Abstract
Trigeminal neuropathic pain is a facial pain syndrome associated with trigeminal nerve injury. However, the mechanism of trigeminal neuropathic pain is poorly understood. This study aimed to determine the role of transient receptor potential vanilloid 1 (TRPV1) in heat hyperalgesia in a trigeminal neuropathic pain model. We evaluated nociceptive responses to mechanical and heat stimuli using a partial infraorbital nerve ligation (pIONL) model. Withdrawal responses to mechanical and heat stimuli to vibrissal pads (VP) were assessed using von Frey filaments and a thermal stimulator equipped with a heat probe, respectively. Changes in withdrawal responses were measured after subcutaneous injection of the TRP channel antagonist capsazepine. In addition, the expression of TRPV1 in the trigeminal ganglia was examined. Mechanical allodynia and heat hyperalgesia were observed in VP by pIONL. Capsazepine suppressed heat hyperalgesia but not mechanical allodynia. The number of TRPV1-positive neurons in the trigeminal ganglia was significantly increased in the large-diameter-cell group. These results suggest that TRPV1 plays an important role in the heat hyperalgesia observed in the pIONL model.
Keywords: TRPV1, trigeminal neuropathic pain, heat hyperalgesia.
Introduction
Idiopathic trigeminal neuralgia and trigeminal neuropathic pain are severe and constant orofacial pain syndromes. Because of such pain, the patient's quality of life is remarkably decreased. Idiopathic trigeminal neuralgia is caused by sensory nerve root compression and characterized by sudden onset of stabbing pain attacks 1, 2. In contrast, trigeminal neuropathic pain is associated with trigeminal nerve injury, leading to chronic pain such as burning and aching 3, 4. Although antidepressants and anticonvulsants are effective in palliating idiopathic trigeminal neuralgia, these drugs have little effect on the palliation of trigeminal neuropathic pain 5.
Neuropathic pain including trigeminal neuropathic pain indicates mechanical and heat hyperalgesia, and enhances heat sensitivity. A number of peripheral nerve injury models have been developed, such as spinal nerve ligation (SNL) model 6, chronic constriction nerve injury (CCI) model 7 and partial sciatic nerve injury (PSL) model 8. However, only a few animal models in the trigeminal system have been reported. Trigeminal neuropathic pain models have been developed in which the infraorbital nerve (ION) 5, 9 or inferior alveolar nerve (IAN) 10 is injured. These models demonstrate mechanical allodynia and heat hyperalgesia.
Transient receptor potential vanilloid 1 (TRPV1) has been identified as a capsaicin-, heat- and proton-sensitive ion channel 11-13. TRPV1 is highly expressed in small-diameter primary sensory neurons in dorsal root ganglia (DRG) and trigeminal ganglia (TG) 11, 13. TRPV1 is reported to be involved in inflammation 14, cancer 15, 16 and neuropathic pain 17-19. In orofacial pain, TRPV1 is reported to be involved in tooth pulp inflammation 20, 21, temporomandibular disorders (TMD) 22, oral cancer 23 and IAN injury pain 24. These findings indicate that TRPV1 participates in neuropathic pain and may be involved in trigeminal neuropathic pain. Moreover, analysis of TRPV1 expression may contribute to the development of therapeutic drugs and methods for treating trigeminal neuropathic pain.
In this study, to examine the mechanisms of trigeminal neuropathic pain, we validated a partial infraorbital nerve ligation (pIONL) model by the evaluation of changes in nociceptive responses to mechanical and heat stimuli. In addition, we investigated the changes of TRPV1 expression in TG neurons.
Materials and Methods
Experimental animals
Twenty-seven male Sprague-Dawley rats (SLC, Hamamatsu, Japan) weighing 150-350 g were used in this study. All procedures for animal care were approved by the Animal Management Committee of Matsumoto Dental University (permission No. 177). All animal experiments were performed in compliance with the Guidelines for Proper Conduct of Animal Experiments, established by the Science Council of Japan, and the guidelines of the International Association for the Study of Pain 25.
Partial infraorbital nerve ligation model
A partial ION ligation model was produced as previously described 5. In brief, the ION was partially ligated using 6-0 silk according to Seltzer's model 8. For a control, a sham operation was performed identically except that the nerve was not ligated. All operations were performed aseptically, no antibiotics were administered and all rats recovered uneventfully. After surgery, the rats were given laboratory chow and tap water ad libitum under conventional laboratory conditions 5.
Behavioral testing
The time courses of mechanical and heat sensitivity and the effects of antagonist on behavioral experiments were determined in a partial ligation of the ION (pIONL) group and a sham operation group (Sham). Rat whiskers were cut with scissors the day before experiments. Animals were allowed to acclimate to their surroundings for 10-15 minutes before testing. During the assessment of mechanical and heat sensitivity, animals were restrained around the trunk with a towel to calm them and treated gently during the experiments 5.
