Abstract
Background
Brown adipose tissue (BAT) activity on 18F-fluorodeoxyglucose (FDG) PET/CT can introduce an undesirable element of complexity when attempting to discern physiologic activity from more ominous entities. Recent studies have demonstrated several methods to reduce BAT FDG uptake. Benzodiazepines, however, have yet to been proven effective against BAT.
Methods
Twenty-five patients with increased BAT FDG uptake were selected retrospectively from our PET/CT database between November 2004 and January 2011. These patients had been asked to return on a different day for repeat scanning with 5mg of intravenous diazepam, administered ten minutes prior to FDG. Two patients underwent this procedure on a second occasion (for a follow-up scan at a later date), thus resulting in a total of twenty-seven scans from twenty five patients. FDG uptake in BAT was recorded using the maximum standardized uptake value (SUVmax).
Results
The mean basal BAT SUVmax was 10.1 ± 4.6 compared to a mean SUVmax of 2.8 ± 3.3 post IV diazepam (p < 0.0001). Approximately 89% (24 of 27) of scans had no significant residual BAT activity. The three remaining scans had a reduction in SUVmax ranging from 23-64% following diazepam administration. No adverse effects were noted.
Conclusion
We observed a significant reduction in brown fat activity in para-spinal, cervical, mediastinal, para-adrenal, and supra- and infra-clavicular regions on PET/CT following premedication with intravenous diazepam. We feel that IV benzodiazepines should be considered a pharmacologic option for reducing BAT FDG uptake, which in turn, will aid in distinguishing physiologic metabolic activity from pathology.
Keywords: Brown adipose tissue, diazepam, 18F-FDG PET
Introduction
18F-fluorodeoxyglucose (FDG) PET/CT scans are an essential tool for diagnosing, assessing treatment response and following-up of a broad range of malignancies. While FDG PET/CT has been shown to have a very high sensitivity in malignancies such as lymphomas and sarcomas [1,2], physiologic uptake in the digestive tract, thyroid gland, skeletal muscle, myocardium, bone marrow, and genitourinary tract can limit specificity [3].
Physiologic FDG uptake is frequently observed in brown adipose tissue (BAT) with varying degrees of metabolic activity. Hany et al. reported FDG uptake in the adipose tissue of the neck and supraclavicular regions in 2.5% of 638 patients, while Cohade et al., however, reported an incidence of 4% with the “USA-Fat” pattern in 347 patients [4,5]. Yeung et al reported uptake in other areas such as the perinephric and paravertebral regions, and in perivascular regions of the mediastinum. While the pattern of uptake is characteristically bilateral, particularly in the neck, unilateral intense uptake could be misinterpreted as pathological, such as in lymph node and adrenal metastases.
To distinguish metabolically active brown fat FDG uptake from pathology, various pharmacologic methods have been utilized. Recent literature has indicated that benzodiazepines are not effective in suppressing BAT activity. In contrast, we have had great success in reducing BAT uptake with intravenous (IV) diazepam. Thus, the objective of this observational study is to demonstrate the effectiveness of IV diazepam in a cohort of patients with high BAT FDG activity.
Material and methods
A retrospective review of the oncology PET/CT database from the Nuclear Medicine department at the McGill University Health Center (MUHC) dating November 2004 to January 2011 revealed 25 patients (two of whom had 2 PET/CT studies, for a total of 27 scans) in whom elevated BAT activity complicated diagnostic interpretation (Table 1) from a database of approximately 12000 PET/CTs. These patients had consented to repeat their FDG PET/CT scan on a different day following pre-medication with intravenous diazepam. They are routinely advised not to drive due to the ensuing sedation and are encouraged to be accompanied when possible. Ten minutes prior to FDG administration of 10-15mCi (370-550MBq), the patients were injected with 5 mg diazepam IV. They were scanned 60 minutes after the radiopharmaceutical injection. Results under basal conditions were compared to those from the post-diazepam study, each patient therefore acting as his/her own control. The post-diazepam PET/CT scans were conducted within one week (mean = 6 days) of the baseline study in all but 3 patients, in whom repeat imaging was performed 2-4 weeks later. Furthermore, every exam was performed under identical conditions with the same PET camera.
Table 1.
