35/58(60%) were cured immediately postoperatively and at last follow-up [mean-9.4yrs, range 0.2-22yrs], 27 patients (46%) remained cured. During follow-up 3 patients died, each was found to have liver metastases at surgery. In comparison to the image positive patients, those with negative imaging had lower preop fasting gastrin levels; a longer delay prior to surgery; more frequently had a small duodenal tumors; less frequently had a pancreatic tumor, multiple tumors or developed a new lesion postoperatively and had a longer survival.
Conclusions
Imaging negative sporadic ZES patients are not rare even in the post-SRS period. An experienced surgeon can find gastrinoma in almost every patient (98%) and nearly one-half (46%) are cured, a rate similar to imaging positive tumor patients. Because liver metastases were found in 7%, which may have been caused by a long delay in surgery and all the disease-related deaths occurred in this group, surgery should be routinely undertaken early in ZES patients despite negative imaging studies.
Keywords: Gastrinoma, imaging negative, surgery, outcome
Introduction
Medical therapy has become increasingly effective in the control of gastric acid hypersecretion in Zollinger-Ellison syndrome patients to the present, where acid can now be controlled in almost every patient both acutely and long-term, thus total gastrectomy is rarely needed 1. This approach became possible first with the development and widespread use of the histamine receptor antagonists (cimetidine, ranitidine, famotidine, nizatidine) in the late 1970-1980s and later the development of the long-acting proton pump inhibitors (omeprazole, lansoprazole, rabeprezole, esomeprazole, pantoprazole), which allowed once or twice a day dosing in most patients 1,2,3. The success of medical therapy coupled with the fact that gastrinomas are frequently not localized preoperatively 4,5,6 and that in many patients with ZES the gastrinomas show indolent behavior 7 has led to a number of groups recommending that surgical exploration not be routinely performed or that it only be performed in patients where the preoperative imaging localizes a likely primary tumor 8,9,10,11,12,13,14,15. Recent studies show that the use of PPIs is not only delaying the diagnosis and but also delays the time patients are sent to surgery, so that patients have more advanced disease 3,16.
This latter recommendation has partially developed because of the lack of data on the efficacy of surgery in imaging negative patients with ZES. Some studies 17,18, containing primarily sporadic ZES patients with positive imaging results, have shown that surgical removal of the gastrinoma can increase survival by decreasing disease-related deaths, resulting in a decrease in the postoperative development of liver metastases, which are the major prognosticators of survival in ZES 18,19. However, no studies have specifically dealt with effectiveness of surgery in the imaging negative groups of patients with sporadic ZES.
In the present study we have attempted to address this issue by comparing from our prospective study of 229 ZES patients, the surgical results in 58 sporadic ZES patients with preoperative negative imaging (35 in the pre-Somatostatin receptor scintigraphy era (SRS), and 23 in the post SRS era) to the results of 117 patients with positive imaging operated over the same time period.
Methods
Two prospective databases of patients who underwent surgery to remove gastrinoma and cure ZES were reviewed 5,19. In one, surgery was done at Stanford University hospital (SUH) since 1996 and, in the other, at the National Institutes of Health (NIH) since 1981. The main outcome measures were overall survival, disease-related survival, and time to development of any recurrence and liver metastases.
The diagnosis of ZES was based on measurement of an elevated fasting serum level of gastrin (> 100 pg/ml), an elevated basal acid output (>10 mEq/h), and the results of secretin and calcium provocative tests 20,21. Basal and maximal acid output (BAO, MAO) was determined for each patient using methods described previously. Briefly, each patient had an elevated fasting serum level of gastrin and a concomitant elevated basal acid output. Most patients also had an abnormal secretin test (>120 pg/ml increment in gastrin following iv 2U/kg secretin) 21. These studies confirmed the diagnosis of Zollinger-Ellison syndrome for each patient. After confirming the diagnosis, patients underwent detailed imaging studies (thin slice CT scan with intravenous contrast, MRI with gadolinium, ultrasound 22, and since 1994 each underwent somatostatin receptor scintigraphy (using [111In-DTPA-DPhe1]-octreotide (6 mCi) with whole body, planar, and SPECT views )4,23,24,25, 22, and in selected cases abdominal angiography to determine precise tumor localization and operability as described previously. In some patients if SRS and conventional localization was equivocal, either selective venous sampling for gastrin gradients basally 26 or after secretin injection and hepatic venous sampling 27 was used to regionally localize the tumor 28. Patients were invited to undergo surgery to remove the tumor if they had no co-morbid medical condition markedly limiting life expectancy, had imaging evidence of either operable localized tumor or no tumor identified 29,25,30. In this particular study patients with either preoperative imaging evidence of liver metastases or family history and biochemical evidence of MEN-1 were excluded and only patients with sporadic ZES with either imaging localized or imaging negative gastrinomas were included.
A detailed past history of disease was taken at first admission and past medical/surgical procedures as described previously 6. Time from onset of symptoms to exploration was determined for all patients. The time of diagnosis of ZES was the time the diagnosis was first established by appropriate laboratory studies, when a physician established the diagnosis based on clinical presentation or when the histological diagnosis was established 6.
