Abstract
Molecular imaging allows direct visualization of targets and characterization of cellular pathways, as long as a high signal/background ratio can be achieved, which requires a sufficient amount of probes to accumulate in the imaging region. The Asn-Gly-Arg (NGR) tripeptide selected by phage display can specifically target tumor vasculature. Recognizing the aminopeptidase N (APN or CD13) receptor on the membrane of tumor cells, the peptide can be further internalized into cytoplasma by the endosomal pathway. Hence NGR can serve as an ideal candidate for tumor imaging, once it is conjugated with fluorescent or radiolabeled imaging probes. Herein, we highlight some recent developments of NGR peptide based imaging of tumors. Although still in the preliminary stage, some NGR probes have shown potential as promising agents in future clinical applications.
Keywords: Asparagine-glycine-arginine (NGR), arginine-glycine-aspartic acid (RGD), isoaspartate-glycine-arginine (isoDGR), cancer, imaging, tumor angiogenesis, vasculature, aminopeptidase N (APN/CD13)
Introduction
Cancer is one of the major health-related issues in the United States and all around the world. In average, one in four deaths in America is caused by cancer [1]. Hence extensive efforts have been taken towards the related cancer therapy and cancer diagnostics. Whereas traditional approaches to locate, target and destroy tumor cells are limited by the restricted access to tumors [2,3], a novel strategy to target the tumor vasculature turns out to be promising [4,5]. The new strategy is expected to inhibit angiogenesis and the function of existing vasculatures of tumors, thereby leading to their starvation and eventual regression [4]. There are a number of proteins that are not or are only barely expressed in normal blood vessels but are up-regulated in endothelial cells of angiogenic vessels and tumor cells, such as the ανβ3 and ανβ5 integrins [6,7], vascular endothelial growth factor receptor (VEGFR) [8,9], and APN/CD13 [10]. To find peptides targeting angiogenic endothelial cells, the in vivo phage display has been performed and led to the identification of a number of peptide ligands for angiogenesis, among which the arginine-glycine-aspartic acid (RGD) peptide and the asparagine-glycine-arginine (NGR) peptide are the most famous ones with their targeting receptors well-characterized. RGD peptide specifically binds to the ανβ3 integrin, and is three-fold less in affinity compared to the other peptide NGR which primarily binds to aminopeptidase N (APN/CD13) [5,11-14].
The NGR peptide is reported to have the greatest tumor selectivity [11] and NGR-based drug delivery has been under rapid developments over the past decades [14-17]. An anti-cancer drug doxorubicin (DOX) coupled to an NGR peptide displays enhanced anti-tumor effects with even lower toxicity than the free drug itself [13]. While intensive attention has been paid to therapeutic development, interest is increasingly being directed toward imaging-related research with NGR. Molecular imaging techniques permit direct visualization of targets and characterization of cellular activity by using contrast agents or so-called probes that specifically bind to targets in order to generate detectable signals in the target location [12]. NGR peptide imaging in vivo would not only provide more insight into NGR’s targeting process, including bio-distribution and pharmacokinetics, but also reveal angiogenic activities related to tumor progression and malignancy [12]. Besides diagnostic purposes, the sensitive detection of tumor regions by the combination of labeled probes and quantitative imaging methods [18] also makes it feasible to monitor a tumor’s response to therapy, which is important to biomedical research.
In this review, we comprehensively discuss the current development of NGR peptides as tools for tumor imaging. NGR peptides in linear and cyclic forms have been conjugated either directly to imaging probes or indirectly to polymers that are later on modified or uploaded with probes. A wide variety of imaging methods such as magnetic resonance imaging (MRI), two-photon laser scanning microscopy (TPLSM), two-dimensional (2-D) planar fluorescence reflectance imaging (FRI), and three-dimensional (3-D) fluorescence mediated tomography (FMT) have been also included here.
The NGR motif for tumor targeting
Given that the NGR peptide can selectively bind to APN/CD13 either immune-captured or expressed on the surface of cells, the receptor of the tumor-homing NGR peptide was suspected to be APN/CD13. The receptor APN/CD13 was further confirmed by the finding that antibodies against APN/CD13 competed with NGR peptide in in vivo tumor targeting [19]. Since APN/CD13 is widely-expressed in different cell lines including epithelial cells, mast cells, fibroblasts and muscle cells, as well as different locations such as cell membranes, cytoplasma, plasma, and stromal fibrillar components of some connective tissues, the detailed mechanism of NGR’s specific tumor-targeting still remained unclear [20]. Until recently, different APN/CD13 isoforms were disclosed to exist in different cells and organs based on the immune-reactive patterns [20]. Further, an APN/CD13 isoform expressed in tumor blood vessels was discovered to recognize NGR peptide while other isoforms in normal cells/organs did not [20]. Besides the special APN/CD13 isoform, an alternative tumor-homing mechanism for NGR peptide was also proposed following the observation that through rapid deamidation of asparagine [21,22], NGR could be converted to isoaspartate-glycine-arginine (isoDGR) that antagonized the ανβ3 integrin, another upregulated biomarker in the endothelial cells of angiogenic vessels [14,22]. The binding of isoDGR inhibited ανβ3 integrin-mediated endothelial cell adhesion, proliferation and related tumor development [22].
