Abstract
Sarcomas are a biologically complex group of diseases that exhibit variable responses to single or combination therapy. 18F-FDG PET imaging contributes to sarcoma treatment response assessment as an objective semiquantitative biomarker of response. In this review, background and experience in 18F-FDG PET as a biomarker that successfully identifies tumor response is assessed.
Keywords: 18F-FDG PET, sarcoma, imaging treatment response
Introduction
Sarcomas are a group of tumors derived from bone, muscle and other connective tissues. Patients with these tumors have highly variable characteristics and clinical outcomes. The pathobiological basis of their behavior is their complexity which is probably a result of a substantial variety of genetic abnormalities. These consist of broad categories of tumor specific translocations which contribute to tumor diagnostic criteria, and sarcoma subtypes that have severe abnormalities in genetic and chromosomal instability [1]. Sarcomas in almost all body locations present unique challenges for diagnosis and management. These challenges have presented opportunities for evaluation and validation of new imaging techniques. Positron Emission Tomography (PET) with fluorodeoxyglucose (18F-FDG) has been evaluated for use in sarcoma imaging over the years, but more recently this imaging procedure has been investigated in treatment response evaluation [2].
Sarcoma pathology, treatment, and imaging
A review of cancer treatment response for sarcoma as with other cancers begins with consideration of the pathological basis of the tumor process. Sarcomas are complex pathobiological processes that may exhibit a wide range of blood flow, cell proliferation rate, cell viability, inflammation, pH, oxygenation. Because of these processes, treatment response can differ significantly from a standard treatment combination or from treatments with different mechanisms of action. The ability of 18F-FDG PET to identify treatment response is an important goal for the sarcoma patient population. These tumors often do not change size in response to neoadjuvant chemotherapy because they can be made up of tissue elements that do not change in tumor response. The tissues that undergo very slow changes in size reduction are those most often composed of bone, cartilage, scar and myxoid material. Consequently, the RECIST criteria for treatment response does not apply well to this group [3,4]. 18F-FDG shows an advantage in response determination, as described by Evelevitch [5]. In this work, a 60% decrease in tumor 18F-FDG uptake compared to baseline had a sensitivity of 100% and a specificity of 71% for histologic response, whereas RECIST criteria for response applied to the same group showed a sensitivity of 25% and a specificity of 100%. In sarcoma clinical practice, tumor treatment response requirements must provide information on the nature and timing of this process [6]. Patients with large intermediate and high grade tumors receive neoadjuvant chemo- and possibly radio- therapy. If a clinical, imaging and histopathological response is observed, then similar adjuvant treatment will be continued. However, if responses are not observed, then second line or experimental therapies will be considered because the patient is highly likely to be at even higher risk for metastases and shortened survival. Since clinical and, often histopathological response evaluations can be somewhat subjective, imaging tumor response quantitatively can provide clinically relevant objective information for treatment planning for a sarcoma patient. For sarcoma patients where treatment choices are limited and often highly toxic, newer therapies that are directed at specific molecular targets may be cytostatic, and result in tumor growth arrest which can be observed effectively with 18F-FDG PET. These changes may indicate effective therapy for a patient, as opposed to direct cell killing mechanisms and tumor shrinkage, and may indicate improved patient outcome [7].
