Abstract
Neurodegenerative diseases encompass complex cell signaling disturbances that initially damage neuronal circuits and synapses. Due to multiple protective mechanisms that are enacted to counteract the onset of neurodegenerative diseases, there is often a prolonged period without noticeable impairments during their initiation. Since severe cognitive deficit or vision loss takes place after that period there is an opportunity to harness endogenous protective mechanisms as potential therapeutic approaches. The activation of the biosynthesis of the docosanoid mediator neuroprotectin D1 (NPD1) is an early response to the upsurge of protein misfolding and other neuroinflammatory events. This overview discusses the potent neuroprotective and inflammation-modulating bioactivity of NPD1. This lipid mediator represents an early response to neurodegenerations, aiming to restore homeostasis.
Keywords: Misfolding, retinal degenerations, Alzheimer’s disease, Huntington’s disease, epilepsy, docosahexaenoic acid, ataxin1, huntingtin, CAG repeats, APP, Bcl-2 proteins
Introduction
Alzheimer’s disease, retinal degenerations and other neurodegenerative diseases are complex progressive disorders that involve in their pathophysiology multiple signaling dysfunctions that converge on the mitochondria, endoplasmic reticulum stress responses, caspase and caspase-independent forms of cell damage, all of which lead to synaptic damage and ultimately neuronal cell death [1, 2, 3, 4]. Neuroinflammation [5, 6] and protein misfolding [7] are early events in many neurodegenerative diseases. Since the initial stages of these diseases span several years, the identification of the key pathogenic steps as well as potential means to modulate those events are of interest to design protective and/or therapeutic approaches to slow down the initiation and progression of neurodegenerative diseases.
The significance of the selective enrichment in omega-3 essential fatty acids (docosahexaenoyl – DHA- chains of membrane phospholipids, 22C and 6 double bonds) in the nervous system (eg. synaptic membranes, dendrites and photoreceptors) has remained, until recently, incompletely understood [8, 9, 10, 11, 12, 13, 14, 15]. While studying mechanisms of cell survival in neurodegenerations, a docosanoid synthesized from DHA by 15-lipoxygenase-1 was identified [16, 17] and dubbed neuroprotectin D1 (NPD1,10R,17S-dihydroxy-docosa- 4Z,7Z,11E,13E,15E,19Z hexaenoic acid). This mediator is a docosanoid because it is derived from a 22C precursor (DHA), unlike eicosanoids, which are derived from the 20 C arachidonic acid family of essential fatty acids. Endogenous NPD1 biosynthesis is promptly induced in response to oxidative stress [17, 18], protein misfolding/proteotoxicity [19], seizures [20], brain ischemia-reperfusion [16, 21], and by neurotrophins [22]. NPD1 is bioactive in experimental brain damage, oxidative-stressed retinal pigment epithelial (RPE) cells, and in human brain cells exposed to amyloid-β peptide [18]. Thus, NPD1 is a protective sentinel made on demand in early stages of neural injury and one of the very first defenses activated when cell homeostasis is threatened by neurodegenerations [8, 23]. Here we provide an overview of experimental examples that highlight the specificity and potency of NPD1, spanning beneficial bioactivity during neuroinflammatory and proteotoxic events critical during the initiation and early progression of neurodegenerations.
The expansion of unstable translated CAG Ataxin-1 82Q or huntingtin 72Q activates endogenous NPD1 synthesis
Errors in DNA replication result in pathological large polyglutamine tracts that impair folding, stability and bioactivity, often triggering damage, unstable translation and cell death [23, 24]. The expansion of CAG repeats causes a subset of at least nine neurodegenerative disorders. The expression of Ataxin-1 82Q mutants, that cause spinocerebellar ataxia type-1 (SCA1), triggers the endogenous synthesis of NPD1 (Fig.1). This was ascertained by LC-MS/MS-based mediator lipidomics [19]. When huntingtin 72Q was expressed under similar conditions, NPD1 synthesis was also activated [19]. To test the hypothesis that those increases in NPD1 synthesis were protective responses, the addition of NPD1 to singular cultures was explored. The idea to be tested here was that the magnitude of the consequences of expressing the misfolded protein was greater than the protection ability of the endogenously produced NPD1. This possibility was explored by adding exogenous NPD1. Figure 2 displays the remarkable ability of 50mM NPD1 in exerting anti-apoptotic bioactivity under these conditions. Moreover, when the serpin family growth factor PEDF was added along with 100mM DHA, protection from apoptosis also took place (Fig. 2). Under these conditions NPD1 is endogenously made, as it was previously shown under basal conditions [22].