Assessment of mechanical sensitivity
Mechanical sensitivity of rat's vibrissal pad (VP) was tested as previously described 5. von Frey filament (26 g; North Coast Medical Inc., Morgan Hill, CA) was applied to the VP. The frequency of nociceptive response (head withdrawal or vocalization) was measured from 5 trials. For each trial, the filament was applied at 5-second intervals. The nociceptive threshold was defined as the minimum force needed to evoke nociceptive responses in at least 60% of the trials 26. The same procedure was performed on days 2, 4, 6, 8, 10, 12, 14, 21 and 28 after pIONL.
Assessment of heat sensitivity
The thermal response was assessed by application of heat stimuli to VP using a stimulator equipped with a thermal probe (THERMAL STIMULATOR UDH-300s, UNIQUE MEDICAL CO., LTD., Tokyo, Japan). During the assessment of heat sensitivity, the glycerol (Sigma) was applied to VP to increase thermal conductivities. Briefly, the thermal probe (diameter: 5 mm) was placed manually on the VP, and the probe temperature was increased at a rate of 1.0ºC/sec to a maximum of 58ºC (cut-off), which was established in order to prevent tissue damage. Animals were confined so as not to withdraw or flick their snouts when their VP was placed on the thermal probe maintained at room temperature before testing. Withdrawal threshold was defined as the temperature at which animals withdrew (head withdrawal or vocalization) their VPs. Withdrawal temperature was measured from 5 trials. For each trial, the filament was applied at 5-second intervals.
Animals were tested before pIONL to assess basal levels. The same procedure was performed on days 2, 4, 6, 8, 10, 12, 14, 21 and 28 after pIONL.
Peripheral administration of TRPV1 antagonist
The effects of the TRP channel antagonist on behavioral experiments were studied 25-28 days after ligation. The TRP channel antagonist capsazepine (Biomol International, Plymouth Meeting, PA) was dissolved in 100% dimethylsulfoxide (DMSO, Sigma) 26. The EC50 value for capsazepine against TRPV1 on the inward currents was 7.8 μM in oocyte 27. The peripheral administration of capsazepine and the concentration used in this study have been proven to be effective in previous studies 16. Fifty μL of capsazepine was subcutaneously administered in small doses at a few separate bilateral VP locations in various directions to make sure that the drugs would reach all over the VP. In control experiments, 50 μL of 100% DMSO was administered. Withdrawal responses were measured 30 minutes after capsazepine injection. Before the administration of capsazepine or DMSO, animals were tested to assess the basal level of withdrawal responses.
Immunohistochemistry
TG were fixed and processed for immunohistochemistry as in a previous study 28. Animals were perfused with cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). For the immunohistochemistry of TG, rabbit polyclonal antiserum against synthetic rat TRPV1 (Trans Genic, Kumamoto, Japan) was used after dilution at a concentration of 1:50 in 0.1 mol/L PBS containing 4% normal goat serum and 0.3% Triton-X 100 (Sigma). Sections were reacted with the reagent for 2 days at 4°C. After rinsing, species-directed secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit IgG; 1:500, Invitrogen, Carlsbad, CA) were applied to the sections (120 min). After being rinsed with 0.1 M PBS, the sections were cover-slipped in mounting medium (Diagnostic BioSystems Pleasanton, CA, USA) and examined under a fluorescence microscope equipped with a digital camera. No specific labeling was observed in the absence of a primary antibody.
The numbers and sizes of TRPV1-positive cells were automatically counted and measured using the computer-assisted imaging analysis system Image J 29. The TRPV1-positive cells were arbitrarily classified by cell diameter into 3 groups (< 20, 20-30, and > 30 μm) 23. The ratios of TRPV1-positive cells in each animal were calculated by the following formula: (total number of positive cells in 5 sections of TG/total number of all cells in 5 sections of TG) × 100. The calculation was performed for each group of cells classified by cell size 5.
Statistical analysis
Data are expressed as mean ± standard error (S.E.). To determine the significance of differences in mechanical sensitivity and heat sensitivity (Fig. 1 and Fig. 2) among Sham and pIONL groups, two-factor repeated-measure analysis of variance (ANOVA) was performed. The Huynh-Feldt ε correction was used to evaluate F ratios for repeated measures involving more than one degree of freedom. Holm-corrected post hoc Welch test was applied (10 hypotheses between Sham and pIONL group at each day in Fig. 2; 6 hypotheses, pre vs. DMSO, DMSO vs. 10 pmol/50 μL of capsazepine and DMSO vs. 100 pmol/50 μL of capsazepine in each operation in Fig. 2).