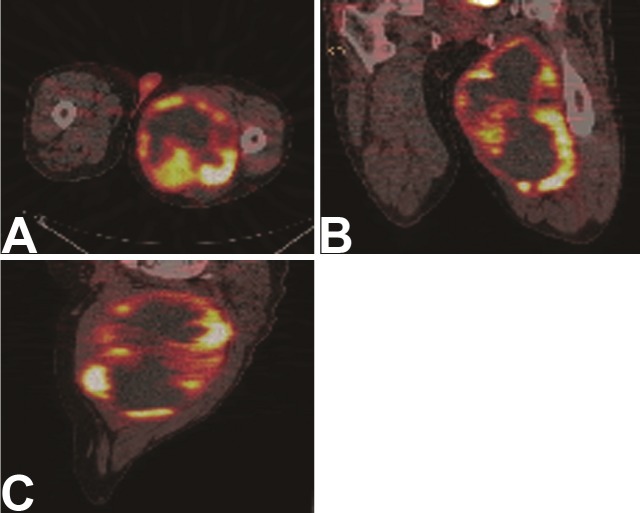
Tumor cellular necrosis fraction is considered the hallmark of treatment response to chemotherapy in sarcomas. However, histopathologic over-interpretation of tumor cellular necrosis in a tumor specimen may result in cases where necrosis was present as a distinguishing feature of the primary tumor. Figure 1 shows an example of a primary sarcoma where significant necrosis is present prior to therapy. For this reason, reliable treatment response imaging in sarcoma requires a baseline pre-treatment scan for comparison. Necrosis can take the form of coagulative necrosis, or hemorrhagic necrosis when response results in resolution of this process. Scarring is a common treatment response and is also common in radiation treatment. Compared to necrosis, this treatment response is metabolically active and can cause significant 18F-FDG uptake. In cases where granulation tissue formation precedes scarring, the inflammatory cells present may also elevate the apparent tumor bed tissue metabolism. Activated white cells can show as much as a ten fold difference in 18F-FDG uptake, complicating image interpretation.
Figure 1.
FDG PET/CT images of a patient with a large left proximal thigh high grade sarcoma. The tumor was largely necrotic at presentation with a thin rim of viable tissue. A. Coronal images; B. Axial images; C. Sagittal images.
So far imaging studies in sarcoma response imaging with 18F-FDG have not addressed the confounding effects of levels of inflammation on scan interpretation. Prospective studies with histopathologic correlation could address this issue. An important part of treatment response interpretation in sarcoma is identifying the treatment agent mechanism, tumor sub-type, and timing of the scan observation in relation to the course and type of treatment. Early after therapy, observations may reveal very different findings from those obtained after the biological mechanisms involved in treatment response have reached a more static state. In particular, radiation responses to tumor and surrounding tissues may have very different timescales in relation to the end of the final therapy response. In fact, early detection of treatment response that indicates improved patient outcome for newer therapies is an area of active research [7]. The ability of 18F-FDG PET to identify treatment response is an important goal for the sarcoma patient population.
Sarcoma 18F-FDG PET response imaging
We advocate the use of the PET tumor SUV to measure sarcoma uptake and treatment response monitoring, as do other groups [8,9]. If the 18F-FDG PET study is performed in a standard consistent manner, the SUV is a robust value for comparison of one imaging study to another in the same patient at a later time, as well as patients in different groups. The optimum parameters for 18F-FDG imaging in cancer were recently described in a report of an NCI consensus committee, where standard techniques for the use of 18F-FDG as a biomarker for cancer treatment is presented [10]. Use of 18F-FDG PET as a biomarker or surrogate endpoint for patient outcome is the basis for clinical research studies that aim to determine the sensitivity and specificity of the method for following response to treatment, and assessing normal tissue damage as a result of treatment. In sarcoma, where so few effective treatment strategies exist, but there is a great potential for new therapy strategies, 18F-FDG PET can be a powerful tool for non-invasive treatment effectiveness evaluation [11-13]. Table 1 summarizes studies reporting 18F-FDG PET in sarcoma imaging.
Table 1.