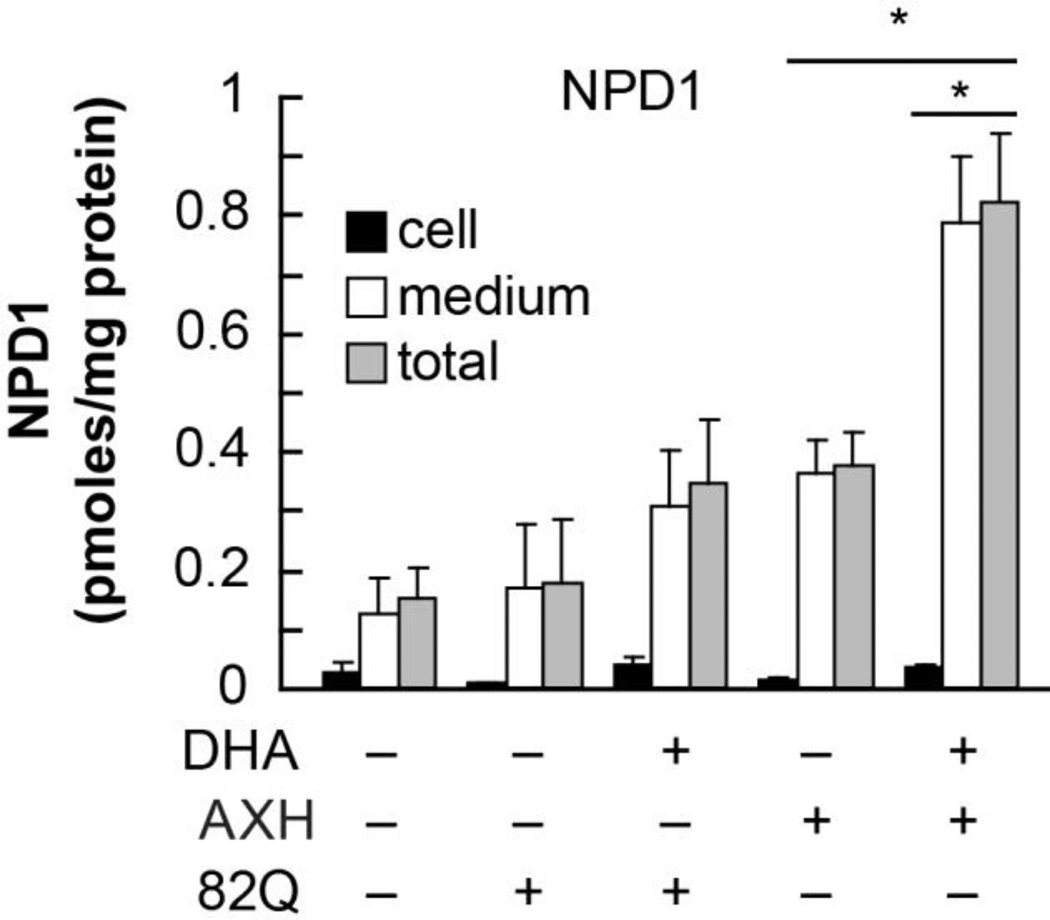
Figure 1. Endogenous NPD1 biosynthesis is enhanced upon expression of Ataxin-1 82Q in RPE cells.
