Abstract
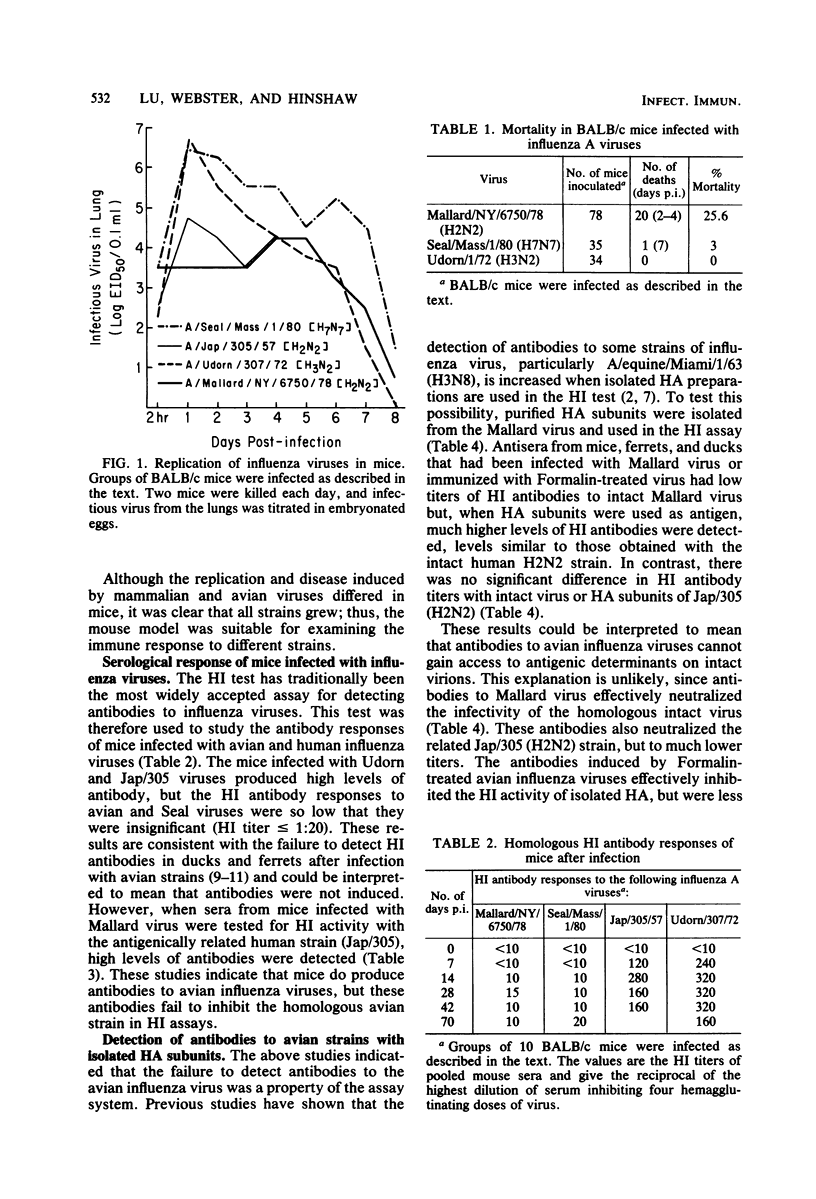
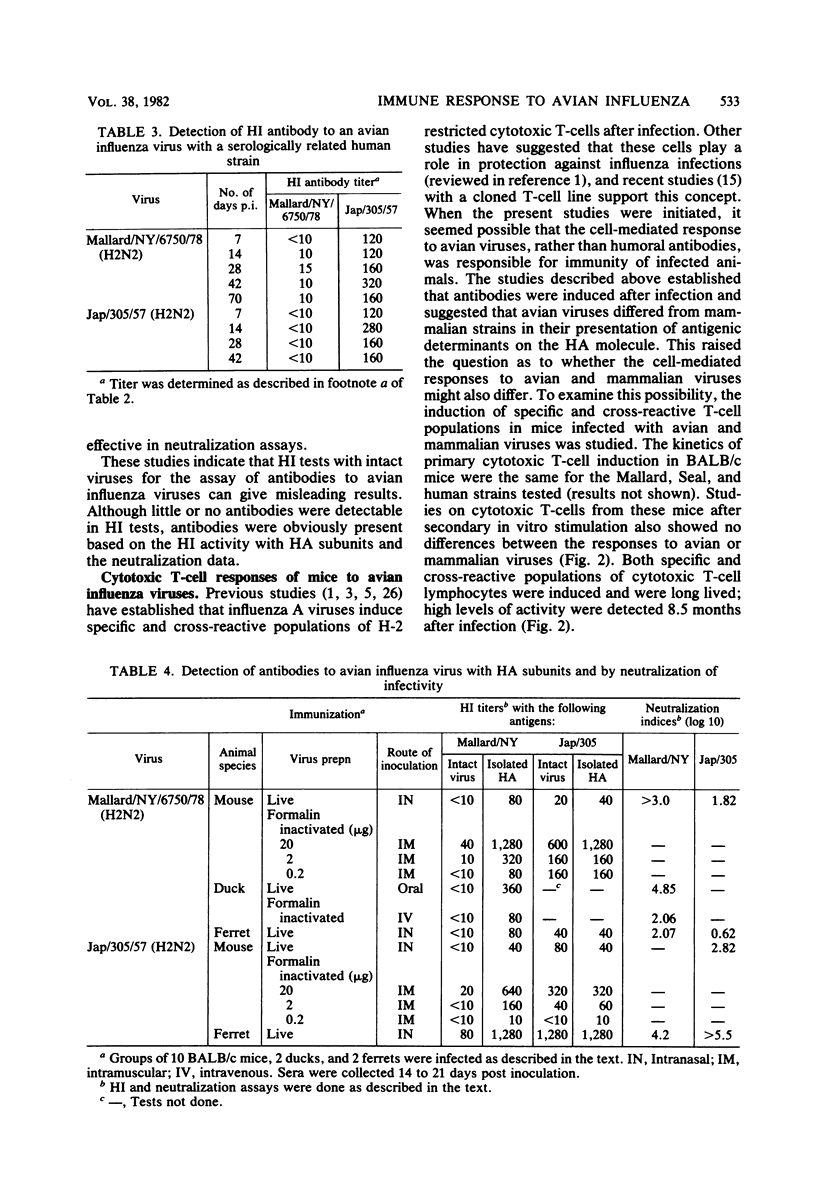
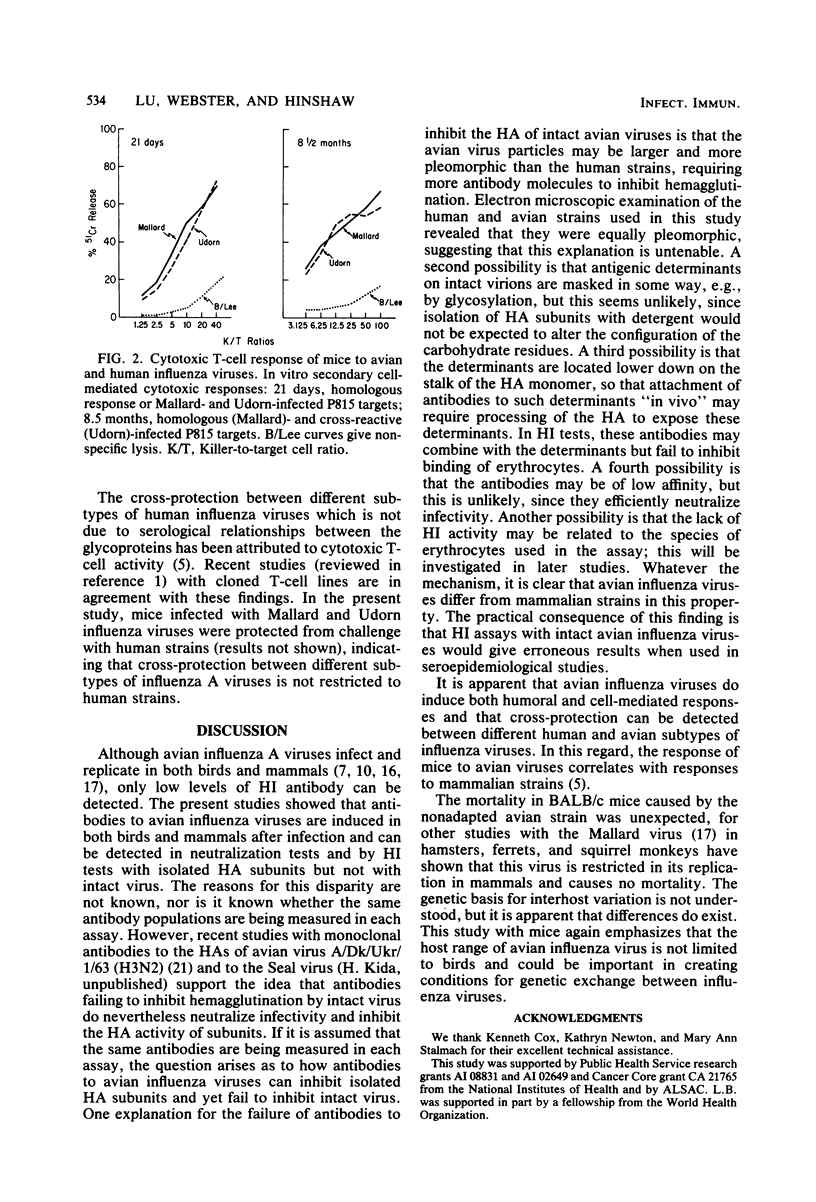
Avian influenza viruses replicate in a variety of mammals and birds, yet hemagglutination inhibition tests show that postinfection sera from these animals (e.g., ferrets and ducks) have insignificant levels of antibodies (Hinshaw et al., Infect. Immun. 34:354-361, 1981). This suggested that avian influenza viruses, in contrast to mammalian viruses, may not induce a significant humoral response. Studies reported here indicate that avian influenza viruses do induce high levels of antibodies in ferrets, ducks, and mice and produce long-lived memory for cytotoxic T-cells in mice. The failure to detect hemagglutination-inhibiting antibodies to avian viruses was explained by the finding that antibodies to avian influenza viruses were not detectable in hemagglutination inhibition tests with intact virus yet were readily demonstrable when hemagglutinin subunits were used. In addition, these sera contained high levels of neutralizing antibodies to the avian virus. These findings suggest that the hemagglutinins of avian and mammalian influenza viruses may differ in their accessibility to antibodies or the biological consequence of antibody attachment or both. The practical consequence of these studies is that hemagglutination inhibition tests with intact avian viruses fail to detect antibody and do not correlate with virus neutralization. The avian virus used in these studies, A/Mallard/NY/6870/78 (H2N2), replicated and caused mortality in BALB/c mice, emphasizing that the host range and virulence of avian viruses extends to mammals. The above findings suggest that avian viruses could infect mammals in nature, yet seroepidemiological studies with conventional hemagglutination inhibition tests could give misleading results.