Listings of selected literature references on 18F-FDG PET response for sarcomas.
| Tumor/Reference | Date | Result | Response Detected |
|---|---|---|---|
| Gastrointestinal Stromal Tumors | |||
| Stroobants S | 2003 | 92% at 1 year | GIST Identifies progression free survival |
| Antoch G | 2004 | 90% at 1 month 100% at 3 and 6 months | GIST Tumor response detection |
| Gayed I | 2004 | Scans at 2 months from treatment | GIST Therapy response prediction |
| Jager PL | 2004 | Sens 93% | GIST Response prediction |
| Holdsworth CH | 2007 | Reduction in SUV of 40% | GIST Predict survival |
| Sarcomas | |||
| Schulte M | 1999 | Reduction in FDG uptake | Bone sarcomas Tumor necrosis correlation |
| Hawkins DS | 2002 | Reduction in SUV | Bone sarcomas Histologic response correlation |
| Schuetze SM | 2005 | Reduction in tumor SUV | Soft tissue sarcomas Survival prediction |
| Evilevitch V. | 2006 | Reduction in tumor SUV | Sarcomas Correlation with tumor necrosis |
Abbreviations: Sens = Sensitivity; GIST = Gastrointestinal Stromal Tumors
As Table 1 shows, a number of investigations have demonstrated utility of 18F-FDG PET in various aspects of sarcoma treatment response imaging. However, as with other tumor FDG imaging, there are limitations to its application. Small tumors (<3 cm) and tumors that have only mild elevations in FDG uptake may be difficult to visualize and distinguish from surrounding tissues. Some of these tumor types would be low grade lyposarcomas and chondrosarcomas, as well as those containing large proportions of myxoid elements.
Gastro-intestinal stromal tumors
The most dramatic example of use of 18F-FDG PET for a biomarker for treatment response assessment is in gastrointestinal stromal tumors (GIST) treated with Gleevec. GIST are 18F-FDG avid and can yield impressive PET images. Goerres et al. found that median survival of patients who demonstrated an 18F-FDG PET response was 100% at 2 years compared to a group with residual tumor uptake after treatment. The study also demonstrated ability to separate patients by time to tumor progression based on 18F-FDG tumor uptake levels [14]. Early response to Gleevec in the GIST population detected by 18F-FDG PET has also been shown. As much as a 65% decrease in tumor 18F-FDG uptake was demonstrated at the end of one week of effective therapy, and as high as a 95% response detected by one month after treatment initiation has been found by other groups. Response detection using CT criteria was less accurate, including no significant CT responses noted in 18F-FDG responsive patients [15-21]. These investigators recommend 18F-FDG PET imaging for GIST patients at baseline to observe for maximum tumor activity levels, and for accurate staging. Repeat imaging is suggested in the first month after therapy initiation to observe for response and to predict treatment effect. Another image may be helpful if treatment resistance is suspected, and a new baseline tumor uptake and location for treatment observation needs to be established. 18F-FDG imaging for GIST treatment response evaluation has been incorporated into the guidelines for GIST management determined by an international consensus conference [22].
Soft tissue sarcomas
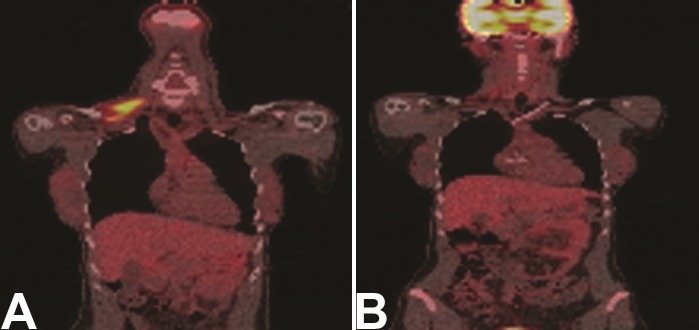
Treatment response in other sarcomas using 18F-FDG PET has also been demonstrated in a number of studies. Soft tissue sarcomas represent the majority of sarcomas that occur in adults. Treatment response imaging for this group of patients is emerging [23-27]. In an early study, in an extremity soft tissue sarcoma group treated with adriamycin based neoadjuvant chemotherapy, Schuetze et al. showed that separating patients by their 18F-FDG response (>40%) showed a significant difference (p=0.02) in survival for each of the groups [28]. Patients in the 18F-FDG PET non-response group had a 90% risk of disease recurrence at 4 years compared to 18F-FDG responders, defined as >4% decrease in 18F-FDG from baseline. This data, and that of others has provided an argument for the effectiveness and survival increase in soft tissue sarcoma patients treated with neoadjuvant chemotherapy prior to tumor resection. Figure 2 shows an example of a patient with neurofibrosarcoma in the base of the neck who had a treatment response documented by 18F-FDG PET. This imaging technique can be used to identify patients with tumor resistance during the course of therapy, who might benefit from treatment intensification, or early resection.
