Abstract
Nanoparticles are used to solve the current drug delivery problem. We present a high-performance method for efficient and selective action on nucleic acid target in cells using unique TiO2·PL-DNA nanocomposites (polylysine-containing DNA fragments noncovalently immobilized onto TiO2 nanoparticles capable of transferring DNA). These nanocomposites were used for inhibition of human influenza A (H3N2) virus replication in infected MDCK cells. They showed a low toxicity (TC50 ≈ 1800 μg/ml) and a high antiviral activity (>99.9% inhibition of the virus replication). The specificity factor (antisense effect) appeared to depend on the delivery system of DNA fragments. This factor for nanocomposites is ten-times higher than for DNA in the presence of lipofectamine. IC50 for nanocomposites was estimated to be 1.5 μg/ml (30 nM for DNA), so its selectivity index was calculated as ~1200. Thus, the proposed nanocomposites are prospective for therapeutic application.
Fragments of nucleic acids (NA) are widely used in life sciences including research methods and medical practice. NAs are among the most important sources for the development of a novel group of pharmaceuticals. NA-based drugs for antisense and antigene application include oligonucleotides, their analogues, ribozymes, DNAzymes, aptamers, small interfering RNAs (siRNAs), and plasmids containing transgenes1,2. This class of compounds is extremely promising for drug therapy aimed to a wide range of diseases including infectious, cancer, neurological disorders such as Parkinson's and Alzheimer's diseases, cardiovascular disorders, etc. [e.g.2,3]. NA-based drugs can be developed for individualized medicine. NA-based drugs can selectively suppress gene expression, inhibit activity of certain enzymes, or implement other special functions4,5. The significant advantage of this type of drugs over currently available low molecular weight pharmaceuticals is their selective recognition of molecular targets. The effect of antisense oligonucleotides is known to be in their binding to complementary regions of a target RNA to form DNA/RNA hybrid duplexes, which leads to blocking the function of the target RNA or its cleavage by cellular RNase H1,2,6. The important advantages of the antisense approach is that NA-drugs can be easily redirected to any target nucleic acids, while the search for new low-molecular drugs usually takes years.
However, because of a low ability to penetrate into cells, these compounds still have not found wide application in medicine. Poor cellular uptake and rapid in vivo degradation of NA-based drugs necessitate designing delivery systems to facilitate cellular internalization and preserve their activity7.
Currently, the transport of exogenous NA to cells can be achieved by using vehicles, which can be separated into two categories: viral and non-viral vectors. The former provides a high transfection rate and a rapid transcription of a foreign material incorporated in the viral genome8. On the other hand, viral vectors present a variety of potential problems to the patients such as toxicity and immune and inflammatory responses. Insertional mutagenesis and oncogenic effects can occur when these vectors are used in vivo9.
Cell-penetrating peptides as non-viral vectors are widely used for delivering various biomolecules into cells including plasmid DNAs, oligonucleotides, siRNAs, peptide-nucleic acids, proteins, etc. Cell-penetrating peptide-based technology is described in recent reviews7,10. Liposomes and cationic polymers are other major types of non-viral gene delivery vectors, which have been under intense development during recent years because of their low immunogenicity and the simplicity of preparation and modification11,12. However, the transfection efficiency of these vectors is still relatively low, compared to viral vectors13. Besides, cationic liposome/DNA complexes exhibit inflammatory toxicity associated with their systemic administration14. A variety of supramolecular nanocarriers including various polymeric nanoparticles are used to deliver antisense oligonucleotides and siRNAs, as more fully described in recent reviews15,16,17. Although cationic polymers, branched dendrimers, cationic liposomes, and cell-penetrating peptides are today under intensive studies, their efficiency as non-viral vectors for cell transfection is still low.
Current achievements of nanotechnology began to be used to solve the drug delivery problem, concerning particularly nucleic acid fragments. Recent advances in clinical trials concerning nanoparticle drug delivery systems are described in18,19,20.
Titanium dioxide is widely used in medicine as biocompatible and non-toxic material21. TiO2 nanoparticles (~5 nm) are known to penetrate through cell membranes22,23. According to FDA (Management of the Food and Drug Administration, United States), TiO2 is recognized in 1966 as safe and harmless substance to humans. Compounds on the basis of TiO2 are widely used in cosmetics and as food supplements. Moreover, recent studies have shown that TiO2 nanoparticles at relatively low doses (up to 200 µg/ml) do not show any noticeable toxicity and no harmful effects on mammalian cells24, bacteria25, and animals26. Cytotoxicity of TiO2 nanoparticles used in this work did not exceed the level of natural death of MDCK cells27. TiO2 nanoparticles have been shown to be non-toxic in rats at low doses (5 mg/kg body weight)28. The above properties of titanium dioxide make it attractive to use as a vehicle of drugs, particularly, antisense oligonucleotides and their analogues into mammalian cells.