Primary human RPE cells were transfected with 82Q. Cells (black bars), NPD1 content in media (white bars) and total (grey bars) was measured by LC MS-MS with or without the addition of DHA. * p<0.005. (Figured modified and published with permission from Journal of Biological Chemistry (2012) 287(28):23726-39 “Ataxin-1 Poly(Q)-induced Proteotoxic Stress and Apoptosis are Attenuated in Neural Cells by Docosahexaenoic Acid-derived Neuroprotectin D1”; Calandria JM, et al.)
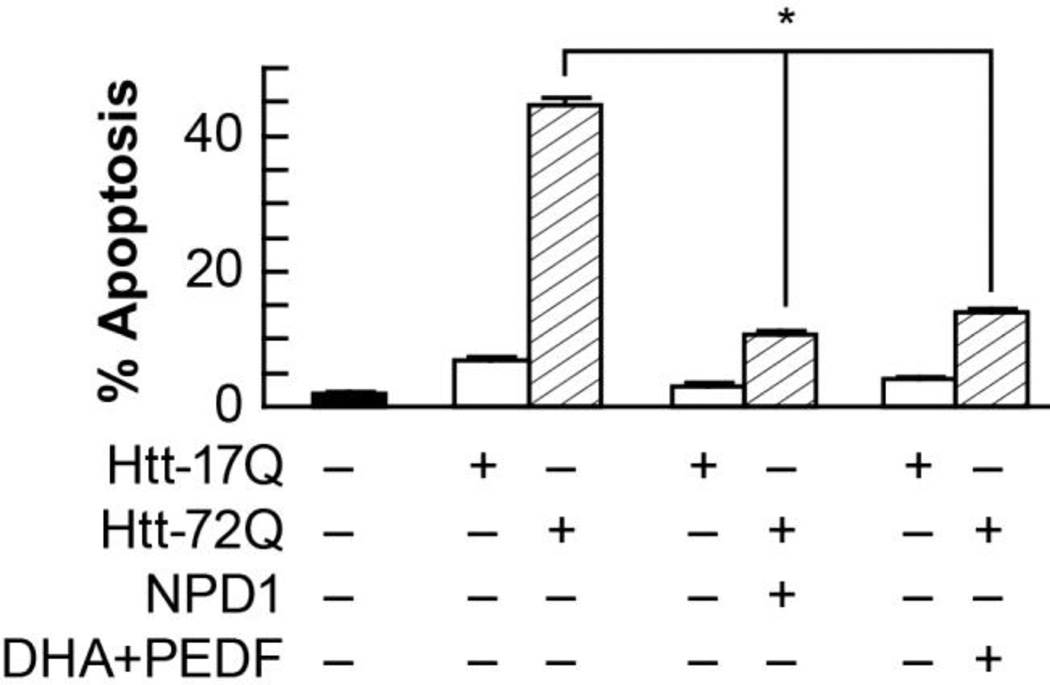
Figure 2. NPD1 prevents huntingtin-17Q-induced apoptosis in ARPE-19 cells.
ARPE-19 cells transfected with an expression construct containing ht-72Q were treated with 50 nM NPD1, or DHA (100 nM) along with PEDF (10 ng/mL). Apoptosis percentage was calculated by dividing pyknotic over the total count of cells. Results are averages ± SD. * p< 0.0005. (Figured modified and published with permission from Journal of Biological Chemistry (2012) 287(28):23726-39 “Ataxin-1 Poly(Q)-induced Proteotoxic Stress and Apoptosis are Attenuated in Neural Cells by Docosahexaenoic Acid-derived Neuroprotectin D1”; Calandria JM, et al.)