Figure 2.
FDG PET/CT images of a patient with a neurofibrosarcoma in the right neck base. Several image levels show the tumor location and extent. A. Baseline images. B. Images acquired following neoadjuvant chemo and radiation therapy. The tumor is nearly in complete response evidenced by lack of FDG uptake.
Ewing’s sarcomas
A similar finding has been shown in the Ewing’s sarcoma population reported by Hawkins et al. [29]. Patients whose tumors increased SUV ratios between the baseline and pre-resection (after neoadjuvant chemotherapy) scans had significantly improved survival. In this patient group, in the future, good responders may be identified for less toxic treatment protocols. Studies in imaging osteosarcoma for treatment response have also been conducted. Early studies by Schulte et al. showed that changes in tumor 18F-FDG uptake correlated with tumor necrosis levels in patients treated with neoadjuvant chemotherapy. Implications for limb salvage surgery were described, as such complex tumor resection procedures might not be considered in non- responders [30]. Others have recently shown similar results for 18F-FDG imaging in osteosarcoma [31,32].
Pediatric sarcomas
Pediatric sarcomas are usually bony tumors, but have been considered in several studies, as the treatment for pediatric patients differs somewhat compared to protocols for adults. Similar to their findings in the Ewings sarcomas in this mixed group of osteosarcoma and Ewings sarcoma patients, Hawkins et al. found that the ratios in 18F-FDG uptake between baseline and post neoadjuvant therapy completion correlated with histiologic response [33]. Noting a need for prospective imaging studies to be conducted in the pediatric sarcoma populations, both Franzius and McCarville published data on small pediatric sarcoma subgroups to demonstrate the clinical utility of 18F-FDG imaging [34,35]. The latter group included a rhabdymyosarcoma sub-group, a tumor subtype where most patients are treated under cooperative group therapy protocols. Correlation of longer survival with 18F-FDG changes in response to therapy in a small retrospective study in patients with rhabdomyosarcoma was also noted, but underscored the difficulty of performing prospective studies in pediatric tumor groups, where patients with these serious tumors are seen infrequently [36].
Summary
18F-FDG imaging in treatment response can make a significant contribution to sarcoma patient care in identifying clinically relevant responses, or lack thereof, and in providing predictive information for patient outcome. Although fortunately, the sarcomas are a less frequent form of malignancy in the population, it affects the entire human age span and in aggregate they constitute a large number of affected individuals. Currently, treatment for the more aggressive high grade tumors, and locally recurrent lower grade tumors still often results in less than optimum patient outcomes. Future clinical trials for newer treatment combinations for sarcoma patients will be benefited with the incorporation of 18F-FDG PET imaging treatment response information as a part of their treatment strategies as they have for patient with other cancer types. For the individual patient, 18F-FDG PET can provide critical information of treatment response, and further treatment planning.
Acknowledgments
Supported by NIH/NCI grants R01 CA065537-15 and P01 CA042045-21.