The operative techniques have been described previously 30,31,5,18. The pancreas and duodenum were widely exposed by dividing the inferior border of the body and tail of the pancreas and performing an extended Kocher maneuver during which the right colon and hepatic flexure were mobilized away from the pancreas and duodenum. Intraoperative ultrasound of the pancreas and duodenum was systematically performed on all patients 32. The duodenum was routinely opened longitudinally and closed transversely in all patients unless a gastrinoma was located in the body or tail of the pancreas 5. A detailed inspection for peripancreatic, periduodenal, or portohepatic lymph nodes was carried out, and these were routinely removed. Tumors in the pancreatic head were enucleated. Tumors in the pancreatic body and tail were resected with a distal pancreatectomy splenectomy. If a large pancreatic head tumor was present and could not be enucleated, a pancreaticoduodenectomy was performed. If liver metastases were present, they were biopsied and excised by either wedge resection or anatomical resection. Postoperatively, patients underwent evaluation for disease-free status immediately after surgery (i.e., 2 weeks post-resection), within 3 to 6 months post-resection, and then yearly 33,6,25,34. Yearly evaluations included conventional imaging studies (CT, ultrasound, MRI, and angiography, if necessary); somatostatin receptor scintigraphy since 1994; assessment of fasting serum level of gastrin, secretin stimulated gastrin level and acid output. Complete disease-free status (or cure) is defined as normal fasting serum levels of gastrin, negative secretin test and no evidence of tumor on postoperative imaging studies including CT and SRS 30,5,6,34. A recurrence post-resection was defined as occurring in a patient who was initially disease-free, but then lost disease-free status on follow-up evaluation by developing positive imaging studies or recurrent elevated fasting serum gastrin levels 25.
All continuous variables were reported as mean ± standard error of the mean. Survival analysis was done using the Kaplan–Meier method and two-group comparisons using log-rank tests. Proportions are compared statistically by Fisher exact test. Statistical analyses were performed by means of the SAS statistical software package and significance was defined as two-tailed P value less than 0.05.
Results
339 patients with ZES were identified which includes 110 patients with MEN-1 and 229 sporadic patients. This analysis excludes patients with MEN-1 such that of the 229 sporadic ZES patients 58 had negative preoperative imaging and those are compared to 117 with positive imaging who underwent the same operation. Of the imaging negative cohort 35 patients were from the pre-SRS era and had negative conventional imaging studies (CT, MRI, ultrasound) and angiography, whereas 23 were from the post-SRS period 23 and had both SRS as well as the imaging studies described for the pre-SRS group. Of the 58 image negative patients, 33 (57%) were male, the mean age was 42.6 years and 48.8 at onset or diagnosis of ZES, the main presenting symptom was upper abdominal pain and none of these variables was different from the imaging positive cohort (Table 1). However, the proportion with prior abdominal surgery (10%) and acid-related surgery (3.4%) was significantly lower in the imaging negative group compared to the imaging positive group 44% and 21%, respectively (Table 1). This was not true for hiatal hernia surgery that was higher proportionally in the imaging negative group, 8.6% vs 0.8%. The fasting serum gastrin level was significantly lower for the imaging negative group, but the delta secretin and the BAO were not different (Table 1).
Table 1.
Comparison of Patient Clinical Characteristics and laboratory results preoperatively in patients with or without imaging positive studies.
| Characteristic | Number (%) | ||
|---|---|---|---|
| IMAGE NEG | IMAGE POS | Significance | |
| Total number | 58 | 117 | |
| Male | 33 (57%) | 76 (65%) | NS |
| Ages (yrs) | |||
| Age ZES onset (yrs) | |||
| Mean ± SEM | 42.6 ± 1.6 | 41.7 ± 1.1 | NS |
| [range] | [14.3-64.9] | [11.0-64.6] | |
| Age ZES Diagnosis (yrs) | |||
| Mean ± SEM | 48.8 ± 1.4 | 47.8 ± 0.9 | NS |
| [range] | [14.2-67.9] | [15.0-69.4] | |
| Main Presenting symptoms (%) | |||
| Pain | 48(83%) | 101(86%) | NS |
| Diarrhea | 46 (79%) | 94 (80%) | NS |
| GERD | 32 (55%) | 44 (38%) | NS |
| MEN1 present | 0 (0%) | 0 (0%) | NS |
| Previous abdominal surgery | 6 (10%) | 51(44%) | <0.0001 |
| Acid related surgery | 2 (3.4%) | 25(21%) | 0.0021 |
| Hiatal hernia repair | 5 (8.6%) | 1(0.8%) | 0.0081 |
| Other | 1 (1.7%) (a) | 27 (23%) (a) | <0.0001 |
| Fasting gastrin level (pg/mL) | |||
| Mean ± SEM | 1198 ± 449 | 3713 ± 1555 | |
| median | 522 | 673 | |
| [range] >10-fold increased |
[144-26,000] 9 (15.5%) |
[78-175,300] 40 (34%) |
0.0099 |
| Delta Secretin | |||
| Mean ± SEM | 4558 ± 2370 | 4590 ± 1188 | NS |
| median | 657 | 806 | |
| [range] | [88-103,000] | [40-101,650] | |
| BAO (mEq/Hr) | |||
| Mean ± SEM | 42.8 ± 2.9 (b) | 46.6 ± 2.4(b) | NS |
| [range] | [17.9-95] | [11.1-159] | |
1 patient in imaging negative group had l nephrectomy for renal cell cancer. In the imaging positive group 3 had a colectomy for colon cancer, 8 had an appendectomy, 5 hysterectomy, 5 cholecystectomy, 2 negative laparotomy for PET, 2 for small bowel obstruction, 1 for renal cell cancer, 1 for small bowel perforation after radiological procedure.