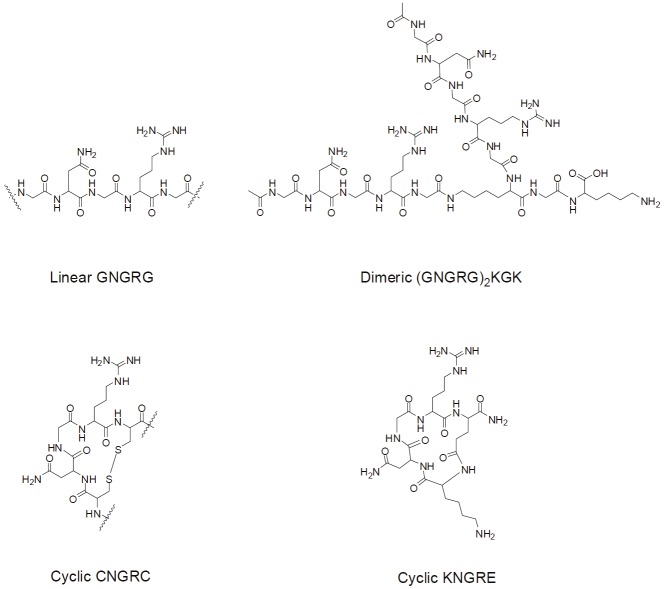
Regarding the antagonist, one pentapeptide containing NGR flanked by one amino acid at both ends turned out to be sufficient for binding activity (Figure 1) [13]. Both linear and cyclic forms of NGR peptides were initially reported, among which the cyclized CNGRC (cNGR) were constructed by the disulfide bonding of the two cysteines [13]. cNGR was revealed to provide not only increased affinity but also the specificity to APN/CD13 [23]. When both forms of NGR were coupled to the tumor necrosis factor (TNF-α) , the cNGR-TNF-α exhibited more than 10-fold higher anti-tumor activities than the linear derivative [24]. Detailed studies revealed that a bent geometry involving glycine and arginine existed in the cyclic form, and was also favored thermodynamically in the linear form, which was believed to be essential for tumor targeting [24]. Up to now, a diverse set of NGR structures has been designed and applied in drug delivery and tumor imaging.
Figure 1.
Structure of common NGR peptides.
For the linear type, two linear NGR epitopes can be conjugated through their carboxyl ends to the amino groups in the branch and N-terminal of lysine, so that a dimeric NGR peptide is constructed [25] (Figure 1). While the disulfide-bond of cNGR may be unstable to biodegradation and chemical modification, a more robust cyclization is created through the coupling of the amino group in lysine’s N-terminal to the carboxyl branch of the glutamic acid residue of KNGRE peptide [26] (Figure 1). Most of the NGR developed so far has shown promising efficacies in tumor targeting, thereby paving the avenue for NGR-directed tumor diagnostics and therapy.
NGR peptides directly conjugated to imaging agents
Fluorescent dye-conjugated NGR peptides
The very early design of NGR conjugated probes was reported by Dirksen et al [27], where they extended the carboxyl terminal of cNGR with glycines and a thioester. The N-terminal cysteine functionalized diethylenetriaminepentaacetic acid (DTPA) was then coupled to the NGR peptide’s thioester by a native chemical ligation. The resulting cysteine after coupling and spontaneous rearrangement could be further conjugated with a maleimide-modified fluorescent dye, Oregon Green 488 (OG488), for optical imaging. In addition, the DTPA ligand attached at the end of the peptide could be complexed to gadolinium (III) for MRI. This bimodal target-specific contrast agent was believed to afford both MRI and optical imaging of angiogenesis. Unfortunately, there has been no imaging results reported so far using this bimodal agent.
Lysine can be also incorporated into the N-terminal of linear or cyclized NGR peptide, where its amino-ended branch was coupled to OG488 [26]. The resulting OG488-cyclic KNGRE exhibited a 3.6 fold-higher affinity than the OG488-linear KNGRG in APN/CD13-positive tumor cells, which was in agreement with the reported trend [24]. Accordingly the cNGR derivative showed stronger punctuate fluorescence than the linear peptide derivatives in APN/CD13-positive cells, as characterized by epifluorescent microscopy (Figure 2A, 2B). It is noteworthy that both NGR-OG488 derivatives failed to stain APN/CD13-negative tumor cells, demonstrating their specificity to the APN/CD13 receptor. Moreover, the much reduced fluorescence of cNGR-OG488 treated cells at 4°C than at 37°C further revealed that the cellular uptake and internalization of NGR peptide was through endosomal uptake [26,28-31] (Figure 2C, 2D).
Figure 2.