In the previous paper, we described new nanocomposites consisting of DNA fragments noncovalently fixed on TiO2 nanoparticles through the polylysine linker (TiO2·PL-DNA)29. These nanocomposites were shown by confocal microscopy to penetrate into mammalian cells without any transfection agents and physical impact. Delivery of nucleic acid fragments into cells is just the first step of their use in living systems. However, the real value of the proposed nanocomposites can be revealed only when they are tested on cells and animals.
Here we present a high-performance method for the site specific effect on target nucleic acids in eukaryotic cells using unique TiO2·PL-DNA nanocomposites. These nanocomposites show a low toxicity, and after penetration into cells infected by influenza A virus, demonstrate a high specific antiviral activity.
Results
The synthesis of TiO2 nanoparticles in amorphous and crystal forms (anatase, brookite), the preparation of nanocomposites consisting of these nanoparticles and noncovalently immobilized polylysine (PL) or polylysine-containing DNA fragments (PL-DNA), and characteristics of the designed TiO2·PL and TiO2·PL-DNA nanocomposites were described in a recent paper29. It has been shown that the efficiency of immobilization of oligonucleotides onto TiO2 nanoparticles, the ability of designed TiO2·PL-DNA nanocomposites to form complementary complexes and penetrate into cells without any transfection agents or physical impact do not depend on the nanoparticles form. Here we used nanocomposites based on one form of nanoparticles (anatase). PL-DNA conjugates30 were immobilized on anatase to give TiO2·PL-DNA bearing 20 nmol of DNA per 1 mg of anatase.
The biological activity of the designed nanocomposites was demonstrated by an example of inhibition of influenza A virus replication in infected MDCK cells. The most widespread human influenza A virus (H3N2) was used in our experiments. Influenza A virus contains eight single-stranded RNA segments of negative polarity as the genome. After infection of cells with influenza virus, viral RNA (−strand) segments are transcribed into mRNAs (+strand) and replicated into complementary RNAs (+strand)31. RNA segment 5 encodes nucleoprotein (NP), which plays a key role in the incorporation of viral genome in cell nuclei of an infected body, thereby, in the following replication and assembly of the virus32. In addition to coding regions, all eight segments of viral (-)RNA are known to contain the conservative sequence CCUGCUUUUGCU3′at the 3′-end33,34.
The noncoding 3′-terminal region of segment 5 was chosen as a target for the designed nanocomposite. It should be noted that the choice of this target is very reasonable because, according to literature data, morpholino oligomer and siRNA addressed to this conservative region of NP gene showed the highest antiviral activity among the other morpholino oligomers34 and 20 siRNA molecules35. Oligonucleotide 5′GCAAAAGCAGGGTAGATAATCp (DNA1) complementary to the chosen region was used in our work to construct nanocomposites. As a control, we used oligonucleotide 5′GATCAACTCCATATGCCATGTp (DNA2) with a random sequence.
Antiviral activity of TiO2·PL-DNA nanocomposites
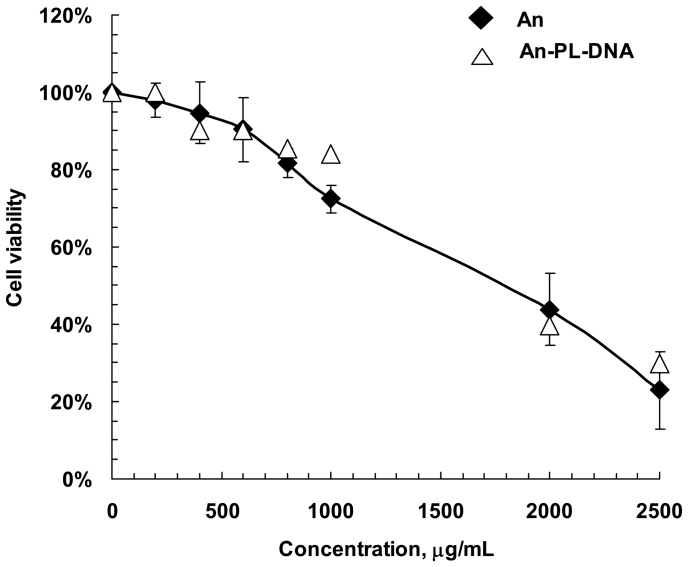
At first, we tested the effect of TiO2 nanoparticles and TiO2·PL-DNA nanocomposite on viability of MDCK cells in the absence of virus. The concentration of samples resulting in 50% cell death (TC50) for TiO2and TiO2·PL-DNA1 was estimated to be ~1800 μg/ml (Fig. 1). It should be noted that just nanoparticles are responsible for the effect, and PL-DNA conjugates are hardly involved in this action.
Figure 1. Effect of TiO2 nanoparticles and TiO2 ·PL-DNA1 nanocomposites on viability of MDCK cells estimated by trypan blue staining.
Experiments were performed in three independent series. An designatesTiO2 nanoparticles in the anatase form.