Basal acid output data are shown for patients without previous acid related surgical procedures and include data from 49 patients in imaging negative and 95 patients in imaging positive groups.
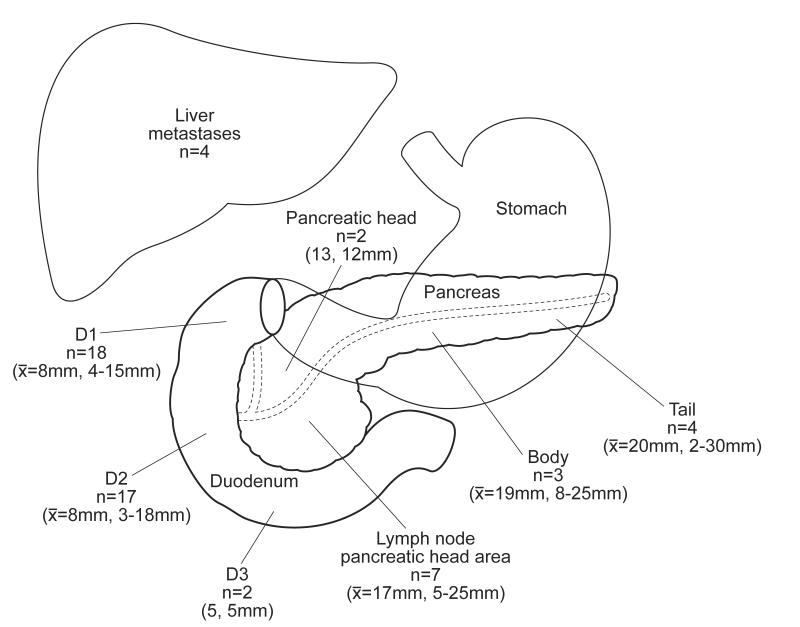
57 of 58 (98%) of the imaging negative patients had gastrinoma excised at surgical exploration, and although there was a trend (p=0.059), this was not different from the imaging positive patients of whom 90.5% had gastrinoma excised (Table 2). Of the imaging negative patients, significantly more tumors were found in the duodenum 64% compared to 37%, p=0.0008; further, significantly less tumors were found in the pancreas 15.5% compared to 30%, p=0.039. In the imaging negative group, there were no truly extra-pancreatic, extra-duodenal tumors compared to 10.2% in the imaging positive group p= 0.008 (Table 2). The exact distribution of gastrinomas found at surgery in the preoperative image negative patients is shown in Figure 1. Most are found in the first and second portion of the duodenum (61%), while another important group (29%) is lymph nodes that are found in the area of the pancreatic head. There is a uniform distribution of pancreatic gastrinomas throughout the pancreas. The size of the imaging negative tumors is smaller than the ones that are imaged preoperatively (1 cm vs. 1.9cm). A significantly higher proportion of these tumors are less than 1 cm (62% compared to 20.5%) and a significantly lesser proportion is greater than 3 cm (1.7% vs. 18%) (Table 2). Despite the fact that the imaging was negative, a similar proportion had biopsy proven liver metastases (6.8%) as the imaging positive patients (7.7%). The surgical procedures and complications (34% both groups) were similar in the imaging negative and imaging positive cohort (Table 3). A higher proportion of imaging negative patients waited over 10 years from onset of ZES to surgery (38% vs 25%, p=0.036). The procedures performed were similar in the two groups except the imaging negative patients had a greater proportion of proximal pancreaticoduodenectomies 6.8% vs. 0.8%, p=0.024). The operative deaths were the same with no deaths in the imaging negative group and 1 (0.8%) in the imaging positive group.
Table 2.
Surgical/pathology findings.
| Characteristic | Number (%) | ||
|---|---|---|---|
| IMAGE NEG | IMAGE POS | significance | |
| Patient number | 58 | 117 | |
| PET found at surgery | 57 (98%) | 106 (90.5%) | 0.059 |
| Primary PET location (surgery) | |||
| Location | |||
| Pancreas | 9 (15.5%) | 35 (30%) | 0.039 |
| Duodenum | 37 (64%) | 43 (37%) | 0.0008 |
| Lymph node (a) | 7 (12%)(a) | 13 (11%)(a) | 0.85 |
| Other(b) | 0 (0%) | 13 (10.2%)(b) | 0.008 |
| Unknown(c) | 5 (8.6%)(c) | 24 (20.5%)(c) | 0.047 |
| >1 primary tumor | 0 (0%) | 7 (6%) | 0.0036 |
| Primary tumor size (cm) | |||
| Mean ± SEM | 1.09 ± 0.09 | 1.9 ± 0.2 | <0.01 |
| [range] | [0.2-3] | [0.4-8] | |
| ≤ 1 cm | 36 (62%) | 24 (20.5%) | <0.00001 |
| ≥3 cm | 1 (1.7%) | 21 (18%) | 0.0024 |
| Tumor extent at surgery | |||
| Primary only | 28 (54%) | 45 (38%) | 0.21 |
| Primary plus lymph node involvement | 20 (34%) | 51 (44%) | 0.25 |
| With liver involvement ± lymph node involvement | 4 (6.8%) | 9 (7.7%) | 0.83 |
| Lymph node metastases only | 4 (6.8%) | 11 (9.4%) | 0.57 |
Primary lymph node gastrinoma was defined as previously reported and included a patient in which only lymph nodes (s) were removed who had normal fasting gastrin levels, secretin test result, and imaging postoperatively. (see reference 46)
Other primary locations includes in the imaging positive group 13 patients with primary tumors in: ovary (n=1),; liver (n=4); pylorus (n=2); heart (n=1); common bile duct (n=2); omentum (n=2); lung cancer (n=1) .