Clinical features of cases included in this study
| ID | Age | Sex | SUVmax (baseline) | SUVmax (diazepam) | Scan Interval (days) | Temp Baseline (C) | Temp Diazepam (C) | Site of SUVmax |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 40 | F | 7.3 | 1.1 | 2 | 10.8 | 12.9 | Infraclavicular |
| Patient 2a | 31 | F | 10.8 | 0.9 | 2 | -1.6 | -6.0 | Supraclavicular |
| Patient 2b | 34 | F | 5.4 | 1.4 | 2 | 13.4 | 18.9 | Supraclavicular |
| Patient 3 | 26 | M | 8.1 | 1.1 | 1 | 9.3 | 13.0 | Supraclavicular |
| Patient 4 | 21 | F | 7.7 | 2.4 | 2 | -2.5 | -8.2 | Infraclavicular |
| Patient 5 | 61 | F | 8.6 | 1.3 | 3 | -7.8 | -10.9 | Posterior neck |
| Patient 6 | 47 | M | 14.7 | 3.7 | 5 | 10.6 | 1.7 | Paraspinal occipital-cervical junction |
| Patient 7 | 24 | M | 19.4 | 10.6 | 3 | -6.8 | -3.9 | Infraclavicular |
| Patient 8 | 19 | F | 11.5 | 1.7 | 7 | -2.0 | -17.1 | Supraclavicular |
| Patient 9 | 68 | F | 16.2 | 3.1 | 6 | 7.3 | 9.5 | Supraclavicular |
| Patient 10 | 33 | F | 14.5 | 2.1 | 8 | 1.8 | 8.3 | Neck |
| Patient 11 | 64 | F | 5.3 | 1.1 | 5 | 6.7 | 9.0 | Neck |
| Patient 12 | 33 | F | 21.1 | 16.2 | 14 | 6.7 | 5.4 | Infraclavicular |
| Patient 13 | 40 | F | 10.8 | 1.1 | 1 | 13.7 | 11.6 | Supraclavicular |
| Patient 14 | 26 | F | 8.0 | 2.1 | 7 | -3.9 | -6.8 | Mediastinum |
| Patient 15 | 21 | F | 5.8 | 1.4 | 14 | 13.6 | 16.0 | Paraspinal thoracic |
| Patient 16a | 21 | M | 7.1 | 1.3 | 11 | 11.1 | 2.2 | Right axilla |
| Patient 16b | 25 | M | 8.0 | 1.5 | 7 | 15.7 | 18.4 | Neck |
| Patient 17 | 33 | F | 5.0 | 1.7 | 7 | 21.1 | 16.3 | Left axilla |
| Patient 18 | 32 | F | 5.3 | 1.6 | 7 | 2.1 | 7.8 | Right peri-adrenal |
| Patient 19 | 19 | M | 12.5 | 1.6 | 24 | 19.4 | 17.3 | Supraclavicular |
| Patient 20 | 34 | F | 7.0 | 2.5 | 3 | 6.2 | 10.6 | Posterior neck |
| Patient 21 | 37 | F | 16.8 | 6.1 | 5 | 11.6 | 3.1 | Supraclavicular |
| Patient 22 | 70 | F | 4.0 | 1.1 | 1 | 17.5 | 16.2 | Supraclavicular |
| Patient 23 | 42 | F | 8.8 | 2.3 | 1 | 21.5 | 22.1 | Paraspinal thoracic |
| Patient 24 | 24 | F | 12.1 | 3.5 | 7 | -17.1 | -8.0 | Paraspinal occipital-cervical junction |
| Patient 25 | 21 | F | 11.1 | 1.3 | 7 | 26.1 | 18.6 | Left axilla |
|
| ||||||||
| Mean | 35 | 10.1 | 2.8 | 6.0 | ||||
All patients were scanned with the Discovery ST PET/CT scanner (GE Healthcare, Canada). Patients fasted for at least 6 hours prior to imaging and were euglycemic upon receiving FDG. No urinary bladder catheterization was performed. Oral contrast (barium) was administered. The PET emission scan, extending from the head to the mid-thigh, was acquired for 5 min per field of view, each covering 14.9 cm, at an axial sampling thickness of 3.75 mm/slice. A 16-slice helical CT acquisition was operated with an X-ray tube voltage peak of 140 keV, 80 mA (whole body), 70 mA (legs), a 1.75:1 pitch, a slice thickness of 3.75 mm and a rotational speed of 0.8 s/ rotation. The patient was allowed to breathe normally during the PET and CT acquisitions. PET images were reconstructed with CT-derived attenuation correction using ordered subset expectation maximization (OSEM) software. Only the maximum standardized uptake value (SUVmax) corrected for body weight was reported.