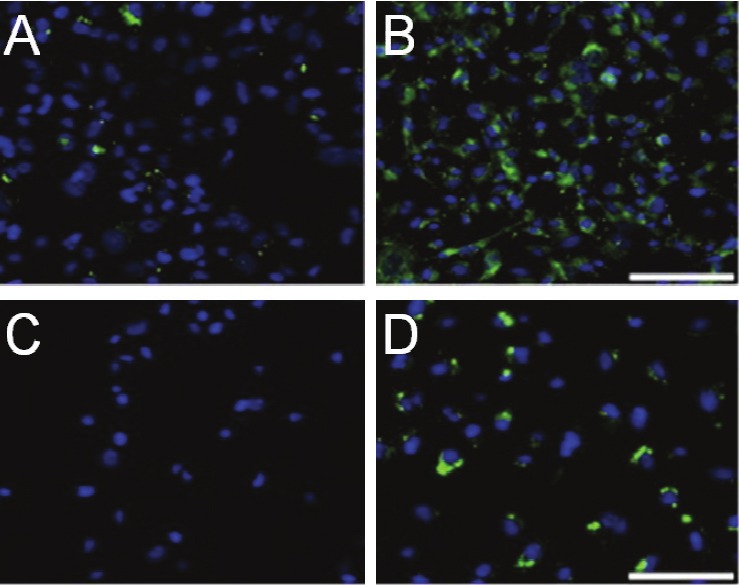
Imaging with NGR-OG488. Epifluorescence microscopy of (A) linear NGR-OG488 (B) cNGR-OG488 in HT-1080 cells at 37 °C after 2h incubation. Green color is from NGR-OG488, blue color is from nuclear staining agent 4'-6-Diamidino-2-phenylindole (DAPI). Scale bar = 100μm. Epifluorescence microscopy of the internalization of cNGROG488 by HT-1080 cells at (C) 4 °C (D) 37 °C after 30 min incubation. Scale bar = 100 μm. Adapted from reference [26].
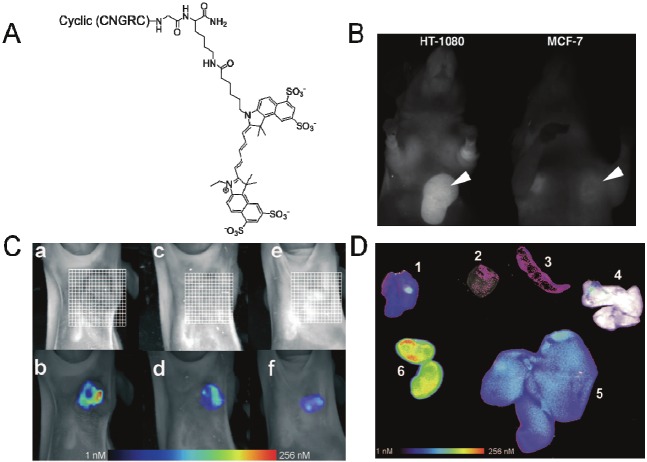
Besides the OG488 dye, fluorescent dye Cy 5.5 was also conjugated to the amino-ended branch of lysine at the carboxyl terminal of cNGR [18] (Figure 3A). The cNGR-Cy5.5 exerted distinct affinity to APN/CD13-positive HT-1080 cells but not to APN/CD13-negative MCF-7 cells. Revealed by fluorescence microscopy, cNGR-Cy5.5 was cell membrane-associated upon initial binding, but underwent endocytosis and nuclear staining with long-time incubation. cNGR-Cy5.5 could be clearly visualized by FRI and FMT imaging in vivo in the HT-1080 xenografts and the target/background ratio can be decreased by competition with unlabelled cNGR peptide (Figure 3B, 3C). Direct imaging of the excised organs implied that the cNGR-Cy5.5 was distributed not only in the tumor but also in the kidney and liver (Figure 3D), indicating rapid blood-clearance and renal excretion that may reduce its toxicity during the clinical application of fluorescent cNGR [18]. Nonetheless, the overaccumulation of cNGR-Cy5.5 in kidney and liver, together with the medium affinity of cNGR-Cy5.5 to targets may limit its accumulation in tumor regions, thus reducing the sensitivity of detection [18].
Figure 3.
In vivo imaging with NGR-Cy 5.5. (A) Chemical structure of Cy 5.5-labeled NGR-peptide. (B) In vivo fluorescence reflectance imaging (FRI) 24 h after the injection of NGR-Cy 5.5 to HT-1080 and MCF-7 xenografts. Arrows indicated the tumors. (C) Top part: fluorescence-mediated tomography (FMT) 24 h after injection of NGR-Cy 5.5 to mice bearing (a) HT-1080, (e) MCF-7, (c) mice bearing HT-1080 that were pre-injected with100-fold unlabeled peptide 10 min ahead of injection with NGR-Cy5.5; Bottom part: FMT 60 min after injection of NGR-Cy5.5 to (b)(d)(f) that are prepared under the same conditions to (a)(c)(e), respectively. (D) Overlay of white light and FRI images for organs of HT-1080 bearing mice 24 h after injection of NGR-Cy5.5; (1) HT-1080 tumor (2) heart (3) spleen (4) lung (5) liver (6) kidneys. Adapted from reference [18].