Unknown includes in the imaging negative and positive groups, respectively: 4 and 11 patients with only lymph node metastases; 0 and 2 patients) with only liver metastases, found determined as described in Methods and 1 and 11 patients with no tumor found.
Figure 1.
The exact distribution of the non-imaged gastrinomas.
Table 3.
Surgical procedures and complications.
| Characteristic | Number (%) | ||
|---|---|---|---|
| IMAGE NEG | IMAGE POS | significance | |
| Patient number | 58 | 117 | |
| Time to onset ZES to surgery (yrs) | |||
| Mean ± SEM | 8.9 ± 1.1 | 7.6 ± 0.6 | |
| Time>10 yrs | 22 (38%) | 29(25%) | 0.036 |
| Age Surgery (yrs) | |||
| Mean ± SEM | 51.3 ± 1.4 | 48.9 ± 1.0 | NS |
| [range] | [26.2-71.0] | [14.6-73.3] | |
| Type Primary surgery | |||
| Biopsy only | 0 (0%) | 4 (3.4%) | 0.15 |
| Enucleation | 6 (10.3%) | 12 (10.2%) | 0.99 |
| Resection | 49 (84%) | 101 (86%) | 0.74 |
| Partial pancreatectomy | 6 (10.3%) | 13 (11%) | 0.88 |
| Whipple resection | 4 (6.8%) | 1 (0.8%) | 0.024 |
| Liver resection | 3 (5.2%) | 18 (15%) | 0.051 |
| Wedge resection | 2 (3.4%) | 7 (6%) | 0.47 |
| Lobectomy/segmentectomy | 1 (1.7%)(a) | 11 (9.4%) | 0.059 |
| Surgical complications | |||
| Surgical death | 0 (0%) | 1 (0.8 %)(a) | 0.47 |
| Complications | 20 (34%)(b) | 40 (34%)(b) | 0.96 |
One patient in the imaging positive group died postoperatively from a pulmonary embolus.
Complications for the imaging negative and positive groups include respectively; pancreatitis (5,3); abscess (1,10); fistula (2,18); pneumonia (2,2); postop motility disorder (2,2); phlebitis (1,5); wound infection (4,6); hepatitis (1,0); postoperative bleeding (0,1).
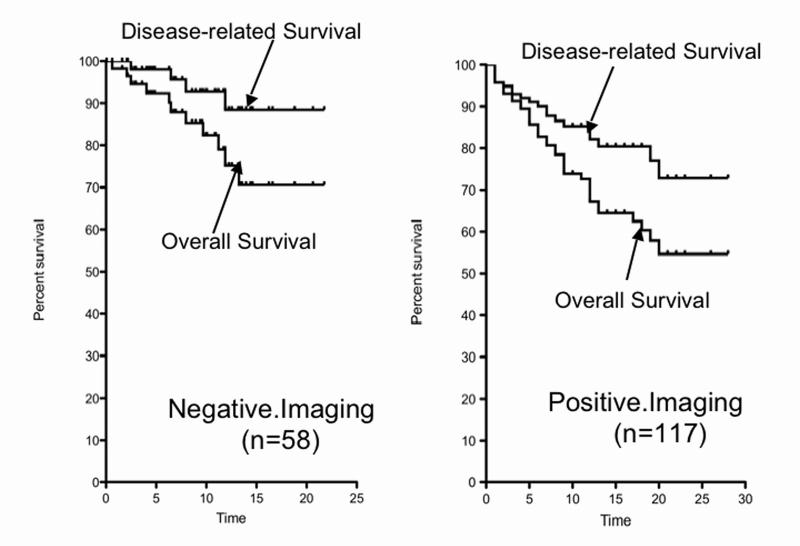
The mean postoperative follow-up is approximately 10 years and it is similar between the two groups (Table 4). The proportion of patients who are alive at last follow-up and had conversely disease-related deaths showed a trend to superiority in the image negative groups (p=0.062), but it did not reach statistical significance. The proportion of patients who are disease-free is 48% in the imaging negative group and is not different from the 35% in the imaging positive group. There was no difference in the proportion of patients who developed liver metastases after surgery, nor the time to development of liver disease; however, the number of patients developing new imageable lesions following surgery was only 21% in the imaging negative group and 41% in the imaging positive group (p=0.008). The overall and disease-related survival was not significantly different from each other for the imaging negative patients, but it was significantly better when compared to the same results for the imaging positive patients (Table 5 and Figure 2). Imaging negative patients had an overall 20-year survival of 71% compared to 58% for the imaging positive patients and the disease-related survival was 88% compared to 73% (p=0.015).