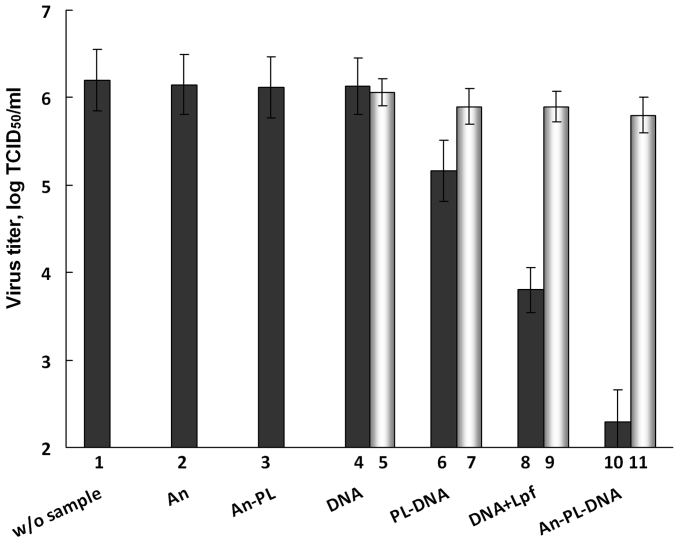
The nontoxic concentration of nanocomposites 5 μg/ml corresponding to 0.1 µM for oligonucleotides was used to study the antiviral activity of the proposed TiO2·PL-DNA nanocomposites. Nanocomposite TiO2·PL-DNA1 containing oligonucleotide directed to the 3′-noncoding regions of initial viral (-)RNA and various controls including samples bearing a random DNA2 sequence were assayed for the antiviral activity against human influenza A virus H3N2. MDCK cells were infected at a moi of 0.05 TCID50/cell. Transfection of cells with nanocomposites was carried out in the absence of serum. The nanocomposites and controls were incubated with cells for 4 h prior to infection. The results are presented as log TCID50/ml in Fig. 2 and as TCID50/ml in Table 1.
Figure 2. Inhibition of replication of virus A/Aichi/2/68 (H3N2).
Virus titer (log TCID50/ml) in the presence of nanocomposites and controls. Without sample (1), TiO2 nanoparticles (2), TiO2 ·PL nanocomposite (3); DNA1 and DNA2 fragments (4, 5), PL-DNA1 and PL-DNA2 conjugates (6, 7), DNA1and DNA2 fragments in the presence of LlipofectamineTM 2000 (lpf) (8, 9), and TiO2 ·PL- DNA1 and TiO2 ·PL-DNA2 nanocomposites (10, 11). Light-coloured columns correspond to samples with noncomplementary oligonucleotides. Concentrations of nanocomposites and controls in the medium were 5 μg/ml for TiO2 nanoparticles and 0.1 μM for DNA fragment. Experiments were performed in three independent series.
Table 1. Antiviral activity of studied DNA-containing samples.
| Virus titer, TCID50/ml | Reduction of virus titer, % | Reduction of virus titer, n-fold | Specificity factora | |||||
|---|---|---|---|---|---|---|---|---|
| DNA1b | DNA2b | DNA1 | DNA2 | DNA1 | DNA2 | |||
| row | Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| a | DNA | 1,348,963 | 1,148,154 | 15 | 28 | 1 | 1.4 | 1 |
| b | PL-DNA | 147,911 | 794,328 | 90.7 | 50 | 11 | 2.0 | 5 |
| c | DNA + lpfc | 6,310 | 793,349 | 99.6 | 50 | 251 | 2.0 | 130 |
| d | TiO2·PL-DNA | 200 | 630,957 | 99.99 | 60 | 8,000 | 2.5 | 3,160 |
| e | w/o sample | 1,584,893 | ||||||
aratio of the TCID50/ml values for DNA2- and DNA1-containing samples.
bDNA1 and DNA2 are DNA fragments complementary and noncomplementary to viral RNA, respectively.
cLipofectamineTM 2000.
The nanoparticles bearing no oligonucleotides were practically inactive independently on the presence or the absence of the PL residue (Fig. 2, columns 2 and 3). Unbound DNA1 and DNA2 fragments also showed very low antiviral activity (Fig. 2, columns 4 and 5; Table 1, row a). This is with agreement with literature data: phosphodiester oligodeoxynucleotides failed to inhibit replication of influenza A at concentration up to 80 µM36. The maximal antiviral efficacy (99.99% reduction of virus titer) demonstrated TiO2·PL-DNA1nanocomposite bearing complementary DNA1 (Fig. 2, column 10; Table 1, row d): the virus titer was reduced by a factor of 8,000 (by about four orders of magnitude). PL-DNA1 conjugate without nanoparticles showed significantly lower efficiency (by a factor of 740) than the same conjugate immobilized onto nanoparticles (compare columns 6 and 10 in Fig. 2 and rows b and d in Table 1). It should be noted that DNA1in TiO2·PL-DNA1 nanocomposite demonstrated ~30-times higher antiviral activity than the same oligonucleotide in the presence of lipofectamine widely used as the transfection agent (compare columns 8 and 10 in Fig. 2 and rows c and d in Table 1). The antiviral activity of TiO2·PL-DNA nanocomposites bearing complementary and noncomplementary DNA fragments differed by a factor of about 3000 (compare columns 10 and 11 in Fig. 2 and cells 1d and 2d in Table 1).