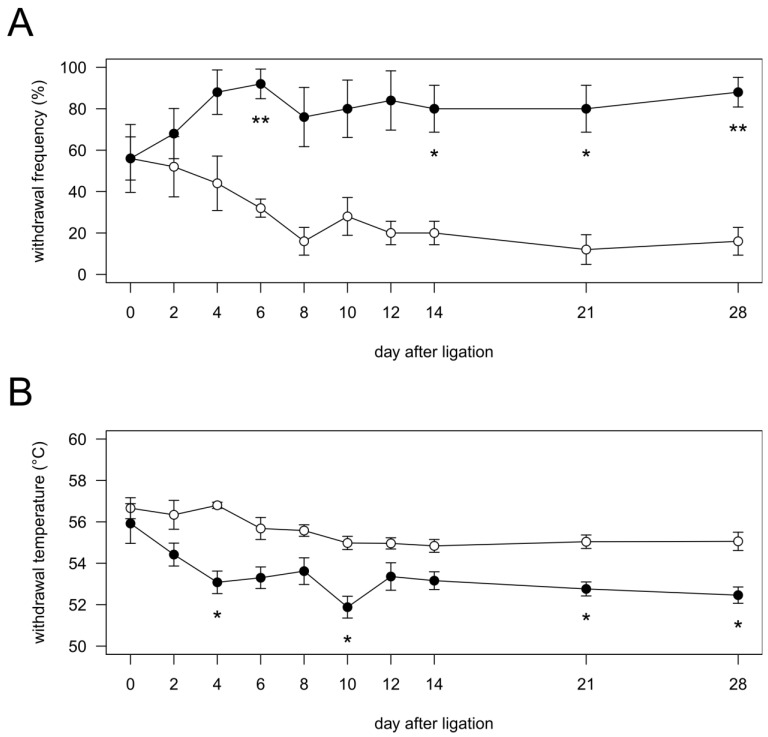
Figure 1.
Time course of the changes in mechanical (A) and heat (B) sensitivities in vibrissal pad after pIONL. Vibrissal pads ipsilateral to nerve injury and sham operation were stimulated with noxious stimuli. Open circles, Sham group (n = 5); closed circles, pIONL group (n = 5). P values were calculated by two-factor repeated-measure ANOVA corrected with Holm method (10 hypotheses). * P < 0.05 and ** P < 0.01 (Sham vs. pIONL).
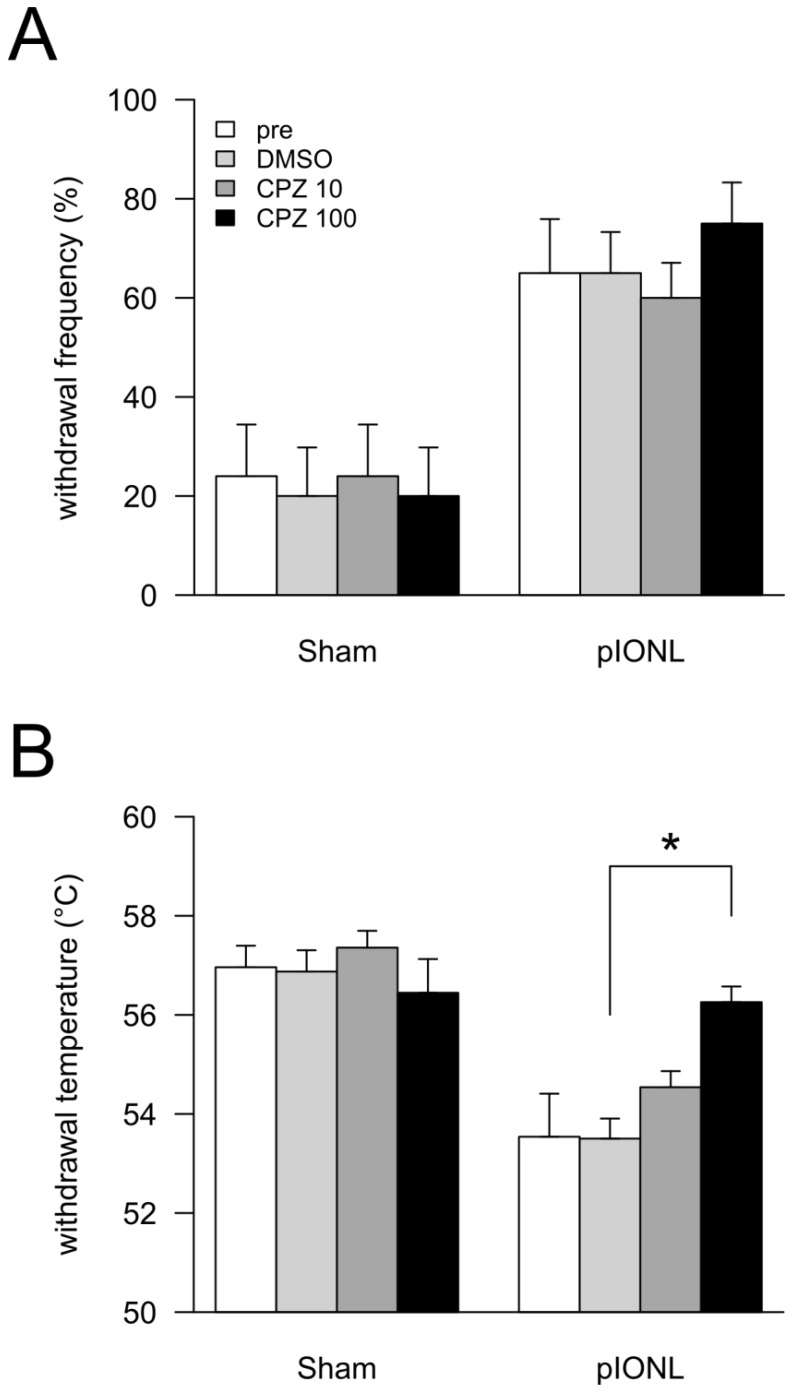
Figure 2.