Table 4.
Postoperative course, surgical result and follow-up.
| Characteristic | Number (%) | ||
|---|---|---|---|
| IMAGE NEG | IMAGE POS | significance | |
| Patient number | 58 | 117 | |
| Status Last follow-up | |||
| Alive | 47 (81%) | 79 (67%) | 0.062 |
| Dead | 11 (19%) | 38 (32%) | |
| Disease-related death | 4 (6.9%) | 20 (17%) | 0.066 |
| Years surgery to death | |||
| Mean ± SEM | 4.3 ± 1.3 | 8.1 ± 0.6 | 0.01 |
| [range] | [0.6-13.2] | [0.6-20.7] | |
| Years surgery to disease-related death | |||
| Mean ± SEM | 7.2 ± 1.9 | 7.1 ± 1.3 | 0.99 |
| [range] | [2.4-11.9] | [0.6-20.7] | |
| Duration of follow-up (yrs) | |||
| Time from surgery to last follow-up (yrs) | |||
| Mean ± SEM | 9.5 ± 0.7 | 11.6 ± 0.6 | |
| [range] | [0.1-21.8] | [0.1-28.1] | |
| Time from onset ZES to last follow-up (yrs) | |||
| Mean ± SEM | 18.8 ± 1.2 | 19.2 ± 0.9 | |
| [range] | [4.2-49.5] | [1.4-42.1] | |
| Postoperative status | |||
| Disease-free | |||
| Immediate Postop | 36 (63%) | 63 (54%) | 0.31 |
| Last Followup | 28 (48%) | 41 (35%) | 0.09 |
| Not disease free last followup | 30 (52%) | 76 (64%) | 0.09 |
| Recurrence | 8 (14%) | 22 (19%) | 0.41 |
| Liver metastases during follow-up | |||
| No. developing liver metastases | 6 (10%) | 19 (17%) | 0.36 |
| Time to development (yrs) | |||
| Mean ± SEM | 3.7 ± 0.9 | 3.1 ± 0.6 | |
| [range] | [0.8-7.2] | [0.2-7.2] | |
| No. developing new lesions during follow-up | 12 (21%) | 48 (41%) | 0.008 |
Table 5.
Survival: Overall and Disease-related.
| % Survival [95 % CI](1) | |||
|---|---|---|---|
| A. Imaging Negative (n=58) ((2),(4)) | 5 yrs | 10yrs | 20 yrs |
| Total survival | 90 [78-96] |
82 [68-91] |
71 [51-83] |
| Disease-related survival | 98 [83-99] |
93 [79-98] |
88 [71-96] |
| B. Imaging positive (n=117) ((3),(4)) | |||
| Total survival | 86 [77-91] |
74 [64-81] |
58 [46-68] |
| Disease-related survival | 91 [84-95] |
85 [76-91] |
73 [58-83] |
Percentage survival from surgery calculated from data from 58 patients with negative imaging preoperatively and 117 patients with positive imaging from survival curves shown in Fig. 2.
For the Imaging Negative patients during the followup (9.5 ± 0.72 [range-0.1-21.8 yrs] from surgery, 11 patients died from any cause (overall survival)(Fig.1) and 4 died from a Disease-related cause (Fig. 1).
For the Imaging Positive patients during the followup (11.6 ± 0.6 [range-0.1-28.1 yrs] from surgery, 38 patients died from any cause (overall survival)(Fig.1) and 20 died from a Disease-related cause (Fig. 1).
The differences between the Overall survival and Disease-related survival were not significant (p=0.069, HR-2.56, 95 CI-0.92-7.1) for theImage Negative patients. Howcver, they were significantly (p=0.015, HR-1.93, 95 CI-1.14-3.2) different from the Image positive patients
Figure 2.
Kaplan Meier plot of the disease-related and overall survival for the imaging negative and imaging positive. Imaging negative patients had an overall 20-year survival of 71% compared to 58% for the imaging positive patients and the disease-related survival was 88% compared to 73% (p=0.015).