NPD1 attenuated prototoxicity of CAG repeat containing proteins
NPD1 also decreased phospho-Ser -776 in Ataxin-1. We speculate that in agreement with our previous findings that NPD1 may work by increasing PP2A activity. Thus the lipid mediator may counteract PP2A inhibition, allowing the 82Q form to be de-phosphorylated and cleared or relocated into the spliceosome. The fact that Anp32 was proposed to have a stronger interaction with the expanded form rather than with the wild type Ataxin-1 makes this protein an excellent target candidate for NPD1 signaling. Thus, in addition to the expansions in the poly-glutamine tract, AXH has an important role in the functionality of Ataxin-1. AXH, a self-folding domain present in Ataxin-1, is responsible for the protein-protein interactions between Ataxin-1 and other transcription factors, such as the capicua homolog CIC protein. The sequestration of the complex partners formed by Ataxin-1 by its inactive counterpart may be involved in the loss of function observed in neurodegenerations. Brother of Ataxin-1 (Boat), another member of the AXH domain-containing protein family, is an example of the proposed loss of function. Boat is an in vivo binding partner of Ataxin-1 that is also affected by the malfunction of Ataxin-1 82Q. Therefore, the expression of AXH alone in our cells resulted in increased apoptosis. Furthermore, AXH expression aggravated the cytotoxicity induced by Ataxin-1 82Q. Unlike the sequestration scenario, in which the complexes are formed but are inactive, AXH induces toxicity in this case by increasing disassembly of the complex, thus promoting inactivation of its partners. NPD1 signaling promotes survival by modulating a set of genes that homeostatically control cell fate and regulate proteostasis. NPD1 reversed the toxicity of Ataxin-1 82Q as well as of Huntingtin 72Q in our cells [19]. Since protein misfolding and proteotoxic stress takes place in early stages of several neurodegenerative diseases, we have explored these events as possible NPD1-targets in cell culture models (human RPE cells and primary neuronal mix cultures). We have studied the expansion of unstable translated CAG repeats that encode polyglutamine tracts that cause spinocerebellar ataxia type 1 and Huntington’s disease, which are Ataxin-1 Poly-Q and huntingtin Poly-Q.
Seizures trigger NPD1 synthesis and this docosanoid attenuates epileptogenesis