All samples containing complementary fragment DNA1 demonstrated the higher antiviral activity as compared to those bearing noncomplementary fragment DNA2 (compare columns 6 and 7, 8 and 9, and 10 and 11 in Fig. 2 and columns 1 and 2, 3 and 4, and 5 and 6 in Table 1). We evaluated the ratio of the TCID50/ml values for these two types of nanocomposites (Table 1, column 7), which can be considered as a specificity factor indicating the antisense effect.
The results show that the specificity factor depends on the method of the delivery of the samples into cells. DNA1 and DNA2 without any transfection agent almost do not penetrate into the cells, and their antiviral activity is equally low. PL-DNA1 and PL-DNA2 penetrate into cells more efficiently, and the difference, although still low, between their antiviral actions is observed. When lipofectamine is used for transfection, complementary DNA1 becomes ~130 times more efficient than noncomplementary DNA2. The maximal difference (3160 times) was detected if DNA fragments were delivered into cells in TiO2·PL-DNA1 and TiO2·PL-DNA2 nanocomposites.
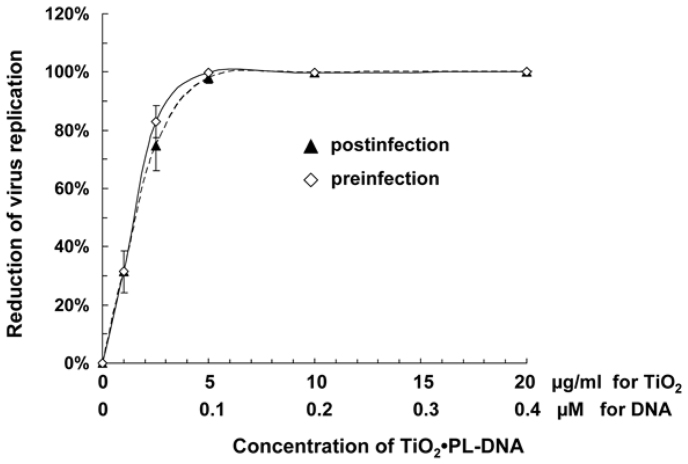
Dose-response studies
The dose-response effect of the TiO2·PL-DNA1 nanocomposite was studied in the pre-infection and post-infection assays. In the pre-infection treatment, MDCK cells were pretreated with the TiO2·PL-DNA1sample for 4 h followed by infection with A/Aichi/2/68 virus at a multiplicity of infection (moi) of 0.1 TCID50/cell for 1 h. After washing cells from the virus-containing medium and the subsequent incubation for 48 h, the virus titer was measured by the hemagglutination (HA) technique. In the post-infection treatment, MDCK cells were initially infected with influenza A virus for 1 h as above and washed, followed by incubation with TiO2·PL-DNA1 nanocomposite for 4 h. After 48-h incubation, the evaluation of virus titer was performed as in the previous case.
Concentrations of the nanocomposite causing 50% inhibition of viral production (IC50) were evaluated to be ~1.5 µg/ml for the nanocomposite (~0.03 µM for DNA1) in the pre-treatment or post-treatment assays (Fig. 3).
Figure 3. Dose-response curves of inhibition of influenza virus replication in MDCK cells by TiO2 ·PL-DNA1 nanocomposite in the pre-treatment or post-treatment assays.
Experiments were performed in two independent series for each treatment.
The data on cell viability and dose-response study allowed us to derive the selectivity index value, i.e. the ratio of TC50 to IC50, (SI ≈ 1200).
Discussion
The nucleic acids-based drugs are the foundation of a new medicine because they selectively recognize the complementary regions of nucleic acid targets and affect their functions in cells that allows for the inhibition of the expression of certain genes responsible for ailments without affecting host genes. As compared to low molecular weight pharmatheuticals, NA-based drugs have also other advantages such as simple design, easy preparation, high specificity, and low side effects.
However, NA-based drugs still have not found wide application in medical practice because of the low ability to penetrate into cells.
In the previous paper29, we described new nanocomposites consisting of DNA-based drugs noncovalently fixed on inorganic nanoparticles used as transporters. Polylysine-containing DNA fragments were immobilized with a high efficiency onto titanium dioxide nanoparticles to give TiO2 ·PL-DNA nanocomposites. We assumed that PL-DNA are immobilized onto TiO2 nanoparticles due mainly to electrostatic interactions between negatively charged TiO2 surface37,38 and positively charged amino groups of polylysine at physiological pH values (pH ~7) (Fig. 4). The important role of electrostatic interactions in the binding of PL-DNA conjugates to titanium dioxide is confirmed by the fact that oligonucleotides without the PL linker are hardly immobilized onto TiO2 nanoparticles, while the attachment significantly increases in the presence of cetyltrimethylammonium bromide, which masks the negative charge of the phosphate groups of oligomers (data not shown). The formed TiO2·PL-DNA nanocomposites were shown by confocal microscopy to penetrate into mammalian cells without any transfection agents and physical impact29.