Quantum dot-conjugated NGR peptides
Quantum dot (QD) is a type of semiconductor nanocrystal that has electrons confined to mathematical points [32], and can be readily synthesized to bear diameters around 1nm-10nm [33]. Common QD features in core-shell architecture, with its core composed of heavy metals such as cadmium or lead to make a narrow band-gap for electron excitations [34]. The shell coating, on the contrary, is comprised of materials of higher band-gap to confine the excitation/emission only in the core, enhance the quantum yield of core emission and protect QD from photo-bleaching [34]. Compared to traditional dyes, QD’s emission is brighter, with narrow emission spectra and is tunable based upon its size [34,35]. Moreover, QD’s optical performance is more stable [34] and durable [36]. Given these advantages, QD-based imaging has been applied in living cells and animal models [36-40], among which QD labeled with antibodies or targeting peptide can lead to specific imaging of various receptors [37,38] and organs [39].
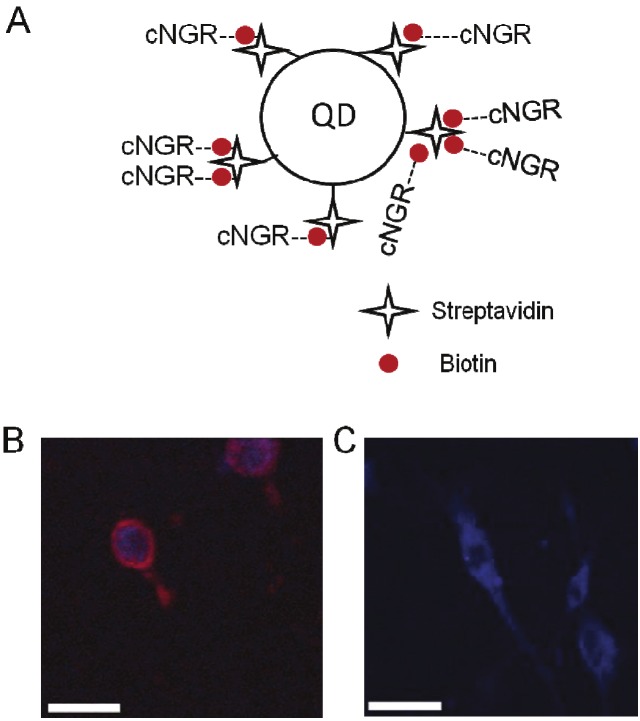
To develop QD-conjugated NGR peptides, the carboxyl terminal of cNGR was extended with glycines and a lysine whose amino branch was conjugated to OG488 or biotin [11]. The biotin-tagged NGR can then multi-valently label the QDs that had been pre-modified with streptavidin (Figure 4A). When applied to tumor imaging by in vivo fluorescence microscopy and TPLSM, the higher intrinsic fluorescence and lower bleaching rate of QD than OG488 dye made the cNGR-QD highly specific in APN/CD13-rich cells (Figure 4B, 4C). In vivo, the fluorescence of cNGR-QD is greater in intensity and longer in persistence than the cNGR-OG488 dye. Judged by fluorescent antibody, APN/CD13 was preferentially expressed in angiogenic areas of heart and the NGR-directed probes can exclusively co-localize with APN/CD13 in cardiac angiogenesis [11].
Figure 4.
In vitro imaging of cNGR-QD. (A) Structure of QD labeled by cNGR peptide. (B) (C) Fluorescent imaging of CD13-positive murine endothelial (2F-2B) cell line (B) and CD13-negative murine emangioendothelioma (EOMA) cell line (C) incubated with cNGR-QD for two hours. Red color is from QD; blue color is from the cell-nuclei stain with Syto44. Scale bar = 25 μm. Incubations of 2F2B cells with cNGR-OG488, cNGR only or with QD-streptavidin resulted in images similar to (C). Figures (B)(C) are adapted from reference [11].
The streptavidin-modified paramagnetic QD could be simultaneously coupled with biotin-tagged cNGR and biotin-tagged Gd(III)-DTPA, so that quantitative MRI was conducted to noninvasively assess the tumor’s angiogenic activity [12]. This dual-modified contrast agent was highly selective, showing a three-fold higher MRI contrast in the tumor rim than control QD, while giving little signal at muscle tissue and tumor core where no angiogenic activity was expected (Figure 5A, 5B). The accurate discrimination of the tumor’s rim-core heterogeneity by the dual-modified QD demonstrated its successful quantization of the extent of the tumor angiogenesis. Further, the dual-modified QD could be colocalized with endothelial cells in tumor vasculature but not in normal muscle, when characterized by ex vivo TPLSM, implying the contrast agent’s specificity [12] (Figure 5C, 5D).
Figure 5.