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- BERLIN B. S., MCQUEEN J. L., MINUSE E., DAVENPORT F. M. A METHOD FOR INCREASING THE SENSITIVITY OF THE HEMAGGLUTINATION-INHIBITION TEST WITH EQUINE INFLUENZA VIRUS. Virology. 1963 Dec;21:665–666. doi: 10.1016/0042-6822(63)90244-7. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Andrew M. E., Braciale V. L. Heterogeneity and specificity of cloned lines of influenza-virus specific cytotoxic T lymphocytes. J Exp Med. 1981 Apr 1;153(4):910–923. doi: 10.1084/jem.153.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions on Original Antigenic Sin. II. Proof in lower creatures. J Exp Med. 1966 Sep 1;124(3):347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw V. S., Bean W. J., Jr, Webster R. G., Easterday B. C. The prevalence of influenza viruses in swine and the antigenic and genetic relatedness of influenza viruses from man and swine. Virology. 1978 Jan;84(1):51–62. doi: 10.1016/0042-6822(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Hinshaw V. S., Bean W. J., Webster R. G., Sriram G. Genetic reassortment of influenza A viruses in the intestinal tract of ducks. Virology. 1980 Apr 30;102(2):412–419. doi: 10.1016/0042-6822(80)90108-7. [DOI] [PubMed] [Google Scholar]
- Hinshaw V. S., Webster R. G., Easterday B. C., Bean W. J., Jr Replication of avian influenza A viruses in mammals. Infect Immun. 1981 Nov;34(2):354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida H., Yanagawa R., Matsuoka Y. Duck influenza lacking evidence of disease signs and immune response. Infect Immun. 1980 Nov;30(2):547–553. doi: 10.1128/iai.30.2.547-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G., Gagnon A., Geraci J. R. Isolation of an influenza A virus from seals. Arch Virol. 