Figure 4. Cartoon scheme of TiO2 ·PL-DNA nanocomposites.
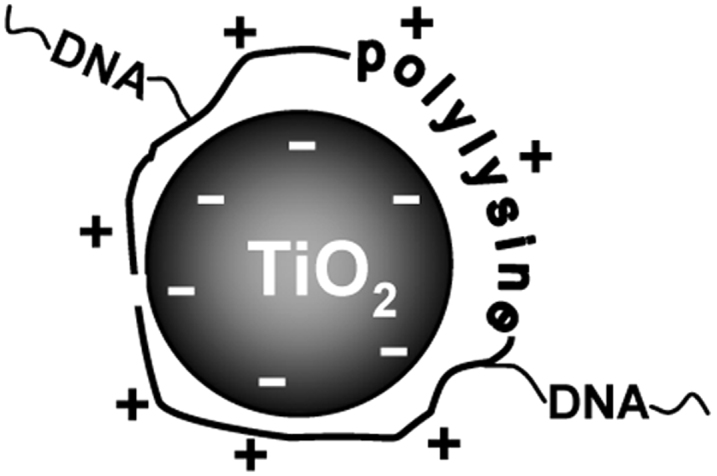
DNA fragments are covalently attached to the polylysine linker (positively charged), which is immobilized to the surface of TiO2 nanoparticles (negatively charged) due to electrostatic interactions.
Here we studied the next key stage of using NA-based drugs, namely, the interaction with target nucleic acids inside cells.
We examined TiO2·PL-DNA nanocomposites bearing DNA fragment complementary to 3′-uncoding region of segment 5 of (-)RNA of influenza A virus (H3N2) for their antiviral activity (Fig. 2). This nanocomposite showed the high antiviral effect (99.99% inhibition of virus replication at 0.1 µM concentration of DNA fragment in nanocomposite (5 µg/ml) at 0.05 moi. DNA fragment in the proposed nanocomposite was more active than the same oligonucleotide in the presence of lipofectamine widely used as the gold standard transfection agent. The antiviral activity of nanocomposite bearing noncomplementary DNA fragment was ~3000 times less active that demonstrates a significant specificity, i.e. antisense effect. It was revealed (Fig. 2, Table 1) that the specificity factor that is the ratio of the activity of noncomplementary and complementary DNA fragments (antisense effect) depended on their delivery system. This effect was more pronounced for the proposed nanocomposite as compared to other samples containing the DNA fragment. This may be due to the more efficient delivery of DNA fragments within the nanocomposite, their higher accessibility for the interaction with target nucleic acids, and enhanced stability inside cells as compared to other DNA-containing samples.
The proposed nanocomposites appeared to be low toxic for MDCK cells (TC50 ≈ 1800 µg/ml), and at the working concentration of 5 μg/ml they were practically nontoxic (Fig. 1). It is important to note that it is the TiO2 nanoparticles that are responsible for the toxic action of nanocomposites, and DNA fragments do not contribute to this effect (Fig. 1). In opposite, the contribution of TiO2 nanoparticles in antiviral activity is very low, if at all. The IC50 value (indicating 50% inhibition of viral replication) for the nanocomposite was evaluated from the data of dose-response study to be ~1.5 µg/ml (Fig. 3). These data allowed us to derive the selectivity index value, i.e. the ratio of TC50 to IC50 for the designed nanocomposite (SI ≈ 1200). In comparison, the selectivity index for morpholino oligonucleotides was estimated as ~4034. High values of the selectivity index and the specificity factor indicate good prospects for using TiO2 ·PL-DNA nanocomposites in medical practice.
Some researchers studied the antiviral activity of oligonucleotides and their derivatives. Oligonucleotides with native phosphodiester bonds did not reveal antiviral activity at concentration, which were 270 times higher than we used36. Transfection of MDCK cells infected with influenza A virus with phosphorothioate antisense oligonucleotides aimed (4 µM) to different regions of the NP gene in the presence of lipofectamine led to the reduction of the virus titer by 1–1.2 log39. The same authors showed the similar antiviral effect of antisense oligonucleotides delivered into MDCK cells with the antibody delivery system40. In our case, the reduction of the virus titer achieves 3.9 log. A morpholino oligonucleotide with the same sequence as DNA1 was also shown34 to be efficient against different strains of influenza A virus, although its efficiency was significantly lower than in our case (the inhibitory effect was 88–98% at 15 µM concentration). Moreover, morpholino oligonucleotides should be conjugated to an arginine-rich peptide to ensure the delivery into cells. The concentrations of morpholino or phosphorothioate oligonucleotides were higher by factors of 40–150, and their efficiency in the virus inhibition was significantly lower than in our case.