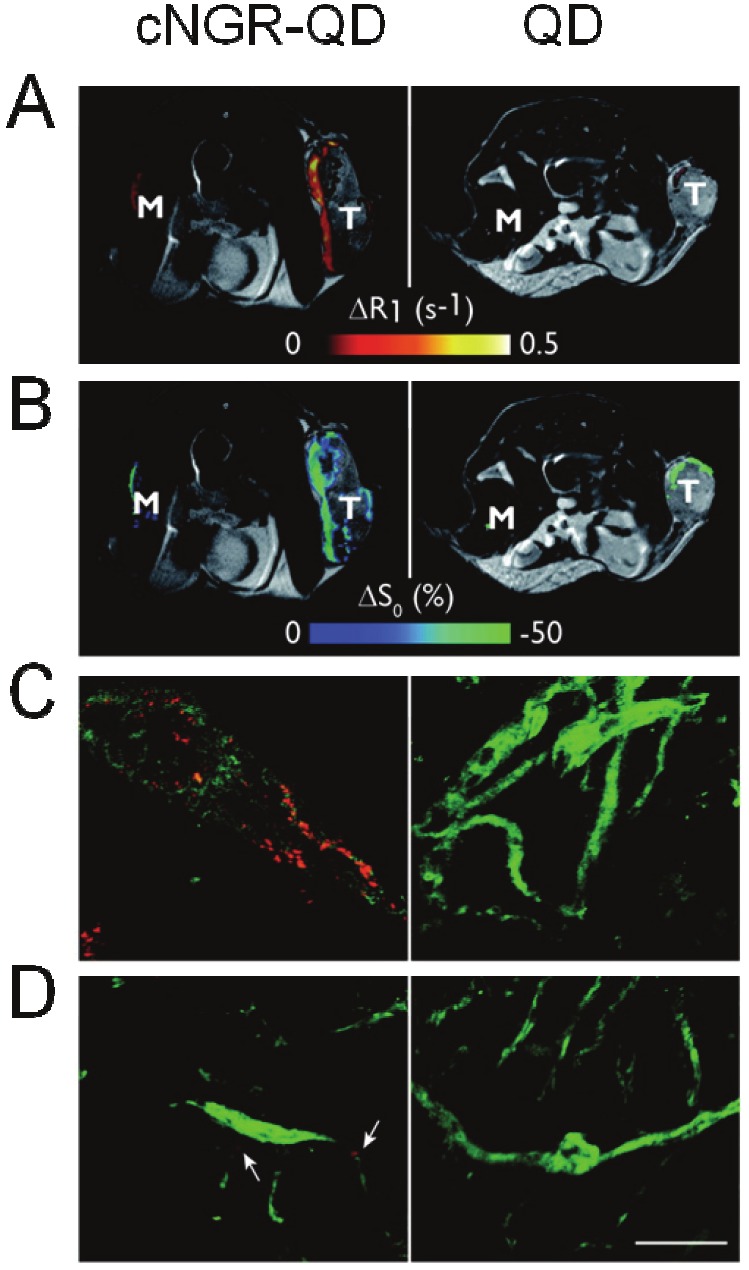
MRI and TPLSM images of cNGR-QD and unlabeled QD in tumor-bearing mice. (A) MRI images with merged view of ΔR1 for tumor (T) and muscle (M) injected with cNGR-labeled or unlabeled QDs. (B) MRI images with merged view of ΔS0 for tumor (T) and muscle (M) injected with cNGR-labeled or unlabeled QDs. (C) Representative TPLSM images of tumor showing QD signal (red) and the endothelial marker, FITC-CD31antibody signal (green) that can be co-localized. (D) Representative TPLSM images of normal muscle tissue showing QD signal (red) and FITC-CD31antibody signal (green). White arrow points out some weak cNGR-QD signal (red). Scale bar = 50 μm. The figures are adapted from reference [12].
Polymer-NGR peptide conjugates
Instead of direct conjugation which in most cases can only allow a limited number of fluorescent probes to be attached to NGR peptide, the tumor-homing peptide can be conjugated to a polymer carrier. Serving as a harbor, the polymer carrier can be modified or encapsulated with multiple probes to produce amplified signals for imaging.
Fluorophores encapsulated in polymers
One of the most popular carriers used in current drug delivery system was liposome which was non-toxic and biodegradable [41-43]. Synthetically the NGR containing linear peptide was made with a cysteine at the C-terminal. The 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) can be conjugated with polyethylene glycol (PEG)n, and then further modified to have a maleimide end for final conjugation with the peptide cysteine [4]. The DSPE part of the final product was then dissolved in the solvent and specially treated to form the liposome [4], during which the DOX was added to make a DOX-encapsulated NGR-modified liposome. The cellular uptake and DOX activity could be characterized by fluorescent microscopy, making use of DOX’s intrinsic fluorescence. The NGR-targeted liposomal DOX specifically bound to the APN/CD13-expressing tumor cells and was internalized through the endosomal pathway. After the break-up of the liposome, DOX was released and observed to localize into the nuclei [4]. The linear or cyclic NGR peptides can be also conjugated to DSPE-PEG through the N-terminal lysine [26]. The resulting multi-valent NGR peptides on the surface of liposome displayed a 10-fold enhanced affinity towards APN/CD13- positive cancer cells versus the peptides alone [26]. Intriguingly, the DOX encapsulated in liposome was susceptible to controlled-release upon temperature changes. Unfortunately, there were no reports about DOX-based fluorescent imaging for this NGR-liposome agent [26].
In another example, additional glycines were added to the N-terminal of cNGR which was then conjugated to a biodegradable di-block copolymer, PEG-poly (D, L-lactide) [44]. The fluorescent probe “1,1'-dioctadecyl-3,3,3',3'- tetramethylindocarbocyanine perchlorate” (DiI) was loaded to the polymer and used for confocal microscopy. The imaging results demonstrated that the NGR-modified polymer exhibited faster cellular uptake than the control polymer in the same cell line. A faster internalization process was also observed at HT-1080 cells than HUVEC cells, presumably due to the higher APN/CD13 expression [44].