Statistical analysis
The student T test was used to compare mean SUVmax values from the pre- and post-diazepam scans. P < 0.05 was considered statistically significant.
Results
The mean age of the cohort was 35 years with a standard deviation of ±15 years, five of whom were men. The most intense site of BAT activity for each patient is listed in Table 1 with the corresponding SUVmax. The neck and clavicular regions were predominantly affected, often symmetrically, although many of these patients had multicompartmental involvement including in the thorax and occasionally in the abdomen. The mean SUVmax under basal conditions was 10.1 ± 4.6 which improved to 2.8 ± 3.3 following diazepam IV (p < 0.0001). Furthermore, 24 of 27 scans (89%) had no residual BAT activity upon visual inspection. The SUVmax was reduced by 64%, 45%, and 23% in the three scans (patients 21, 7, and 12, respectively) with visible residual BAT activity following diazepam. No side effects were experienced aside from somnolence. Consequently, the patients were strongly advised not to drive.
The mean temperature (in degrees Celsius) on the day of the baseline PET/CT and on the post IV diazepam PET/CT in Montreal, Quebec, Canada were obtained from the historical records of Environment Canada, found in Table 1.
Selected images from the cohort were chosen to illustrate the effectiveness of IV diazepam. Classic bilateral BAT uptake is demonstrated in Figure 1 on the baseline scans with complete resolution on the post-diazepam PET/CT. Occasionally, patients have unilateral uptake in BAT (Figure 2). The patient in Figure 3 had locoregional malignancy in the right mediastinum with additional activity in the ipsilateral supraclavicular region. The post-diazepam study cleared the supraclavicular activity thus limiting radiotherapy to a single field in the chest. Similarly, the patient in Figure 4 had an intense focus of uptake in the right axilla, later proven to be metastatic adenocarcinoma, with nearby abundant BAT activity. All except the malignant node resolved on the post-diazepam study. Lastly, The last patient was under investigation for lymphoma and had elevated activity in the right peri-adrenal region. Fortunately, this uptake disappeared following diazepam.
Figure 1.
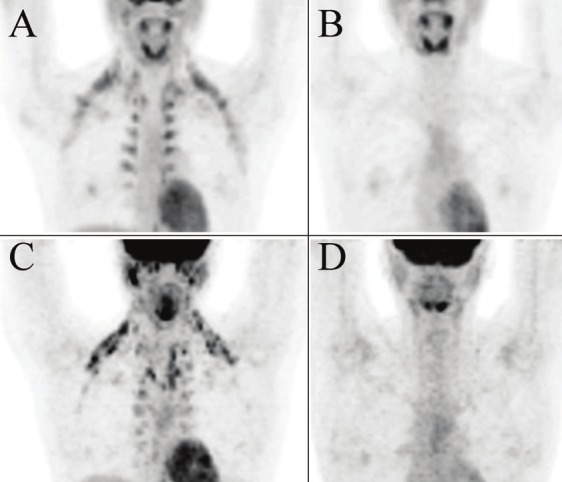
Anterior maximum intensity projections (MIPs) of the upper body from PET/CT studies of a 22 year old woman (patient 15) treated for Hodgkin’s lympoma (A: pre-diazepam; B: post-diazepam) and a 33 year old woman (patient 10) under investigation for malignancy (C: pre-diazepam; D: post-diazepam). Both studies show extensive BAT hypermetabolism in the cervical, axillary, mediastinal and paraspinal regions bilaterally which resolve on the PET/CT scans repeated 1-2 weeks later following diazepam administration.
Figure 2.