Short interference RNAs (siRNA) are under intense investigation as antiviral agents. After electroporation, NP-specific siRNA (5 µM) inhibited virus production by factors of 30,000 or 200 at a moi of 0.001 or 0.1, respectively35. Similar or even slight higher inhibitory effect showed siRNA directed to the M2 gene of influenza A virus41. The inhibitory effect of several NP-specific siRNAs on influenza A virus replication in MDCK cells transfected in the presence of lipofectamine was shown to be 0.8–1.6 log (6–43-fold reduction)42.
The antivirus efficiency of DNA fragments delivered into cells with the proposed TiO2·PL-DNA nanocomposites (~8,000-fold reduction of virus replication at 0.1 µM and 0.05 moi; 3.9 log of the inhibitory effect) is not inferior to (and even exceeds) that of siRNAs, which considered to be the most promising agents among nucleic acid drugs.
Thus, for the firs time, we have shown the highly efficient and selective action on intracellular nucleic acid targets of antisense DNA fragments delivered into cells with TiO2 nanoparticles. The high inhibition of influenza A virus replication with the proposed nanocomposites shows their potency as antisense drugs. The proposed high-performance method for specific effect on nucleic acids in cells may find a wide application for the treatment of not only viral but also other nucleic acid-based diseases, when it is necessary to ensure effective and selective interaction of DNA with intracellular nucleic acid targets.
Methods
Chemicals were obtained from commercial suppliers. We used: PMI-1640 medium, antibiotics (BioloT, Russia); trypsin (Sigma, USA); fetal calf serum (Gibco, USA); L-glutamine, PBS buffer, chicken erythrocytes, MDCK cells, and influenza A virus Aichi/2/68 (H3N2) (Vector, Russia); LipofectamineTM 2000 (Invitrogen, USA). TiO2 nanoparticles in the crystal form (anatase, An) were synthesized as described in19. Oligonucleotides were synthesized by the phosphoroamidite method on ASM-800 DNA synthesizer (Biosset, Russia) using phosphoroamidite monomers (Glen Research, USA). Trypsin (1 µg/ml) and penicillin-streptomycin (100 U/ml) (Sigma-Aldrich, USA) were stored at –80°C.
The synthesis of polylysine-containing oligonucleotides (PL-DNA) and nanocomposites consisting of anatase nanoparticles and PL-DNA conjugates (A[ndot ]PL-DNA) were fulfilled as described in19. The virus was grown in the allantoic cavity of 10-day-old embryonated chicken eggs (Vector, Russia) at 37°C. Allantoic fluid was harvested for 48 h after virus inoculation, aliquoted, and stored at –80°C.
Cell viability assays
MDCK cells in logarithmic phase were seeded at 100000 cells/ml in RPMI-1640 nutrient medium in 96-well plates (100 μl/well) and incubated at 37°C and 5% CO2. Two days after the formation of a continuous monolayer, cells were washed three times with nutrient medium (200 μl/well) containing no serum. The samples of TiO2 nanoparticles or TiO2 ·PL-DNA nanocomposites were diluted with RPMI-1640 medium to the needed concentration (10–1000μg/ml) and incubated with MDCK cells at 37°C and 5% CO2 for two days. Destructive changes in the cells were evaluated by an inverted microscope. MDCK cells without studied samples were used as a control. Cells were stained with trypan blue43, and the number of viable cells was counted in a Goryaev chamber. All experiments were performed in three independent series (Fig. 1).
Comparison of antiviral activity of samples (Preinfection assays)
MDCK cells were seeded as above and incubated with samples under investigation (DNA, PL-DNA, DNA + LipofectamineTM 2000, TiO2, TiO2 ·PL, and TiO2 ·PL-DNA) added in RPMI-1640 (100 µl/well) without trypsin. The final concentration was 0.1 µM for oligonucleotide in all DNA-containing samples and 5 µg/ml for nanoparticles and nanocomposites. Transfection of DNA with lipofectamine was performed according to the manufacturer's protocol. Transfection experiments were carried out in the absence of serum. Cells were incubated at room temperature and 5% CO2 and 100% humidity for 4 h, followed by rinsing with RPMI-1640. Virus A/Aichi/2/68 (H3N2) was then added in each well (100 μl/well) at a multiplicity of infection (moi) of 0.05 TCID50/cell in a medium containing trypsin. After 1-h adsorption at room temperature, virus-containing media were removed; cells were rinsed with RPMI-1640 medium without trypsin, and the same medium containing trypsin was applied to each well (100 µl). Cells were incubated at 37°C and 5% CO2 and 100% humidity for 48 h. Samples of virus culture were collected and their measurements were performed by hemagglutination (HA) assays using round-bottom 96-well plates. Serial ten-fold dilutions of virus samples with RPMI-1640 medium containing 2 μg/ml trypsin were applied to MDCK cells for 48 h. The presence of virus was visually determined in HGR with a 1% suspension of chicken erythrocyte. The titer was evaluated by noting the highest dilution of the virus, which caused the hemagglutination reaction. Wells containing an adherent, homogenous layer of erythrocytes were scored as positive. MDCK infected by A/Aichi/2/68 (H3N2) virus without the treatment with nanocomposites were used as controls. Virus titer in cells treated with nanocomposites was expressed as log TCID50/ml, n = 6 (Fig. 2) and TCID50/ml (Table 1).