Discussion
At the 100th annual meeting of the American Surgical Association in 1980, Dr. Robert Zollinger in the discussion of his paper on the 25 year appraisal of his surgery in patients with ZES 35 stated: “We have to convince our physician friends that it is time to recommend that every gastrinoma be considered a surgical problem. They should not treat the patient with cimetidine indefinitely. It is a basic principle to take out the malignant tumor rather that to treat the end result”. Unfortunately, a number of features of ZES/gastrinomas have led a number of groups to not heed Dr Zollinger’s advice and instead to advocate over the intervening years a completely medical approach or one in which only patients who had imaged possible primary tumors undergo surgical exploration for possible cure8,9,10,11,12,13,14,15. The ZES/gastrinoma features that encouraged this approach included: the development of highly successful medical treatment for the gastric acid hypersecretion 1,2,31-3; the failure to image primary tumors in 30-70% of patients in different series, especially those with duodenal primaries 4,5,6,36,37; the fact that only 20-30% of gastrinomas pursue an aggressive course 38,39; and that until recently the lack of prospective studies showing surgery could cure a significant number of these patients, effect the development of liver metastases or survival 40. Other factors favoring a decrease in the use of early surgery for possible cure in these patients included a long delay in the diagnosis of ZES which is a mean >5 years in some studies 12,19,20, and likely increasing with the widespread use of PPIs16; and the delay in time from diagnosis to surgery of 4-8 years in some studies 19. Recent studies have somewhat dealt with a number of these points in that they report immediate postoperative cure rates in sporadic ZES patients of 50-60% and long term cure rates of 30-40% 5,6,19; an increase in survival of patients undergoing surgical resection 29 and a decrease in the development of liver metastases 18,29, which are the most important prognostic factor for survival7,39. Nevertheless, a recent study 3 reports that the above mentioned factors leading to delays in surgery are still operative in that they conclude from an analysis of their patients diagnosed and treated during different time periods, that at present these patients are being operated later in their disease course with more advanced disease in this era of PPI treatment. 5,7,19,23,26,34,36,39,41,42
The present study attempts to address one of the important implicit premises of the avocation of only recommending operation for image positive patients (i.e., that surgery is less effective in imaging negative ZES patients) by comparing results of 58 patients with negative imaging to 117 patients with positive imaging operated over the same time period. While the study concludes that surgery in imaging negative patients is just as likely to find a primary gastrinoma, the cure rate is as high, and the survival is even better that in the imaging positive patients, there are a few points that could lead to questioning the result. First, one could argue that insignificant number of patients had endoscopic ultrasound (EUS) that has been identified as one of the best studies to localize pancreatic neuroendocrine tumors 37, 44,45. However, in this study in the imaging negative patients duodenal gastrinomas were much more frequent (4-fold) than pancreatic gastrinomas, which is similar to other recent studies 41-44. Numerous studies show that EUS does not visualize most duodenal gastrinomas 44,45 seeing only 35% in one review of five series 37. Furthermore, all of these patients had careful endoscopic examination of the duodenal area to attempt to identify any submucosal gastrinoma 45. In addition, intraoperative ultrasound has also been poor at imaging duodenal gastrinomas 42. This may be because the duodenum has a mixed background with solid, liquid and gas in which it is difficult to detect sonolucent neuroendocrine tumors like gastrinomas. Therefore, this data supports the conclusion that even if EUS were performed prospectively on these patients it would not have identified a significant additional number of patients preoperatively. Furthermore, it could be argued if SRS was performed in those operated on, before it became available, that additional duodenal lesions might have been detected and therefore the results are not applicable to the present time, during which it is available. This cannot be completely refuted, however, 40% of our patients had negative SRSs in the present study, and furthermore, the SRS frequently misses small duodenal tumors and therefore, because most patients had small duodenal primaries it would have been positive in <50% even if used 4. Somatostatin receptor scintigraphy has been reported to be the single best preoperative localization study for gastrinoma. It has been able to detect approximately 30% of gastrinomas less than 1 cm, 64% between 1 and 2 cm and 96% of those greater than 2 cm. Because, it is a total body exam, it is especially useful for ectopic (extra-pancreatic, extra-intestinal) gastrinomas 4,23. This study corroborates those results because 13 patients (10%) in the imaging positive group had extra-pancreatic, extra-intestinal gastrinoma in ectopic locations including the heart, liver and ovary, while none in the imaging negative group had similar findings.
This is the first study in ZES that has focused on results in imaging negative patients. A common question by patients and referring physicians is why do surgery on these patients with negative imaging studies when they are so well controlled on PPIs? The demographic characteristics of patients with negative imaging studies are remarkably similar to those with imaging positive tumors except they have lower fasting serum gastrin levels and less prior surgery for uncontrolled symptoms of ZES. Further, they have a longer time interval from their disease onset to surgery suggesting that the referring physicians were reluctant to allow them to have surgery. In some studies21 the level of fasting gastrin correlates with gastrinoma size or tumor burden therefore the finding of lower preoperative fasting gastrin levels in the imaging negative patients is consistent with our finding at surgery of smaller tumors in the majority of these patients. The fact that they have had less surgical procedures for complications of ZES is also consistent with smaller tumor burden. This was also demonstrated in the size of tumors removed during surgery that were significantly smaller in the imaging negative group. Further, in this study there was an equal ability to find tumor in the imaging negative group as in the imaging positive group. The location of the primary tumor in the imaging negative group was most often in the duodenum suggesting that the critical maneuver to finding imaging negative gastrinomas is duodenotomy at the time of surgery 42,43. The imaging negative group had less other tumors, less pancreatic tumors, less unknown locations and less greater than 1 cm tumors explaining the negative imaging as radiographic imaging is most dependent on size. The extent of tumor in the negative imaging group is still very worrisome as 4 had liver metastases and 34% had lymph node metastases and both of these can affect subsequent disease-related survival 19,7,29,39. Each of the deaths in the imaging negative patients occurred in patients with liver metastases. The time from onset to surgery was longer in the imaging negative cohort probably because of delay or procrastination related to the negative radiographic imaging. The extent of surgical operation was similar in the two groups except more patients in the imaging negative cohort had Whipple procedures because of more extensive nodal disease with small duodenal primary tumors. The complications were identical and only one surgical death. The most important results are the long-term outcome data based on preoperative imaging positive and negative results. There is no difference in the development of liver metastases in the imaging negative and imaging positive group, but there is a higher overall development post resection of imaged lesions in the preoperative imaging positive group. There is better overall and disease-related survival and a trend to higher cure-rate in the imaging negative group.