Effects of TRPV1 antagonist capsazepine (CPZ) on behavioral experiments. At 25-28 days after pIONL, none (pre), 50 μL of DMSO, 10 pmol/50 μL of CPZ and 100 pmol/50 μL of CPZ were intraperitoneally injected. After 30 min, mechanical (A) and heat (B) sensitivities in vibrissal pad were examined. P values were calculated by two-factor repeated-measure ANOVA corrected with Holm method (6 hypotheses). * P < 0.05, ** P < 0.01 (pre vs. DMSO, DMSO vs. 10 pmol/50 μL of CPZ and DMSO vs. 100 pmol/50 μL of CPZ in each operation).
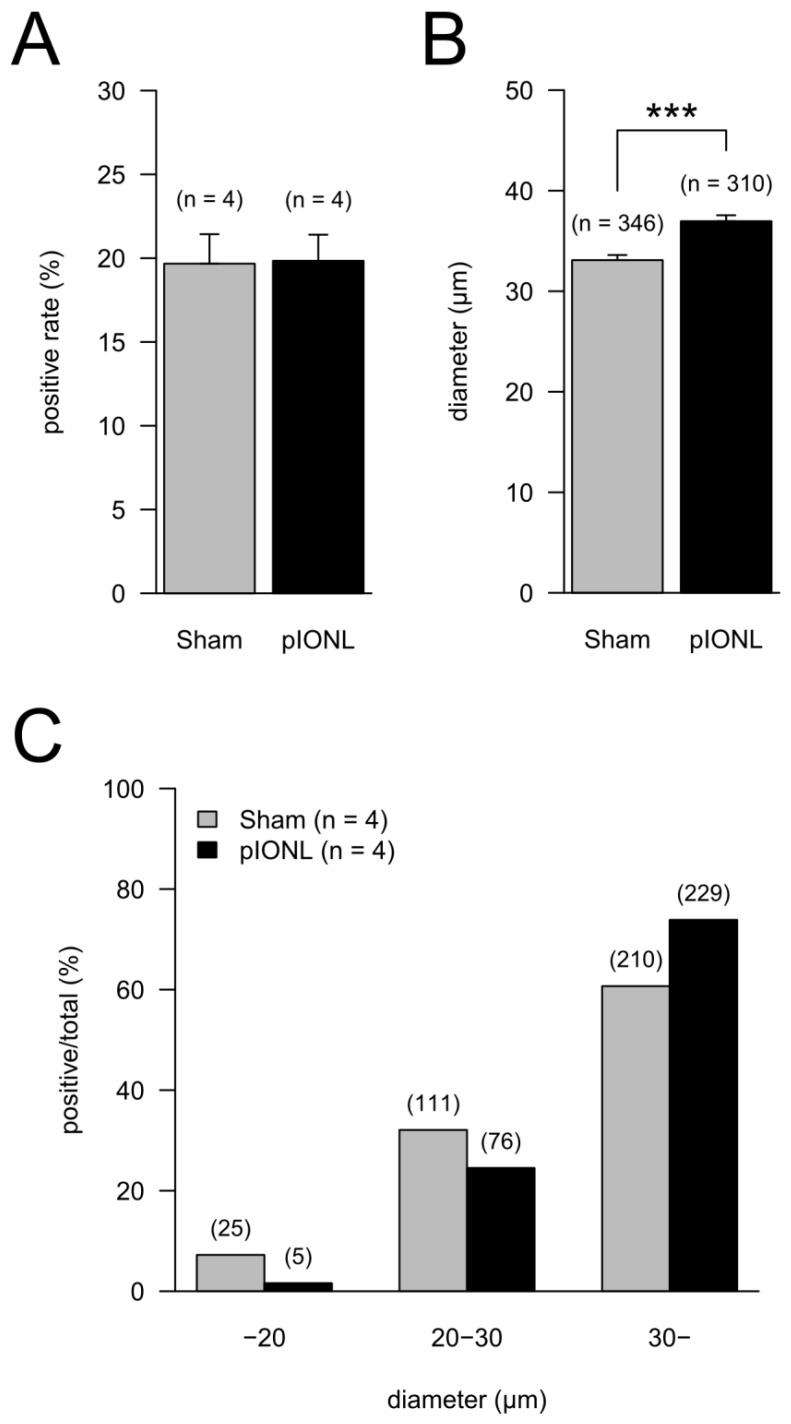
Welch test was performed to determine whether the ratios (Fig. 4A) and the major axis (Fig. 4B) of TRPV1-positive cells in TG were significantly different among Sham and pIONL groups. Fisher's exact test was performed to determine whether the ratios of TRPV1-positive cells classified by cell size were significantly different among Sham and pIONL groups (Fig. 4C).