Dose-response studies
Preinfection assays
MDCK cells at ~80% confluence were incubated with the TiO2·PL-DNA1 nanocomposite taken in RPMI-1640 medium without trypsin (100 µl/well) at various concentrations (1, 2.5, 5, 10, 20, and 50 µg/ml). After 4-h incubation at 37°C and 5% CO2 and 100% humidity, preparations were removed; cells were rinsed with RPMI-1640 medium without trypsin. Virus A/Aichi/2/68 was then added in each well in RPMI-1640 medium (100 µl) containing trypsin (2 µg/ml) at a moi of 0.1 TCID50/cell. After 1-h adsorption at room temperature, virus-containing media were removed; cells were rinsed with RPMI-1640 medium without trypsin, and the same medium containing trypsin was applied to each well (100 µl). Cells were incubated at 37°C and 5% CO2 and 100% humidity for 48 h. The virus titers were evaluated as above.
Postinfection assays
MDCK cells at ~80% confluence were initially infected with A/Aichi/2/68 (H3N2) virus as described above. After 1-h adsorption at room temperature, virus-containing medium was removed, and cells were rinsed with RPMI-1640 medium without trypsin. Nanocomposite TiO2·PL-DNA1 taken in RPMI-1640 medium without trypsin (100 µl/well) at various concentrations (1, 2.5, 5, 10, 20, and 50 µg/ml) was applied to the infected MDCK cells, followed by the incubation for 4 h at 37°C and 5% CO2 and 100% humidity. The cultural media were then removed, cells were rinsed with RPMI-1640 medium without trypsin, and the same medium containing trypsin (2 µg/ml) was applied to each well (100 µl/well). After 48-h incubation at 37°C, 5% CO2, and 100% humidity, serial ten-fold dilutions of cultural virus-containing liquid from each well were applied to MDCK cells for 48 h to evaluate the virus titer. Results are presented in Fig. 3.
Author Contributions
A.S.L. and M.N.R created nanocomposites and other samples, Z.R.I. and N.V.S. prepared nanoparticles, E.G.M. and V.V.Z. gave a consultation on the antiviral study, N.A.M. and A.A.E. performed the antiviral studies, S.I.B. prepared the figures, V.F.Z. planned the work, A.S.L. and V.F.Z. wrote the manuscript.
Acknowledgments
This work was supported by Russian Foundation for Basic Research grant No. 11-04-01408-a, Integration Project of Siberian Branch of the Russian Academy of Sciences No. 61, and Federal Target Program No. 16.512.11.2267.
References
- Mescalchin A. &, Restle T. Oligomeric nucleic acids as antivirals. Molecules 16, 1271–1296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgers K. B., Sharkey C. M., Warfield K. L. & Bavari S. Oligonucleotide antiviral therapeutics: antisense and RNA interference for highly pathogenic RNA viruses. Antiviral Res 78, 26–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin R. M. & Ferrary N. Emerging oligonucleotide therapies for asthma and chronic obstructive pulmonary disease. Expert Opin. Investig. Drugs 18, 1505–1517 (2009). [DOI] [PubMed] [Google Scholar]
- Silverman S. K. in Functional Nucleic Acids For Analytical Applications, eds. Yingf, L. & Yi, L., N.Y., Springer., 47–108 (2009).
- Bhindi R. et al. DNA enzymes, short interfering RNA, and emerging wave of small-molecule nucleic acid-based gene-silencing strategies. Am. J. Path. 171, 1079–1088 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F. & Swayze E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010). [DOI] [PubMed] [Google Scholar]
- Margus H., Padari K. & Pooga, M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol. Ther. 20, 525–533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. I. Retroviral integration profiles: their determinants and implications for gene therapy. BMB Rep. 45, 207–212 (2012). [DOI] [PubMed] [Google Scholar]
- Bouard D., Alazard-Dany N. & Cosset F-L. Viral vectors: from virology to transgene expression. British J. Pharm. 157, 153–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J. & Neundorf I. Peptide vectors for the nonviral delivery of nucleic acids. Acc. Chem. Res. 45, 1048–1056 (2012). [DOI] [PubMed] [Google Scholar]
- Morille M. et al. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 29, 3477–3496 (2008). [DOI] [PubMed] [Google Scholar]
- Donkuru M. et al. Advancing nonviral gene delivery: lipid- and surfactant-based nanoparticle design strategies. Nanomedicine (Lond) 5, 1103–1127 (2010). [DOI] [PubMed] [Google Scholar]
- Koynova R. & Tenchov B. Recent patents in cationic lipid carriers for delivery of nucleic acids. Recent Pat. DNA Gene Seq. 5, 8–27 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang J. S., Liu F. & Huang L. Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv. Drug Deliv. Rev. 57, 689–698 (2005). [DOI] [PubMed] [Google Scholar]