This study suggests that ZES patients with negative imaging studies can greatly 19benefit from surgical exploration. Tumor is almost always found and removed with acceptable morbidity and minimal mortality. Surgeons doing these procedures should focus primarily on the duodenum and the gastrinoma triangle as most tumors will be found there. However, a complete exploration is necessary as liver metastases and body/tail pancreatic tumors still occur, albeit less frequently. Furthermore, it is essential to routinely sample lymph nodes both in peritumoral areas as well as in the pancreatic head area as this may increase the cure rate, has prognostic significance and is the only means of detecting possible lymph node primary gastrinomas 19,46. Lymph node primary gastrinomas are controversial and may represent a missed duodenal primary with lymph node metastases as some suggest.46 The non-imaged tumors are small in size and usually occur within the duodenum. A critical maneuver is duodenotomy that allows precise detection of the duodenal tumors 41-44. They are not ectopic and they have a similar incidence of lymph node and liver metastases. This type of careful meticulous exploration and resection of preoperative imaging negative tumors should result in improved cure-rate and improved long-term overall and disease-related survival. This happens because negative imaging is associated with a lower incidence of subsequent tumor recurrence.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey A. Norton, Stanford University Medical Center.
Douglas L. Fraker, University of Pennsylvania.
H. Richard Alexander, University of Maryland
Robert T Jensen, NIDDK, NIH.
References
- 1.Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004 Dec;6(6):454–463. doi: 10.1007/s11894-004-0067-5. [DOI] [PubMed] [Google Scholar]
- 2.Jensen RTCG, Brandi ML, et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Functional Pancreatic Endocrine Tumor Syndromes. Neuroendocrinology. 2012;95:98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison ECSJ. Zollinger-Ellison syndrome in the era of effective acid suppression: are we unkowingly growing tumors? Am J Surg. 2003;186:245–248. doi: 10.1016/s0002-9610(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 4.Alexander HR, Fraker DL, Norton JA, et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998 Aug;228(2):228–238. doi: 10.1097/00000658-199808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg. 2004 May;239(5):617–625. doi: 10.1097/01.sla.0000124290.05524.5e. discussion 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999 Aug 26;341(9):635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 7.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999 Feb;17(2):615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy DM. The place of surgery in the Zollinger-Ellison syndrome. N Engl J Med. 1980 Jun 12;302(24):1344–1347. doi: 10.1056/NEJM198006123022404. [DOI] [PubMed] [Google Scholar]
- 9.McNamara D, Lewis T, O’Moran C. Zollinger-Ellison syndrome with fasting hypoglycaemia. J R Soc Med. 1998 Feb;91(2):92–93. doi: 10.1177/014107689809100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschowitz BI. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999 Dec 30;341(27):2096–2097. doi: 10.1056/NEJM199912303412714. [DOI] [PubMed] [Google Scholar]
- 11.Hirschowitz BI, Simmons J, Mohnen J. Clinical outcome using lansoprazole in acid hypersecretors with and without Zollinger-Ellison syndrome: a 13-year prospective study. Clin Gastroenterol Hepatol. 2005 Jan;3(1):39–48. doi: 10.1016/s1542-3565(04)00606-8. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox CM, Seay T, Arcury JT, Mohnen J, Hirschowitz BI. Zollinger-Ellison syndrome: presentation, response to therapy, and outcome. Dig Liver Dis. Jun;43(6):439–443. doi: 10.1016/j.dld.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Collins JS, Buchanan KD, Kennedy TL, et al. Changing patterns in presentation and management of the Zollinger-Ellison syndrome in Northern Ireland, 1970-1988. Q J Med. 1991 Mar;78(287):215–225. [PubMed] [Google Scholar]
- 14.Quatrini M, Castoldi L, Rossi G, Cesana BM, Peracchi M, Bardella MT. A follow-up study of patients with Zollinger-Ellison syndrome in the period 1966-2002: effects of surgical and medical treatments on long-term survival. J Clin Gastroenterol. 2005 May-Jun;39(5):376–380. doi: 10.1097/01.mcg.0000159221.77913.ac. [DOI] [PubMed] [Google Scholar]
- 15.Hung PD, Schubert ML, Mihas AA. Zollinger-Ellison Syndrome. Curr Treat Options Gastroenterol. 2003 Apr;6(2):163–170. doi: 10.1007/s11938-003-0017-6. [DOI] [PubMed] [Google Scholar]
- 16.Corleto VD, Annibale B, Gibril F, et al. Does the widespread use of proton pump inhibitors mask, complicate and/or delay the diagnosis of Zollinger-Ellison syndrome? Aliment Pharmacol Ther. 2001 Oct;15(10):1555–1561. doi: 10.1046/j.1365-2036.2001.01085.x. [DOI] [PubMed] [Google Scholar]