Fluorophores conjugated to polymers
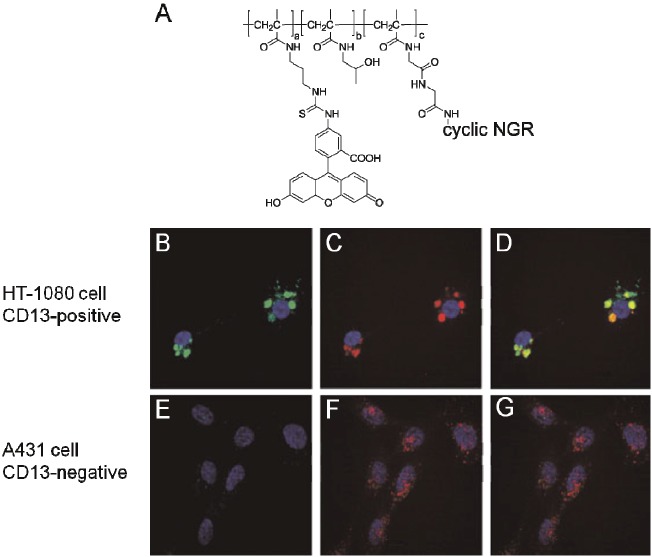
The N-(hydroxypropyl)methacrylamide (HPMA) copolymer was synthesized through copolymerization of HPMA with methacryloyl-glycine-glycine-p-nitrophenyl ester (MA-GG-ONp) and methacryloyl aminopropyl fluorescein-5-isothiocyanate (MAP-FITC) [25]. The fluorescein-isothiocyanate (FITC)-labeled HPMA copolymer was further modified with a series of NGR peptides (linear, dimeric, and cyclic) by replacement of the nitrophenol group at the branch sites of the polymer (Figure 6A). Confocal imaging revealed that the NGR-labeled FITC-HPMA conjugates selectively bound to APN/CD13-positive cells in a concentration-dependent manner, and were internalized to a greater extent in APN/CD13-positive cells through the lysosomal pathway (Figure 6B - 6G). On the other hand, whereas FITC-labeled NGR peptide was subjected to lysosomal trafficking after 4 hours, and then increasingly localized into mitochondria after 17 hours, the FITC-labeled HPMA copolymer seemed to be trapped in endosomal and lysosomal compartments [25]. The above comparison demonstrated NGR’s effect on improving the imaging agent’s cellular distribution by promoting their escape from lysosome and endosome.
Figure 6.
In vitro imaging of FITC-HMPA-cNGR in CD13-positive/negative cells. (A) Structure of FITC-HMPA-cNGR. (B)-(G) Confocal images of (B)(E) FITC-HMPA-cNGR, (C)(F) LysoTracker Red DND-99, and their co-localization image (D)(G) in HT-1080 and A431 tumor cells, respectively. Green color is from FITC; red color is from LysoTracker Red DND-99; blue color is the nuclear staining by DAPI. The figures are adapted from reference [25].
NGR peptides fused to proteins
Cleavage of some cytoskeletal proteins such as actin was found to be involved in apoptosis [45,46] and one fragment of the degraded actin–15 kDa actin was shown to induce the morphological changes of apoptosis in tumor cells [47]. It is therefore postulated that once internalized, the15 kDa actin may bind to the cytoskeleton of tumor cells and induce apoptosis.
While NGR can be chemically conjugated to synthetic polymers, Lei et al [5] illustrated that the NGR peptide can be fused to a 15 kDa actin by using recombinant DNA technology to construct the corresponding plasmid and express it in E. coli. The lysines on NGR-actin were then chemically labeled with FITC. Fluorescent imaging confirmed that NGR lead to the internalization of NGR-actin to HeLa cells and HepG2 cells, and the actin fragment further induced the binding of NGR-actin to cytoskeleton proteins. The resulting tumor apoptosis was then observed.
Conclusion
In this review, we have summarized the current development of NGR peptide-based agents for tumor imaging. For NGR directly conjugated with probes, the low sensitivity brought by the limited signal/background ratio, as well as the in vivo stability of peptide and fluorophores remain challenging issues. So far the introduction of QD seems to be the most effective solution, given its strong intrinsic fluorescence, its ability to carry multivalent dyes for signal amplification, and its endurable performance. One major concern of QD is its potential toxicity [48] and difficulty in being excreted out from body [49], which hopefully can be overcome by new generations of QDs that are claimed to be non-toxic and renally excretable [49-52].
For polymers conjugated with NGR peptides on the surface, the targeting efficiency and signal intensity are both expected to be increased, due to the multivalent modifications similar to what can happen with QDs. Despite the frequent trials of NGR-dyes for in vivo experiments, most NGR-polymer-dye conjugates are only tested in cells. The related confocal fluorescence was focused on cellular uptake mechanisms, instead of tumor imaging. Additionally, given their potential clinical applications, most polymers are made biodegradable to avoid any toxicity, thereby rendering other concerns about the leakage of encapsulated probes or stability of attached probes, once they are tested in vivo.