- Yu B., Zhao X., Lee L. J. & Lee R. J. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 11, 195–203 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari M., Soltani M., Naahidi S., Karunaratne D. N. & Chen P. Nonviral approach for targeted nucleic acid delivery. Curr. Med. Chem. 19, 197–208 (2012). [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Kawakami S. & Hashida M. Strategies for in vivo delivery of siRNAs: recent progress. BioDrugs 24, 195–205 (2010). [DOI] [PubMed] [Google Scholar]
- Cho K., Wang X., Nie S., Chen Z. G. & Shin D. M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 14, 1310–1316 (2008). [DOI] [PubMed] [Google Scholar]
- Marcato P. D. & Durán N. New aspects of nanopharmaceutical delivery systems. J. Nanosci. Nanotechnol. 8, 2216–2229 (2008). [DOI] [PubMed] [Google Scholar]
- Parveen S., Misra R. & Sahoo S. K. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8, 147–166 (2012). [DOI] [PubMed] [Google Scholar]
- Han W., Wang Y. & Zheng Y. In vivo biocompatibility studies of nano TiO2 materials. Advanced Materials Research 79–82, 389–392 (2009). [Google Scholar]
- Suzuki H., Toyooka T. & Ibuki Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 41, 3018–3024 (2007). [DOI] [PubMed] [Google Scholar]
- Thurn K. T. et al. Endocytosis of titanium dioxide nanoparticles in prostate cancer PC-3M cells. Nanomedicine 7, 123–130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng H. A. & Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 41, 2699–2711 (2006). [DOI] [PubMed] [Google Scholar]
- Heinlaan M., Ivask A., Blinova I., Dubourguier H. C. & Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71, 1308–1336 (2008). [DOI] [PubMed] [Google Scholar]
- Mikkelsen L. et al. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO2. Part Fibre Toxicol. 8, 32–48 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismagilov Z. R. et al. The effect of chemical treatment conditions of titanium dioxide sols on their dispersion and cytotoxic properties. Chem. Eng. Trans. 27, 241–246 (2012). [Google Scholar]
- Fabian E. et al. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch. Toxicol. 82, 151–158 (2008). [DOI] [PubMed] [Google Scholar]
- Levina A. et al. Nanocomposites consisting of titanium dioxide nanoparticles and oligonucleotides. J. Nanosci. Nanotech. 12, 1812–1820 (2012). [DOI] [PubMed] [Google Scholar]
- Levina A., Pyshnaya I., Repkova M., Rar V. & Zarytova V. Oligonucleotide probes containing polylysine residues for fabrication of DNA chips on various solid surfaces. Biotechnol. J. 2, 879–885 (2007). [DOI] [PubMed] [Google Scholar]
- Lamb R. A. & Krug R. M. in Fundamental Virology, eds. Knipe, D.M. & Howely, P.M., Lippincott Williams and Wilkins, Philadelphia., 725–770 (2001).
- Portela A. & Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 83, 723–734 (2002). [DOI] [PubMed] [Google Scholar]
- Fodor E., Pritlove D. C. & Brownlee G. G. The influenza virus panhandle is involved in the initiation of transcription. J. Virol. 68, 4092–4096 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q. et al. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob. Agents Chemother. 50, 3724–3733 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q. et al. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. USA 100, 2718–2723 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter J., Agrawal S., Palese P. & Zamecnik P. Inhibition of influenza virus replication by phosphorothioate oligodeoxynucleotides. Proc. Natl. Acad. Sci. USA 87, 3430–3434 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner R., Michael J., Schwenzer B. & Scharnweber D. Biological nano-functionalization of titanium-based biomaterial surfaces: a flexible toolbox. J. R. Soc. Interface 7, S93–S105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsokechagia T., Cellesi F., Thomas A., Niederberger M. & Tirelli N. Preparation of ligand-free TiO2 (anatase) nanoparticles through a nonaqueous process and their surface functionalization. Langmuir 24, 6988–6997 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang T. et al. Antisense oligonucleotides targeting the RNA binding region of the NP gene inhibit replication of highly pathogenic avian influenza virus H5N1. Int. Immunopharmacol. 11, 2057–2061 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang T. et al. Antisense oligonucleotide inhibits avian influenza virus H5N1 replication by single chain antibody delivery system. Vaccine 29, 1558–1564 (2011). [DOI] [PubMed] [Google Scholar]
- Sui H. Y. et al. Small interfering RNA targeting m2 gene induces effective and long term inhibition of influenza A virus replication. PLoS One 4, e5671 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. et al. Effective small interfering RNAs targeting matrix and nucleocapsid protein gene inhibit influenza A virus replication in cells and mice. Antiviral Res. 76, 186–193 (2007). [DOI] [PubMed] [Google Scholar]
- Osano E., Kishi J. & Takahashi Y. Phagocytosis of titanium particles and necrosis in TNF-alpha-resistant mouse sarcoma L929 cells. Toxicol. In Vitro 17, 41–47 (2003). [DOI] [PubMed] [Google Scholar]