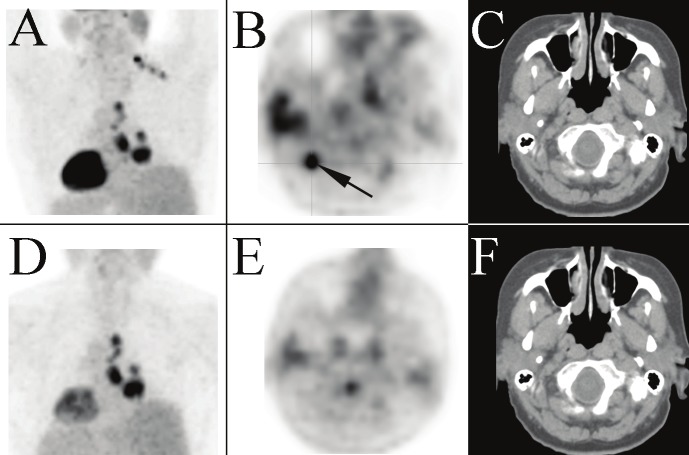
Posterior upper body view of the MIP from a PET/CT performed on a 61 year old woman (patient 5) to stage a suspected primary lung cancer (A). A unilateral cluster of distinct foci are noted at the base of the neck extending down to the supraclavicular region on the right side. The most intense supraclavicular focus is shown on the transaxial PET image (B). There is no corresponding lymph node on the corresponding transaxial CT image (C), presumably hypermetabolic BAT. The right mediastinal activity on the MIP is consistent with the patient’s known disease. The BAT activity resolves on the post-diazepam PET/CT study repeated three days later; the MIP (D) and transaxial PET (E) and CT (F) images of the neck are provided.
Figure 3.
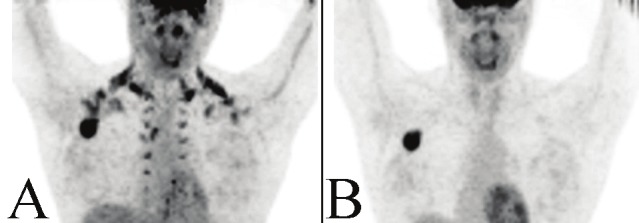
Anterior upper body views of the MIP from a PET/CT performed on a 31 year old woman (patient 2) with poorly differentiated metastatic adenocarcinoma (A). Extensive hypermetabolic activity is noted in both supraclavicular and paraspinal regions with a large intense focus located in the right axilla. The MIP of the post-diazepam PET/CT repeated two days later distinguishes hypermetabolic BAT from pathology as the activity in the aforementioned sites completely resolves with exception to the intense right axillary focus, which was biopsy-proven metastatic adenocarcinoma (B).
Figure 4.
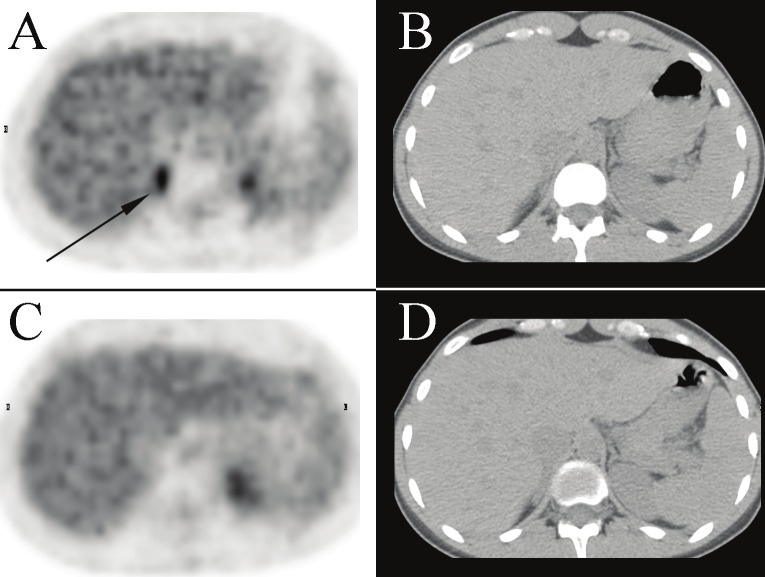
Transaxial images of the PET (A) and correlative CT (B) performed for diagnostic evaluation of lymphoma in a 32 year old woman (patient 18). Focal hypermetabolism is demonstrated in the right peri-adrenal region, which completely resolves on the post-diazepam PET/CT (panels C, D) repeated seven days later. Physioloic activity is noted in the left kidney on both pre- and post-diazepam PET studies. In addition, the patient had bilateral supraclavicular hypermetabolic BAT (not shown) which improved on the post-diazepam study.