Epileptogenesis as a model to explore mechanisms that sustain neuronal network integrity under adverse conditions shows that NPD1 is a protective mediator candidate. Using LC-MS/MS-based mediator lipidomic analysis shows that NPD1 synthesis increases during seizures (Fig. 3) in the hippocampus [20] and that when administered this docosanoid during pharmacologically–induced epileptogenesis it elicited a remarkable attenuation of pathological brain oscillations. This effect reflects modulatory bioactivity of aberrant neuronal networks that lead to spontaneous recurrent seizures. This was further substantiated using multi-microelectrode arrays in freely moving mice, described in a yet unpublished study (Musto AE & Bazan NG). Thus, docosanoid-mediated signaling rescues neuronal network disruptions.
NPD1 is reduced in Alzheimer’s disease brains and redirects APP processing to non-amyloidogenic pathway
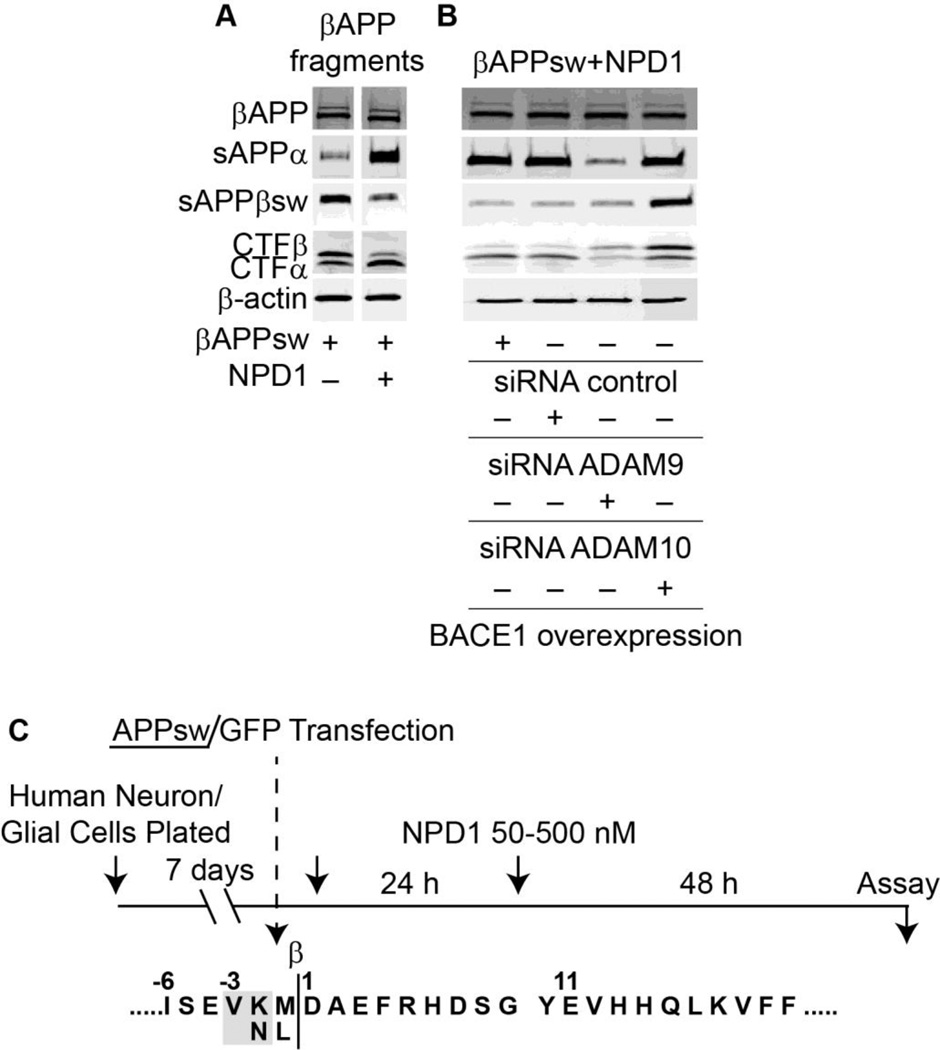
Early stages of AD display a reduced content of NPD1 [25, 26], and 15 lipoxigenase-1 expression is decreased in AD [25]. This enzyme is key for NPD1 synthesis. The silencing of this enzyme in human retinal pigment epithelial cells leads to selective rescue from oxidative stress-induced apoptosis only by NPD1. Since this enzyme catalyzes conversion of arachidonic acid in several eicosanoids, the addition of 12-HETE, 15-HETE and of protective lipoxin A4 fails to rescue 15 lipoxigenase-1 deficient cells from oxidative stress induced cell death [27]. We have therefore explored the significance of NPD1 in cellular models that recapitulate part of the Alzheimer’s pathology. Human neurons and astrocytes challenged by amyloid-β or by overexpressing APPsw (double Swedish mutation that causes familial forms of the disease) show that NPD1 downregulates amyloidogenic processing of amyloid-β precursor protein (Fig. 4). NPD1 switches off pro-inflammatory gene expression (TNF-α, COX-2 and B-94-TNF-α inducible pro-inflammatory element), and promotes neural cell survival. Moreover, anti-amyloidogenic processing by NPD1 targets α- and β-secretases and PPARγ receptor activation [18]. Currently we are using imaging MALDI/TOF-MS to further unravel the lipidome in specific brain regions.
Figure 4. NPD1 shifts βAAP processing to a non-amyloidogenic pathway.
(A) Control or HNG cells over-expressing βAPPsw were treated with increasing concentrations (0, 50, 100, 500 nM) of NPD1 for 48 h and subjected to Western blot detection of holo-βAPP (βAPP holoenzyme), sAPPα, sAPPβsw, CTFα and CTFβ in comparison to β-actin levels in the same sample; (B) Quantification of gel bands in (A) analyzing βAPP fragments with increasing doses of NPD1. Results are means ± SEM (n=4); *p<0.01 vs. βAPPsw control.