Figure 4.
Proportion of TRPV1-positive neurons in TG neurons in the pIONL group (n = 4) and the Sham group (n = 4). (A) The means of ratios of TRPV1-positive cells to all cells in TG neurons. P = 0.952 (Welch test). (B) The mean of the major axis of TRPV1-positive cells. Total TRPV1-positive cell numbers are indicated in parentheses. P < 0.001 (Welch test). (C) Ratios of TRPV1-positive cells classified by cell size (C). Cells were classified into 3 groups (< 20 μm, 20 to 30 μm, > 30 μm) by the major axis of cells. The ratios of TRPV1-positive cells in each group to total TRPV1-positive cell number are shown. P < 0.001 (Fisher's exact test).
All computations were performed with the statistical program R 30, and type II sum of squares was calculated with the package 'car'. Values with p < 0.05 were considered as significantly different.
Results
Change in mechanical sensitivity
In the Sham group, the frequency of nociceptive responses showed no significant changes during the experimental period. In the pIONL group, the frequency of nociceptive responses was significantly increased at days 6, 14, 21 and 28 after pIONL compared with that in the Sham group, indicating the development of mechanical allodynia (Fig. 1A).
Change in heat sensitivity
In the Sham group, withdrawal temperature showed no significant changes during the experimental period. In the pIONL group, withdrawal temperature was significantly decreased at days 4, 10, 21 and 28 after pIONL compared with that in the Sham group, indicating the development of heat hyperalgesia (Fig. 1B).
Effect of capsazepine on mechanical and heat hyperalgesia
We examined the effect of the TRP channel antagonist capsazepine. In the Sham group, peripheral administration of DMSO and capsazepine induced no significant changes in both mechanical and heat sensations (Fig. 2A and B). In the pIONL group, peripheral administration of capsazepine induced no significant changes in the mechanical hyperalgesia (Fig. 2A). In contrast, capsazepine revealed partial inhibition of heat hyperalgesia in a dose-dependent manner. Moreover, 100 pmol/50 μL of capsazepine reversed heat sensation to the sham level (Fig. 2B). Peripheral administration of vehicle (100% DMSO) showed no effect in both mechanical and heat sensations.
Expressions of TRPV1 in trigeminal ganglia
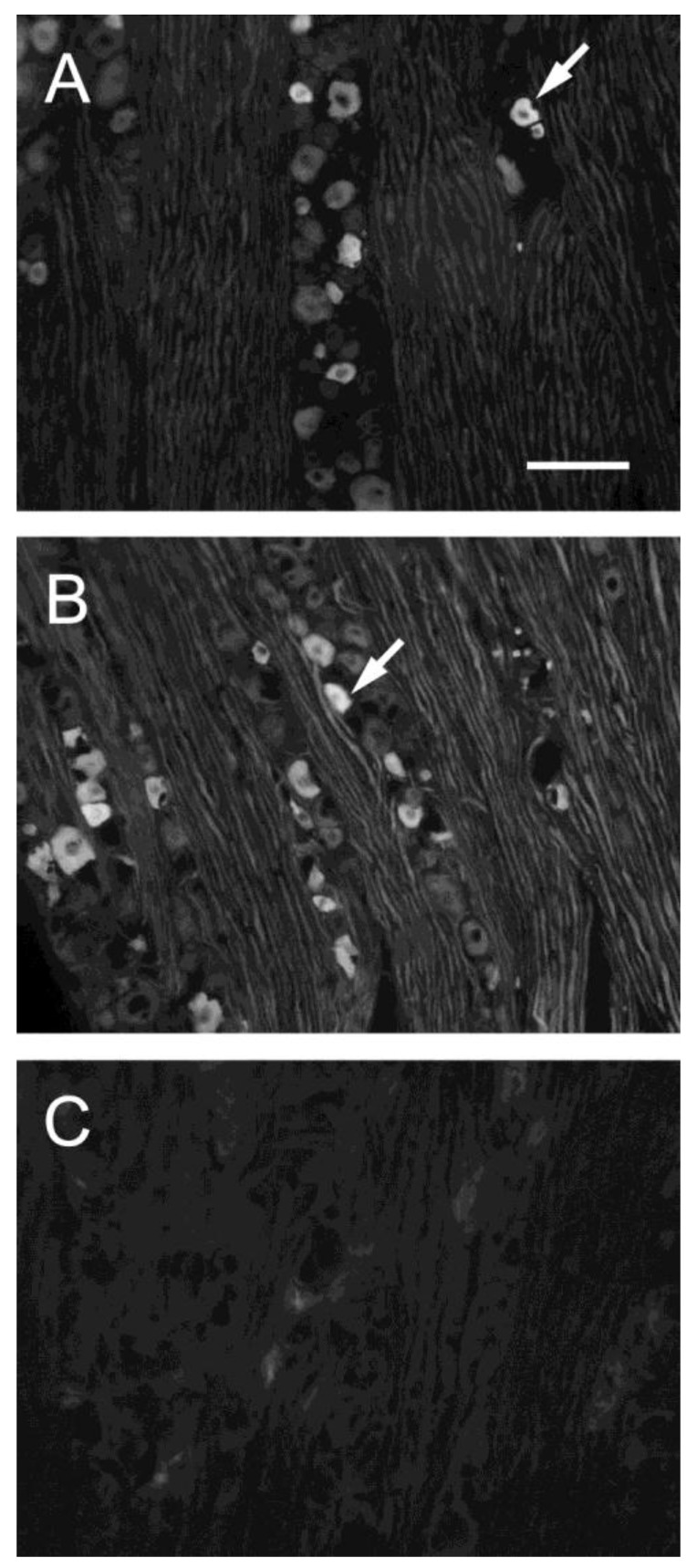
TRPV1-positive cells were intensely stained and were easily distinguished from the nonpositive cells (arrows in Fig. 3). We arbitrarily classified the cell sizes according to the frequency distribution of the ganglion cells and data in the literature 23.