Finally for all the reported imaging studies, the NGR-based probes were co-localized with CD13 receptor, with assumption of no degradation of NGR, which otherwise would result in iso-DGR that binds to ανβ3 integrin instead. Future imaging work may involve the localization of ανβ3 integrin in cells or xenografts and to study its correlation with NGR-labeled probes. Despite current limits and concerns, the development of NGR-labeled probes and polymer conjugates for tumor-specific imaging has opened up a promising field in the research of cancer diagnostics and therapy. Boosted by the rapid progress of imaging techniques, it is conceivable that NGR-peptide related drugs will find profound future applications in the clinic.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Friedmann T. The development of human gene therapy. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 3.Rhim JS. Molecular and genetic mechanisms of prostate cancer. Radiat Res. 2001;155:128–132. doi: 10.1667/0033-7587(2001)155[0128:magmop]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Garde SV, Forte AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, Wu JJ. Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD13-expressing cells and its antitumor effects. Anticancer Drugs. 2007;18:1189–1200. doi: 10.1097/CAD.0b013e3282a213ce. [DOI] [PubMed] [Google Scholar]
- 5.Lei H, Cao P, Miao G, Lin Z, Diao Z. Expression and functional characterization of tumor-targeted fusion protein composed of NGR peptide and 15-kDa actin fragment. Appl Biochem Biotechnol. 2010;162:988–995. doi: 10.1007/s12010-009-8901-8. [DOI] [PubMed] [Google Scholar]
- 6.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. Alpha(v)beta3 and alpha(v) beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. doi: 10.1097/00006123-200108000-00022. discussion 390. [DOI] [PubMed] [Google Scholar]
- 8.Padro T, Bieker R, Ruiz S, Steins M, Retzlaff S, Burger H, Buchner T, Kessler T, Herrera F, Kienast J, Muller-Tidow C, Serve H, Berdel WE, Mesters RM. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2002;16:1302–1310. doi: 10.1038/sj.leu.2402534. [DOI] [PubMed] [Google Scholar]
- 9.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 10.Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, Shapiro LH. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97:652–659. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buehler A, van Zandvoort MA, Stelt BJ, Hackeng TM, Schrans-Stassen BH, Bennaghmouch A, Hofstra L, Cleutjens JP, Duijvestijn A, Smeets MB, de Kleijn DP, Post MJ, de Muinck ED. cNGR: a novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2681–2687. doi: 10.1161/01.ATV.0000245807.65714.0b. [DOI] [PubMed] [Google Scholar]
- 12.Oostendorp M, Douma K, Hackeng TM, Dirksen A, Post MJ, van Zandvoort MA, Backes WH. Quantitative molecular magnetic resonance imaging of tumor angiogenesis using cNGR-labeled paramagnetic quantum dots. Cancer Res. 2008;68:7676–7683. doi: 10.1158/0008-5472.CAN-08-0689. [DOI] [PubMed] [Google Scholar]
- 13.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 14.Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112:2628–2635. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti A, Curnis F. Tumor Vasculature Targeting Through NGR Peptide-Based Drug Delivery Systems. Curr Pharm Biotechnol. 2011 doi: 10.2174/138920111796117373. [DOI] [PubMed] [Google Scholar]
- 16.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev. 2011 doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 17.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18:1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 18.von Wallbrunn A, Waldeck J, Holtke C, Zuhlsdorf M, Mesters R, Heindel W, Schafers M, Bremer C. In vivo optical imaging of CD13/APN-expression in tumor xenografts. J Biomed Opt. 2008;13:011007. doi: 10.1117/1.2839046. [DOI] [PubMed] [Google Scholar]
- 19.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 20.Curnis F, Arrigoni G, Sacchi A, Fischetti L, Arap W, Pasqualini R, Corti A. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res. 2002;62:867–874. [PubMed] [Google Scholar]
- 21.Fuzery AK, Mihala N, Szabo P, Perczel A, Giavazzi R, Suli-Vargha H. Solution state conformation and degradation of cyclopeptides containing an NGR motif. J Pept Sci. 2005;11:53–59. doi: 10.1002/psc.588. [DOI] [PubMed] [Google Scholar]
- 22.Curnis F, Longhi R, Crippa L, Cattaneo A, Dondossola E, Bachi A, Corti A. Spontaneous formation of L-isoaspartate and gain of function in fibronectin. J Biol Chem. 2006;281:36466–36476. doi: 10.1074/jbc.M604812200. [DOI] [PubMed] [Google Scholar]
- 23.Majhen D, Gabrilovac J, Eloit M, Richardson J, Ambriovic-Ristov A. Disulfide bond formation in NGR fiber-modified adenovirus is essential for retargeting to aminopeptidase N. Biochem Biophys Res Commun. 2006;348:278–287. doi: 10.1016/j.bbrc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Colombo G, Curnis F, De Mori GMS, Gasparri A, Longoni C, Sacchi A, Longhi R, Corti A. Structure-Activity Relationships of Linear and Cyclic Peptides Containing the NGR Tumor-homing Motif. J Biol Chem. 2002;277:47891–47897. doi: 10.1074/jbc.M207500200. [DOI] [PubMed] [Google Scholar]