The abundance of anti-apoptotic BCL-2 proteins is positively modulated by NPD1, whereas pro-apoptotic BCL-2 proteins are negatively regulated
The availability of anti-apoptotic BCL-2 proteins is positively modulated by NPD1, whereas pro-apoptotic BCL-2 proteins are negatively regulated, as is microglial activation. NPD1 modulates the protein phosphatase PP2A that targets S62- Bcl-xl. In turn Bcl-xl heterodimerizes with BAX, thus decreasing the availability of this pro-apoptotic BCL-2 protein and leading to cytoprotection [28]. Overall, oxidative stress consequences are attenuated [29, 30]. Moreover, in a model of the wet form of age-related macular degeneration it was found that NPD1 attenuates choroidal neovascularization [31]. Additionally, microglial activation towards a highly ramified phenotype is induced by NPD1 under these conditions [32].
This cell survival cascade and the events that sustain neuronal network homeostatic integrity involves multiple checkpoints and signaling networks that include restoring proteostasis during protein misfolding/ proteotoxicity. NPD1 regulation of targets upstream events of cell survival, neuroinflammatory signaling and transcription in turn promotes homeostatic regulation of synaptic and neural circuitry integrity.
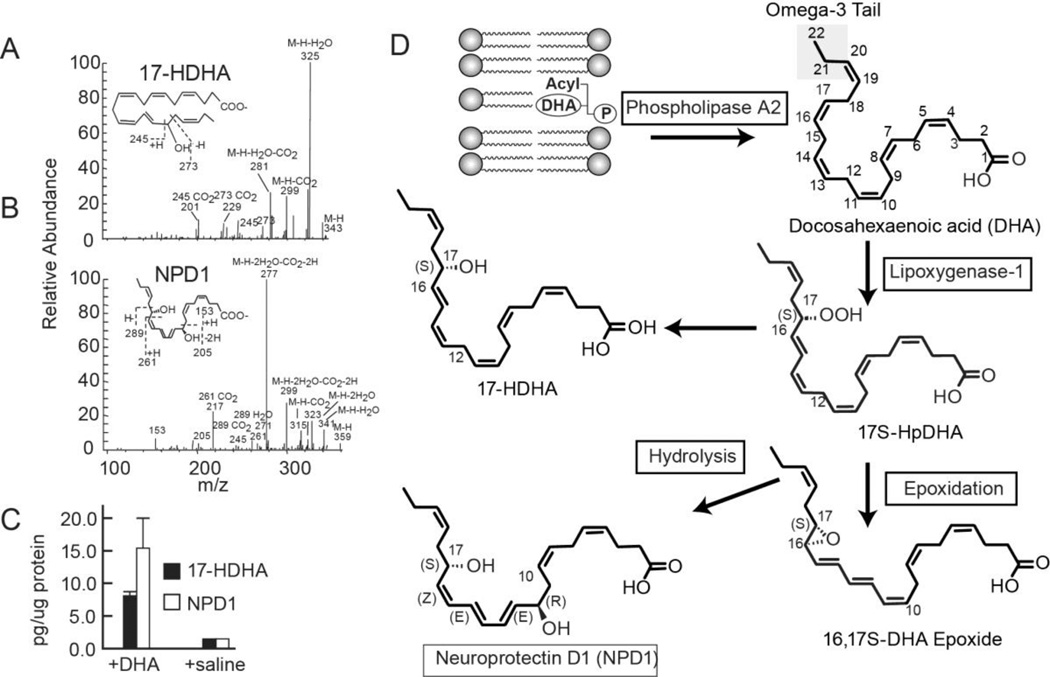
Figure 3. The characterization and quantification of 17-HDHA and NPD1 in the ipsilateral penumbra 3 days after MCAo.
a–b) The fragmentation pattern is depicted and (c) the quantification is presented. The increased content of 17-HDHA and of NPD1 in the ipsilateral penumbra in animals injected with DHA is consistent with the activation of the biosynthesis of NPD1. (d) Enzyme-mediated oxygenation of DHA for the biosynthesis of NPD1. Phospholipase A2 releases DHA from the second C position of phospholipids during brain ischemia-reperfusion. 15-Lipoxygenase-1 catalyzes the synthesis of 17S-H(p)DHA, which in turn is converted to a 16(17)-epoxide and then is enzymatically hydrolyzed to NPD1. (Figured modified and published with permission from Translational Stroke Research (2011) 2:33-41 “Docosahexaenoic acid therapy of experimental ischemic stroke”; Belayev, et al.)