Figure 3.
Fluorescent photomicrographs of TRPV1-immunoreactive cells in TG on sham-operated side (A), nerve ligation side (B) and in the absence of a primary antibody. (C). Arrows indicate TRPV1-positive neuron. Bar, 100 μm.
At 28 days after pIONL, the ratios of TRPV1-positive cells in all TG were not significantly different between Sham and pIONL groups (Fig. 4A). In contrast, the mean of the major axis in the pIONL group was significantly longer than that in the Sham group (Fig. 4B). The ratio of the TRPV1-positive large-diameter-cell group (> 30 μm) in TG significantly increased in the pIONL group (Fisher's exact test, P < 0.001). In addition, in both Sham and pIONL groups, TRPV1-positive large-diameter-cell numbers were higher than in any cell groups (Fig. 4C).
Discussion
This study aims to investigate whether withdrawal temperature by heat stimuli and TRPV1 expression in TG neurons change using a trigeminal pain model (i.e. pIONL model). We found that withdrawal temperature was significantly decreased in the pIONL group, and that capsazepine recovered the heat hyperalgesia. In addition, we found that the ratio of TRPV1-positive cells was increased in large neurons in TG by pIONL. These results suggest that TRPV1 expression in large neurons participated in the development and maintenance of heat hyperalgesia by trigeminal neuropathic pain.
In this study, we observed mechanical allodynia and heat hyperalgesia using a pIONL model (Fig 1). This pIONL model provides consistent and reproducible persistent hyperalgesia, which was shown in neuropathic pain patients. In this study, the determination of withdrawal temperature would promote more research into orofacial pain.
We evaluated the potential role of TRPV1 in chronic pain conditions by examining the anti-hyperalgesic activity of a TRP channel antagonist, capsazepine, in trigeminal neuropathic pain. Capsazepine has been shown to inhibit capsaicin-induced TRPV1 activation competitively across species in vitro 31. Capsazepine inhibited mechanical and heat hyperalgesia associated with a cancer pain model in rat 16. In this study, 100 pmol/50 μL of capsazepine inhibited heat hyperalgesia but not mechanical allodynia (Fig. 2). The anti-hyperalgesic effect of capsazepine in this study was in the same dose range as that in rat cancer pain models 16. In PSL neuropathic pain model, capsazepine recovered mechanical hyperalgesia in the guinea pig but not in the rat 32. These studies suggest that TRPV1 participates in the development of mechanical hyperalgesia or allodynia in neuropathic pain animal models and demonstrate species differences as a potential factor influencing the functional contribution of TRPV1. Therefore, the possible involvement of TRPV1 receptor in the heat hyperalgesia in the pIONL model is suggested.
In this study, TRPV1-positive large-diameter cells in TG increased and medium- and small-diameter cells decreased strikingly by pIONL (Fig. 4C), although there was no significant difference in the ratio of TRPV1-positive cells between pIONL and control animals (Fig. 4A). We consider that our results may explain the behavioral data that pIONL induced sustained mechanical allodynia and heat hyperalgesia. In other pain models such as nerve injury, cancer and inflammation, the ratio of TRPV1-positive cells in large neurons was also increased. In nerve injury model animals, TRPV1 was expressed de novo in myelinated A-fiber in mouse DRG neurons after PSL 18. Similarly, after L5 and L6 SNL, there was a dramatic shift in the size distribution of TRPV1-positive neurons towards large-diameter cells, although the proportion of TRPV1-positive DRG neurons was not changed 33. Although the relationship between DRG cell body diameter and class of afferent is not exact, our data are consistent with these previous reports that TRPV1 is expressed in A-fiber cell bodies de novo after nerve injury. In cancer pain model animals, the ratio of TRPV1-positive cells was increased in the large-cell groups in mouse DRG 15, rat DRG 16 and rat TG 23. However, there was no significant difference in the ratio of total TRPV1-positive cells in rat DRG 16. In inflammation, the ratios of TRPV1-positive cells in small- and medium-sized DRG neurons were increased. These TRPV1-positive neurons were myelinated Aδ-fibers in rat DRG 14. These findings support our assumption that the aberrant TRPV1 expression in TG neurons and the structural plasticity of myelinated A-fiber neurons are important mechanisms causing mechanical allodynia and heat hyperalgesia in trigeminal neuropathic pain. Originally, TRPV1 expression is restricted to small- to medium-sized DRG neurons 34, suggesting that TRPV1 is a polymodal nociceptor with unmyelinated C-fibers and some thinly myelinated fibers. Moreover, the large DRG neurons that innervate Aβ-afferents are not normally associated with nociception 15. In pathological conditions such as nerve injury, cancer and inflammation, therefore, TRPV1 may be upregulated in A-fibers such as Aβ and Aδ neurons and may be activated by noxious heat stimuli through increased TRPV1.