- 17.Norton JA, Fraker DL, Alexander HR, et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006 Sep;244(3):410–419. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraker DL, Norton JA, Alexander HR, Venzon DJ, Jensen RT. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994 Sep;220(3):320–328. doi: 10.1097/00000658-199409000-00008. discussion 328-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krampitz GWNJ, Poultsides GA, Visser B, Sun L, Jensen RT. Lymph nodes and survival in duodenal and pancreatic neuroendocrine tumors. Arch Surg. 2012 doi: 10.1001/archsurg.2012.1261. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000 Nov;79(6):379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006 Nov;85(6):295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krudy AG, Doppman JL, Jensen RT, et al. Localization of islet cell tumors by dynamic CT: comparison with plain CT, arteriography, sonography, and venous sampling. AJR Am J Roentgenol. 1984 Sep;143(3):585–589. doi: 10.2214/ajr.143.3.585. [DOI] [PubMed] [Google Scholar]
- 23.Termanini B, Gibril F, Reynolds JC, et al. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997 Feb;112(2):335–347. doi: 10.1053/gast.1997.v112.pm9024287. [DOI] [PubMed] [Google Scholar]
- 24.Gibril F, Reynolds JC, Chen CC, et al. Specificity of somatostatin receptor scintigraphy: a prospective study and effects of false-positive localizations on management in patients with gastrinomas. J Nucl Med. 1999 Apr;40(4):539–553. [PubMed] [Google Scholar]
- 25.NortonJA H, Chen Y, Visser BC, Poultsides GA, Kunz PC, Fisher GA, Jensen RT. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg. 2011;146(6):724–732. doi: 10.1001/archsurg.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherner JA, Doppman JL, Norton JA, et al. Selective venous sampling for gastrin to localize gastrinomas. A prospective assessment. Ann Intern Med. 1986 Dec;105(6):841–847. doi: 10.7326/0003-4819-105-6-841. [DOI] [PubMed] [Google Scholar]
- 27.Doppman JL. Pancreatic endocrine tumors--the search goes on. N Engl J Med. 1992 Jun 25;326(26):1770–1772. doi: 10.1056/NEJM199206253262609. [DOI] [PubMed] [Google Scholar]
- 28.Doppman JL, Miller DL, Chang R, et al. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology. 1990 Jan;174(1):25–29. doi: 10.1148/radiology.174.1.2294556. [DOI] [PubMed] [Google Scholar]
- 29.Norton JAFD, Alexander HR, Gibri F, Liewehr DJ, Venzon DJ aRJ. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006 doi: 10.1097/01.sla.0000234802.44320.a5. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norton JA, Jensen RT. Role of surgery in Zollinger-Ellison syndrome. J Am Coll Surg. 2007 Oct;205(4 Suppl):S34–37. doi: 10.1016/j.jamcollsurg.2007.06.320. [DOI] [PubMed] [Google Scholar]
- 31.Fraker DL, Norton JA, Saeed ZA, Maton PN, Gardner JD, Jensen RT. A prospective study of perioperative and postoperative control of acid hypersecretion in patients with Zollinger-Ellison syndrome undergoing gastrinoma resection. Surgery. 1988 Dec;104(6):1054–1063. [PubMed] [Google Scholar]
- 32.Norton JA, Cromack DT, Shawker TH, et al. Intraoperative ultrasonographic localization of islet cell tumors. A prospective comparison to palpation. Ann Surg. 1988 Feb;207(2):160–168. doi: 10.1097/00000658-198802000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome. Results of a 10-year prospective study. Ann Surg. 1992 Jan;215(1):8–18. doi: 10.1097/00000658-199201000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishbeyn VA, Norton JA, Benya RV, et al. Assessment and prediction of long-term cure in patients with the Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993 Aug 1;119(3):199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zollinger RMEE, Fabri PJ, et al. Primary peptic ulcerations of the jejunum associated with islet cell tumors. Twenty-five year appraisal. Ann Surg. 1980;192:422–430. doi: 10.1097/00000658-198009000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004 Nov;240(5):757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard TJ, Zinner MJ, Stabile BE, Passaro E., Jr. Gastrinoma excision for cure. A prospective analysis. Ann Surg. 1990 Jan;211(1):9–14. doi: 10.1097/00000658-199001000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stabile BE, Passaro E., Jr. Benign and malignant gastrinoma. Am J Surg. 1985 Jan;149(1):144–150. doi: 10.1016/s0002-9610(85)80024-6. [DOI] [PubMed] [Google Scholar]
- 39.Weber HCVD, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 40.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1991 Jan-Feb;15(1):151–159. doi: 10.1007/BF01658992. [DOI] [PubMed] [Google Scholar]
- 41.Thom AK, Norton JA, Axiotis CA, Jensen RT. Location, incidence, and malignant potential of duodenal gastrinomas. Surgery. 1991 Dec;110(6):1086–1091. discussion 1091-1083. [PubMed] [Google Scholar]
- 42.Sugg SL, Norton JA, Fraker DL, et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993 Aug;218(2):138–144. doi: 10.1097/00000658-199308000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NortonJA AH, Fraker DL, Venzon DJ, Jensen RT. Does the use of routine duodenotomy affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg. 2004;239(5):617–626. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg. 1993 Jul-Aug;17(4):455–462. doi: 10.1007/BF01655104. [DOI] [PubMed] [Google Scholar]
- 45.Thom AK, Norton JA, Doppman JL, Miller DL, Chang R, Jensen RT. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992 Dec;112(6):1002–1008. discussion 1008-1009. [PubMed] [Google Scholar]
- 46.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003 May;237(5):650–657. doi: 10.1097/01.SLA.0000064375.51939.48. discussion 657-659. [DOI] [PMC free article] [PubMed] [Google Scholar]