Discussion
Brown and white adipose tissues differ in their composition. BAT contains multilocular intracellular lipid droplets, whereas white fat contains a single lipid droplet in each adipocyte. Consequently, their functions are unique. White adipose tissue primarily stores energy in the form of fat. BAT is a thermogenic organ oriented toward heat production that helps maintain normal body temperature in newborn infants and regresses with increasing age.[6]. BAT is more active in young subjects; Yeung et al reported a 15% incidence in pediatric patients versus 1.9% in adult patients [7]. Fatty acids are the main metabolic substrate of BAT[8]. Cold exposure will stimulate BAT via adrenergic stimulation[9]. In fact, Cohade et al. showed that BAT was more frequent during the colder period of the year (13.7%; January to March) than during the warmer period (4.1%; April to December)[10].
The cellular density of BAT and rich neurovascular supply accounts for the preferential uptake of FDG over white adipose tissue. Moreover, BAT is richer in mitochondria than white fat, as demonstrated by the greater abundance of cytochrome c, a mitochondrial marker [11]. Virtanen et al assessed tissue-biopsy specimens of brown fat and showed that BAT overexpresses uncoupling protein UCP1 by a factor of 1000 in comparison with white fat [11]. This gene’s function is adaptive thermogenesis, which generates heat instead of ATP [11].
BAT uptake is often bilateral and symmetric and located in distinct locations; FDG uptake has been observed most frequently in the neck (2.3%), paravertebral area (1.4%), perinephric space (0.8%), and mediastinum (0.9%) [7]. However, FDG uptake in BAT is extremely variable and the intensity of FDG uptake does not distinguish BAT from pathologic tissue [10]. For example, one patient in our cohort exhibited a SUVmax of 21 (see Table 1, patient 12) in the lower neck region on the baseline scan.
The methods of BAT FDG uptake reduction have been extensively described by the recent publication by Cohade [12]. These include environmental factors such as avoiding cold exposure and air conditioning the day of the study, wearing warm cloths, maintaining a warm ambient environment and providing blankets, avoiding smoking/nicotine and adrenergic agents, and using pharmacologic agents such as propranolol. Soderlund et al demonstrated complete or near complete resolution of BAT FDG uptake in a series of 11 patients given 80 mg of oral propranolol 2 hours before FDG administration [13].
However, a review of the recent literature suggests the use of benzodiazepines have limited use in reducing BAT FDG uptake; Cohade claims benzodiazepines are not supported as a method to reduce BAT FDG uptake[12]. Tatsumi et al performed rodent studies and showed that FDG uptake in BAT was significantly decreased to less than 30% of the control level after propranolol or reserpine (P < 0.05), but diazepam did not significantly decrease 18F-FDG uptake in BAT [14]. Similarly, Gelfand et al illustrated that low-dose oral diazepam had no effect in reducing BAT FDG uptake in a pediatric population [15]. Sturkenboom at al conducted a randomized controlled trial evaluating the effect of oral diazepam on the neck and upper chest regions and showed there was no significant difference of neck and upper chest uptake between the group of patients receiving diazepam (5 mg orally) versus the group receiving a placebo [16]. All three of these studies utilized oral diazepam. While the onset of action is almost immediate for intravenous diazepam, Toshniwal et al reported a mean of 8.5 minutes for the onset of action of oral diazepam [17]. More importantly, the peak plasma levels of diazepam was observed at 1 min after IV administration in rats and mice [18], whereas peak plasma levels of oral diazepam are reached in 30 minutes to 2 hours [19]. Comparing our experience to the studies using oral diazepam, it is plausible that the superior pharmacokinetics of IV diazepam is responsible for its relative effectiveness in reducing FDG activity in BAT. It may also be possible that IV diazepam is simply more effective at reducing BAT FDG uptake than the oral route.
As BAT FDG uptake is variable depending on the temperature of the given day, we have provided the mean temperature in degrees Celsius for Montreal, Quebec, Canada in table 1. 14 patients returned for their post IV diazepam repeat PET/CT on days when the temperature was colder than the baseline scan. When only examining this cohort of patients, we found that 86 percent (12/14) of cases had no visual evidence of elevated FDG uptake in BAT after IV diazepam. From this we can infer that temperature did not bias the effectiveness of IV diazepam in reducing BAT FDG uptake.