Furthermore, it is shown that TRPV1 expression in “uninjured” neurons was increased in the DRG and TG nerve injury. For instance, in unilateral L5 SNL animals, the number of TRPV1-positive cells was increased in undamaged L4 DRG, and the expression of TRPV1 mRNA was increased in small- and medium-sized cells in undamaged L4 DRG neurons 19. Similarly, after L5 SNL, TRPV1-positive cells were increased in uninjured A-fiber neurons in particular, and were greatly reduced in all damaged fibers in rat DRG 17. Moreover, in a trigeminal neuropathic pain model including the inferior alveolar nerve and mental nerve injury (IAMNT) model, the expression of TRPV1 was increased in the uninjured TG neurons 24. From these reports, we assumed that increased TRPV1 expression in uninjured nociceptive neurons plays an important role in the mechanical allodynia and heat hyperalgesia in the pIONL model.
In contrast, the mechanisms of TRPV1-mediated mechanical allodynia or hyperalgesia remain to be elucidated. Although the role of TRPV1 in mechanical hypersensitivity has been controversial, it is reported that capsazepine attenuates mechanical allodynia or hyperalgesia associated with neuropathy 17, cancer 16 and inflammation 35. These results suggest that the upregulation and/or sensitization of TRP channel receptors other than TRPV1 may also play important roles in mechanical allodynia and heat hyperalgesia. Similarly, recent studies suggested that other TRP channels including TRPV2, TRPV3, TRPV4 and TPPA1 mediate mechanosensation and/or thermal sensation. TRPV2, a TRPV1 homologue, is activated by a very high-threshold heat temperature (> 52ºC), suggesting that TRPV2 acts as a high-threshold temperature sensor in Aδ nociceptors 28, 36. TRPV2 was upregulated in the eccentric exercise (ECC)-induced muscle pain model 26, and capsazepine attenuated mechanical hyperalgesia associated with this model. TRPV3 and/or TRPV4 in keratinocytes might participate in temperature sensation 37-39. In addition, mechanosensation is defective in mice lacking TRPV4 40. TRPA1 has been identified as a cold-sensitive ion channel, was highly expressed in DRG and TG, and was co-expressed with TRPV1 13, 41. Both TRPV1 and TRPA1 are reported to mediate capsaicin- and mustard oil-induced mechanical hypersensitivity in a masseter muscle pain model 22. In DRG from CCI rats, TRPA1 and TRPV2 mRNA were slightly but significantly increased after nerve injury 42. In addition to TRPV1, therefore, TRPV2, TRPV3, TRPV4 and TRPA1 may be involved in mechanical allodynia and heat hyperalgesia in our model.
In conclusion, the increase of TRPV1-positive large-diameter cells in the trigeminal sensory system may play an important role in developing and maintaining heat hyperalgesia induced by trigeminal neuropathic pain. Moreover, TRPV1 might be an effective therapeutic target for orofacial pain syndrome associated with trigeminal nerve injury.
Acknowledgments
We thank A. Prof. Eiji Kondo (Department of Oral Anatomy I, Matsumoto Dental University), Dr. Masayo Okumura (Institute for Oral Science, Matsumoto Dental University) and A. Prof Toshimi Hattori (Department of Pharmacology, Matsumoto Dental University) for help with the immunohistochemistry. This work was supported by a Grant-in-Aid for Scientific Research (No. 22792025) from the Japan Society for the Promotion of Science.
Abbreviations
- TRPV1
transient receptor potential vanilloid 1
- pIONL
partial infraorbital nerve ligation
- VP
vibrissal pads
- SNL
spinal nerve ligation
- CCI
chronic constriction nerve injury
- PSL
partial sciatic nerve injury
- ION
infraorbital nerve
- IAN
inferior alveolar nerve
- DRG
dorsal root ganglia
- TG
trigeminal ganglia
- TMD
temporomandibular disorders
- DMSO
dimethylsulfoxide
- IAMNT
inferior alveolar nerve and mental nerve injury
- ECC
eccentric exercise.