Acknowledgments
(Supported by NIH: NINDS R01 NS046741, NEI R01 EY005121)
References
- 1.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D, Tsai LH. Bridging physiology and pathology in AD. Cell. 2009;137(6):997–1000. doi: 10.1016/j.cell.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegenration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71(1):35–48. doi: 10.1016/j.neuron.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;44(2):216–222. doi: 10.1007/s12035-011-8200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, Spector AA, Xiong ZM. A synaptogenic amide Ndocosahexaenoylethanolamide promotes hippocampal development. Prostglandins Other Lipid Mediat. 2011;96(1–4):114–120. doi: 10.1016/j.prostaglandins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport SI, Ramadan E, Basselin M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an invivo biomarker of brain DHA metabolism and neurotransmission. Prostglandins Other Lipid Mediat. 2011;96(1–4):109–113. doi: 10.1016/j.prostaglandins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto M, Hossain S. Neuroprotective and ameliorative actions of polyunsaturated fatty acids against neuronal diseases: beneficial effect of docosahexaenoic acid on cognitive decline in Alzheimer’s disease. J Pharmacol Sci. 2011;116(2):150–162. doi: 10.1254/jphs.10r33fm. [DOI] [PubMed] [Google Scholar]
- 12.Calon F. Omega-3 polyunsaturated fatty acid in Alzheimer’s disease: key questions and partial answers. Curr Alzheimer Res. 2011;8(5):470–478. doi: 10.2174/156720511796391881. [DOI] [PubMed] [Google Scholar]
- 13.Astarita G, Piomelli D. Towards a whole-body systems [multi-organ] lipidomics in Alzheimer’s disease. Prostaglandins Leukot Essent Fatty Acids. 2011;85(5):197–203. doi: 10.1016/j.plefa.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44(2):203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 15.Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. 2010;68(Suppl.2):102–111. doi: 10.1111/j.1753-4887.2010.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278(44):43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretaseand PPARγ-mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;6(1):e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calandria JM, Mukherjee PK, de Rivero Vaccari JC, Zhu M, Petasis NA, Bazan NG. Ataxin-1 Poly(Q)-induced Proteotoxic Stress and Apoptosis are Attenuated in Neural Cells by Docosahexaenoic Acid-derived Neuroprotectin D1. J Biol Chem. 2012;287(28):23726–23739. doi: 10.1074/jbc.M111.287078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musto AE, Gjorstrup P, Bazan NG. The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia. 2011;52(9):1601–1608. doi: 10.1111/j.1528-1167.2011.03081.x. [DOI] [PubMed] [Google Scholar]
- 21.Belayev L, Eady TN, Khotorova L, Atkins KD, Obenaus A, Cordoba M, Vaquero JJ, Alvarez-Builla J, Bazan NG. Superior Neuroprotective Efficacy of LAU-0901, a Novel Platelet-Activating Factor Antagonist, in Experimental Stroke. Transl Stroke Res. 2011;3(1):154–163. doi: 10.1007/s12975-011-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson FE, Bazan NG. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc. Natl. Acad. Sci. USA. 2007;104(32):13158–13163. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;4(3) doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoghbi HY, Orr HT. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type-1. J Biol Chem. 2009;284(12):7425–7429. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen VQ, Gillen DL, Head E, Cotman CW, Piomelli D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS One. 2010;5(9):e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calandria JM, Bazan NG. Neuroprotectin D1 modulates the induction of pro-inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol. 2010;664:663–670. doi: 10.1007/978-1-4419-1399-9_76. [DOI] [PubMed] [Google Scholar]
- 29.Antony R, Lukiw WJ, Bazan NG. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J Biol Chem. 2010;285(24):18301–18308. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheets KG, Zhou Y, Ertel MK, Knott EJ, Regan CE, Jr, Elison JR, Gordon WC, Gjorstrup P, Bazan NG. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;2(16):320–329. [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]