- 25.Adar L, Shamay Y, Journo G, David A. Proapoptotic peptide-polymer conjugates to induce mitochondrial-dependent cell death. Polym Adv Technol. 2011;22:199–208. [Google Scholar]
- 26.Negussie AH, Miller JL, Reddy G, Drake SK, Wood BJ, Dreher MR. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J Control Release. 2010;143:265–273. doi: 10.1016/j.jconrel.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dirksen A, Langereis S, de Waal BF, van Genderen MH, Meijer EW, de Lussanet QG, Hackeng TM. Design and synthesis of a bimodal target-specific contrast agent for angiogenesis. Org Lett. 2004;6:4857–4860. doi: 10.1021/ol048084u. [DOI] [PubMed] [Google Scholar]
- 28.Hansen GH, Delmas B, Besnardeau L, Vogel LK, Laude H, Sjostrom H, Noren O. The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment. J Virol. 1998;72:527–534. doi: 10.1128/jvi.72.1.527-534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamdouh Z, Giocondi MC, Laprade R, Le Grimellec C. Temperature dependence of endocytosis in renal epithelial cells in culture. Biochim Biophys Acta. 1996;1282:171–173. doi: 10.1016/0005-2736(96)00077-6. [DOI] [PubMed] [Google Scholar]
- 30.Seddiki T, Ollivier-Bousquet M. Temperature dependence of prolactin endocytosis and casein exocytosis in epithelial mammary cells. Eur J Cell Biol. 1991;55:60–70. [PubMed] [Google Scholar]
- 31.Weigel PH, Oka JA. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981;256:2615–2617. [PubMed] [Google Scholar]
- 32.Reed MA. Quantum Dots. Sci Am. 1993;268:118–123. [Google Scholar]
- 33.Murray CB, Norris DJ, Bawendi MG. Synthesis and Characterization of Nearly Monodisperse Cde (E = S, Se, Te) Semiconductor Nanocrystallites. J Am Chem Soc. 1993;115:8706–8715. [Google Scholar]
- 34.Walling MA, Novak JA, Shepard JR. Quantum dots for live cell and in vivo imaging. Int J Mol Sci. 2009;10:441–491. doi: 10.3390/ijms10020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekimov AI, Onushchenko AA. Quantum size effect in three-dimensional microscopic semiconductor crystals. Bellingham, WA, ETATS-UNIS: Society of Photo-Optical Instrumentation Engineers; 2005. [Google Scholar]
- 36.Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjug Chem. 2004;15:79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 37.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 38.Howarth M, Liu W, Puthenveetil S, Zheng Y, Marshall LF, Schmidt MM, Wittrup KD, Bawendi MG, Ting AY. Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat Methods. 2008;5:397–399. doi: 10.1038/nmeth.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci U S A. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozubek A, Gubernator J, Przeworska E, Stasiuk M. Liposomal drug delivery, a novel approach: PLARosomes. Acta Biochim Pol. 2000;47:639–649. [PubMed] [Google Scholar]
- 42.Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90:667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 43.Medina OP, Zhu Y, Kairemo K. Targeted liposomal drug delivery in cancer. Curr Pharm Des. 2004;10:2981–2989. doi: 10.2174/1381612043383467. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Wang Y, Chen X, Wang J, Zhang X, Zhang Q. NGR-modified micelles enhance their interaction with CD13-overexpressing tumor and endothelial cells. J Control Release. 2009;139:56–62. doi: 10.1016/j.jconrel.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 45.Brown SB, Bailey K, Savill J. Actin is cleaved during constitutive apoptosis. Biochem J. 1997;323:233–237. doi: 10.1042/bj3230233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neradil J, Veselska R, Svoboda A. The role of actin in the apoptotic cell death of P19 embryonal carcinoma cells. Int J Oncol. 2005;27:1013–1021. [PubMed] [Google Scholar]
- 47.Mashima T, Naito M, Tsuruo T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene. 1999;18:2423–2430. doi: 10.1038/sj.onc.1202558. [DOI] [PubMed] [Google Scholar]
- 48.Pelley JL, Daar AS, Saner MA. State of Academic Knowledge on Toxicity and Biological Fate of Quantum Dots. Toxicol Sci. 2009;112:276–296. doi: 10.1093/toxsci/kfp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Park J, Kim T, Jang E, Jun S, Jang H, Kim B, Kim SW. Reverse type-I ZnSe/InP/ZnS core/shell/shell nanocrystals: cadmium-free quantum dots for visible luminescence. Small. 2011;7:70–73. doi: 10.1002/smll.201001096. [DOI] [PubMed] [Google Scholar]
- 51.Pons T, Pic E, Lequeux N, Cassette E, Bezdetnaya L, Guillemin F, Marchal F, Dubertret B. Cadmium-free CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano. 2010;4:2531–2538. doi: 10.1021/nn901421v. [DOI] [PubMed] [Google Scholar]
- 52.Zimmer JP, Kim SW, Ohnishi S, Tanaka E, Frangioni JV, Bawendi MG. Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their application to in vivo imaging. J Am Chem Soc. 2006;128:2526–2527. doi: 10.1021/ja0579816. [DOI] [PMC free article] [PubMed] [Google Scholar]