References
- 1.Türp JC, Gobetti JP. Trigeminal neuralgia versus atypical facial pain. A review of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:424–32. doi: 10.1016/s1079-2104(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 2.Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123:64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Eide PK, Rabben T. Trigeminal neuropathic pain: pathophysiological mechanisms examined by quantitative assessment of abnormal pain and sensory perception. Neurosurgery. 1998;43:1103–10. doi: 10.1097/00006123-199811000-00055. [DOI] [PubMed] [Google Scholar]
- 4.Fried K, Bongenhielm U, Boissonade FM, Robinson PP. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–65. doi: 10.1177/107385840100700210. [DOI] [PubMed] [Google Scholar]
- 5.Shinoda M, Kawashima K, Ozaki N, Asai H, Nagamine K, Sugiura Y. P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain. 2007;8:588–97. doi: 10.1016/j.jpain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 7.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 8.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–18. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 9.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci. 1994;14:2708–23. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata K, Imai T, Tsuboi Y, Tashiro A, Ogawa A, Morimoto T, Masuda Y, Tachibana Y, Hu J. Alteration of medullary dorsal horn neuronal activity following inferior alveolar nerve transection in rats. J Neurophysiol. 2001;86:2868–77. doi: 10.1152/jn.2001.86.6.2868. [DOI] [PubMed] [Google Scholar]
- 11.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 12.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 13.Tominaga M. Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1. Yakugaku Zasshi. 2010;130:289–94. doi: 10.1248/yakushi.130.289. [DOI] [PubMed] [Google Scholar]
- 14.Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–6. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- 15.Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M, Sugiura Y. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain. 2005;117:19–29. doi: 10.1016/j.pain.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, Sugiura Y. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain. 2008;9:687–99. doi: 10.1016/j.jpain.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–14. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 18.Rashid MH, Inoue M, Kondo S, Kawashima T, Bakoshi S, Ueda H. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J Pharmacol Exp Ther. 2003;304:940–8. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X3, increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–20. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Chidiac JJ, Rifai K, Hawwa NN, Massaad CA, Jurjus AR, Jabbur SJ, Saade NE. Nociceptive behaviour induced by dental application of irritants to rat incisors: a new model for tooth inflammatory pain. Eur J Pain. 2002;6:55–67. doi: 10.1053/eujp.2001.0305. [DOI] [PubMed] [Google Scholar]
- 21.Tarsa L, Balkowiec-Iskra E, Kratochvil FJ 3rd, Jenkins VK, McLean A, Brown AL, Smith JA, Baumgartner JC, Balkowiec A. Tooth pulp inflammation increases brain-derived neurotrophic factor expression in rodent trigeminal ganglion neurons. Neuroscience. 2010;167:1205–15. doi: 10.1016/j.neuroscience.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–7. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagamine K, Ozaki N, Shinoda M, Asai H, Nishiguchi H, Mitsudo K, Tohnai I, Ueda M, Sugiura Y. Mechanical allodynia and thermal hyperalgesia induced by experimental squamous cell carcinoma of the lower gingiva in rats. J Pain. 2006;7:659–70. doi: 10.1016/j.jpain.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB. Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain. 2008;9:280–8. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 26.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Capsazepine is a novel activator of the delta subunit of the human epithelial Na+ channel. The Journal of Biological Chemistry. 2004;279:44483–44489. doi: 10.1074/jbc.M408929200. [DOI] [PubMed] [Google Scholar]
- 28.Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X3 and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. Pain. 2010;149:393–405. doi: 10.1016/j.pain.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Abràmoff MD, Magalhães PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 30.R Development Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. 2011.
- 31.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–52. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- 33.Ma W, Zhang Y, Bantel C, Eisenach JC. Medium and large injured dorsal root ganglion cells increase TRPV-1, accompanied by increased alpha2C-adrenoceptor co-expression and functional inhibition by clonidine. Pain. 2005;113:386–94. doi: 10.1016/j.pain.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–54. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholas RS, Winter J, Wren P, Bergmann R, Woolf CJ. Peripheral inflammation increases the capsaicin sensitivity of dorsal root ganglion neurons in a nerve growth factor-dependent manner. Neuroscience. 1999;91:1425–33. doi: 10.1016/s0306-4522(98)00706-4. [DOI] [PubMed] [Google Scholar]
- 36.Ahluwalia J, Rang H, Nagy I. The putative role of vanilloid receptor-like protein-1 in mediating high threshold noxious heat-sensitivity in rat cultured primary sensory neurons. Eur J Neurosci. 2002;16:1483–9. doi: 10.1046/j.1460-9568.2002.02231.x. [DOI] [PubMed] [Google Scholar]
- 37.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–46. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- 38.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–82. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–75. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 40.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A. 2003;100:13698–703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 42.Frederick J, Buck ME, Matson DJ, Cortright DN. Increased TRPA1, TRPM8, and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem Biophys Res Commun. 2007;358:1058–64. doi: 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]