1981;68(3-4):189–195. doi: 10.1007/BF01314571. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. 3. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology. 1973 Feb;51(2):383–391. doi: 10.1016/0042-6822(73)90437-6. [DOI] [PubMed] [Google Scholar]
- Lu L. Y., Askonas B. A. Cross-reactivity for different type A influenza viruses of a cloned T-killer cell line. Nature. 1980 Nov 13;288(5787):164–165. doi: 10.1038/288164a0. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Yanagawa R., Noda H. Experimental infection of mink with influenza A viruses. Brief report. Arch Virol. 1979;62(1):71–76. doi: 10.1007/BF01314905. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Hinshaw V. S., Sly D. L., London W. T., Hosier N. T., Wood F. T., Webster R. G., Chanock R. M. Virulence of avian influenza A viruses for squirrel monkeys. Infect Immun. 1982 Sep;37(3):1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Rohde W., Von Hoyningen V., Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978 Jun 1;87(1):13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- Webster R. G. Antigenic hybrids of influenza A viruses with surface antigens to order. Virology. 1970 Nov;42(3):633–642. doi: 10.1016/0042-6822(70)90309-0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Bean W. J., Van Wyke K. L., Geraci J. R., St Aubin D. J., Petursson G. Characterization of an influenza A virus from seals. Virology. 1981 Sep;113(2):712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Schild G. C., Newman R. W., Seagroatt V. An improved single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand. 1977;5(3):237–247. doi: 10.1016/s0092-1157(77)80008-5. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]