While the process by which benzodiazepines alters FDG uptake in BAT has not yet been elucidated, one theory is that benzodiazepines have a central antianxiety effect which may relieve the sympathetic nervous system activity thus reducing FDG uptake in BAT.14 Tatsumi et al even postulated that the central antianxiety effect may not have been present in rats in their study, thereby speculating why diazepam did not demonstrate a statistically significant decrease in BAT FDG uptake in rats.
Our study demonstrated similar results to that of Parysow et al who studied the effect of oral propranolol on BAT activity using PET/CT. In 26 cancer patients, 92 percent had no signs of elevated FDG uptake in BAT after receiving 20mg of oral propranolol 1-2 hours prior to FDG administration [20]. Our cohort showed that 89 percent of cases had no visual evidence of elevated FDG uptake in BAT after IV diazepam. Even in the three cases that demonstrated residual FDG activity on the post IV diazepam scan, the abatement in SUVmax favors BAT over pathology (see Table 1). Taking into consideration the longer patient preparation time and known contra-indications for propranolol (e.g. asthma, COPD, bradycardia or heart block) and that our results are similar to Parysow et al, we feel IV diazepam should be studied further as an effective method of BAT FDG uptake reduction. Physicians interpreting PET/CTs must also be comfortable administering IV diazepam.
Conclusion
Contrary to previous experiences with oral diazepam, the data presented herein supports the use of IV diazepam as an effective premedication to suppress BAT activity on FDG-PET/CT, particularly involving the typical locations in the neck and the mediastinal, para-spinal and para-adrenal regions.
References
- 1.Freudenberg LS, Antoch G, Schutt P, Beyer T, Jentzen W, Muller SP, Gorges R, Nowrousian MR, Bockisch A, Debatin JF. FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–329. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 2.Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE. FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging. 2009 doi: 10.1007/s00259-009-1203-0. [DOI] [PubMed] [Google Scholar]
- 3.Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61–77. doi: 10.1148/radiographics.19.1.g99ja0761. quiz 150-151. [DOI] [PubMed] [Google Scholar]
- 4.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 5.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat ("USA-Fat"): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 6.Lean ME. Brown adipose tissue in humans. Proc Nutr Soc. 1989;48:243–256. doi: 10.1079/pns19890036. [DOI] [PubMed] [Google Scholar]
- 7.Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789–1796. [PubMed] [Google Scholar]
- 8.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cohade C, Mourtzikos KA, Wahl RL. "USA-Fat": prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 11.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 12.Cohade C. Altered biodistribution on FDG-PET with emphasis on brown fat and insulin effect. Semin Nucl Med. 2010;40:283–293. doi: 10.1053/j.semnuclmed.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging. 2007;34:1018–1022. doi: 10.1007/s00259-006-0318-9. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–1193. [PubMed] [Google Scholar]
- 15.Gelfand MJ, O'Hara S M, Curtwright LA, Maclean JR. Pre-medication to block [(18)F] FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–990. doi: 10.1007/s00247-005-1505-8. [DOI] [PubMed] [Google Scholar]
- 16.Sturkenboom MG, Hoekstra OS, Postema EJ, Zijlstra JM, Berkhof J, Franssen EJ. A randomised controlled trial assessing the effect of oral diazepam on 18F-FDG uptake in the neck and upper chest region. Mol Imaging Biol. 2009;11:364–368. doi: 10.1007/s11307-009-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toshniwal N, Halbe A, Iyyer H. Study Of Comparative Effects Of Oral Clonidine Vs Oral Diazepam Pre-Medication On The Extent And Duration Of Sensory Blockade In Patients Undergoing Vaginal Hysterectomy Under Spinal Anaesthesia. The Internet Journal of Anesthesiology. 2009;9 [Google Scholar]
- 18.Diazepam Drug Information Provided by Lexi-Comp, in The Merck Manuals Online Medical Library. 2010 [Google Scholar]
- 19.Sethy V, Francis J, Elfring G. Onset and duration of action of benzodiazepines as determined by inhibition of (3H)-flunitrazepam binding. The Internet Journal of Anesthesiology. 1987;10:117–121. [Google Scholar]
- 20.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med. 2007;32:351–357. doi: 10.1097/01.rlu.0000259570.69163.04. [DOI] [PubMed] [Google Scholar]