References
- 1.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3(9):685–94. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 2.Eary JF, Conrad EU. PET Imaging: update on sarcomas. Oncology. 2007;21(2):249–252. [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42(8):1031–9. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Evilevitch V, Weber WA, Tap WD, Allen-Auerbach M, Chow K, Nelson SD, Eilber FR, Eckardt JJ, Elashoff RM, Phelps ME, Czernin J, Eilber FC. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14(3):715–20. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- 6.Schuetze SM, Baker LH, Benjamin RS, Canetta R. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist. 2008;13(Suppl 2):32–40. doi: 10.1634/theoncologist.13-S2-32. [DOI] [PubMed] [Google Scholar]
- 7.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11(8):2785–808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 8.Eary JF, O’Sullivan F, Powitan Y, Chandhury KR, Vernon C, Bruckner JD, Conrad EU. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002;29(9):1149–54. doi: 10.1007/s00259-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 9.Benz MR, Allen-Auerbach MS, Eilber FC, Chen HJ, Dry S, Phelps ME, Czernin J, Weber WA. Combined assessment of metabolic and volumetric changes for assessment of tumor response in patients with soft-tissue sarcomas. J Nucl Med. 2008;49(10):1579–84. doi: 10.2967/jnumed.108.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47(6):1059–66. [PubMed] [Google Scholar]
- 11.Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46(6):983–95. [PubMed] [Google Scholar]
- 12.Jerusalem G, Belhocine TZ. Metabolic monitoring of chemosensitivity with 18FDG PET. Methods Mol Med. 2005;111:417–40. doi: 10.1385/1-59259-889-7:417. [DOI] [PubMed] [Google Scholar]
- 13.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW, Humm JL, Guillem J, Downey R, Karpeh M, Cohen AE, Ginsberg R. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–171. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 14.Goerres GW, Stupp R, Barghouth G, Hany TF, Pestalozzi B, Dizendorf E, Schnyder P, Luthi F, von Schulthess GK, Leyvraz S. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32(2):153–62. doi: 10.1007/s00259-004-1633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdsworth CH, Badawi RD, Manola JB, Kijewski MF, Israel DA, Demetri GD, Van den Abbeele AD. CT and PET: early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. AJR Am J Roentgenol. 2007;189(6):W324–30. doi: 10.2214/AJR.07.2496. [DOI] [PubMed] [Google Scholar]
- 16.Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R, Dumez H, Silberman S, Mortelmans L, van Oosterom A. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec) Eur J Cancer. 2003;39(14):2012–20. doi: 10.1016/s0959-8049(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 17.Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs) Eur J Cancer. 2002;38(Suppl 5):S60–5. doi: 10.1016/s0959-8049(02)80604-9. Review. [DOI] [PubMed] [Google Scholar]
- 18.Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, Bockisch A, Debatin JF, Freudenberg LS. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45(3):357–65. [PubMed] [Google Scholar]
- 19.Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45(1):17–21. Erratum in: J Nucl Med 2004; 45(11): 1803. [PubMed] [Google Scholar]
- 20.Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumors: best monitored with FDG PET. Nucl Med Commun. 2004;25(5):433–8. doi: 10.1097/00006231-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 21.McAuliffe JC, Hunt KK, Lazar AJ, Choi H, Qiao W, Thall P, Pollock RE, Benjamin RS, Trent JC. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009;16(4):910–9. doi: 10.1245/s10434-008-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, Le Cesne A, McClure J, Maurel J, Nupponen N, Ray-Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16(4):566–78. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 23.Schuetze SM, Rubin BP, Vernon C, Hawkins DS, Bruckner JD, Conrad EU rd, Eary JF. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005;103(2):339–348. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- 24.Schuetze SM. Utility of positron emission tomography in sarcomas. Curr Opin Oncol. 2006;18(4):369–73. doi: 10.1097/01.cco.0000228744.49294.12. [DOI] [PubMed] [Google Scholar]
- 25.Hicks RJ. Functional imaging techniques for evaluation of sarcomas. Cancer Imaging. 2005;5(1):58–65. doi: 10.1102/1470-7330.2005.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khamly KK, Hicks RJ, McArthur GA, Thomas DM. The promise of PET in clinical management and as a sensitive test for drug cytotoxicity in sarcomas. Expert Rev Mol Diagn. 2008;8(1):105–19. doi: 10.1586/14737159.8.1.105. Review. [DOI] [PubMed] [Google Scholar]
- 27.Toner GC, Hicks RJ. PET for sarcomas other than gastrointestinal stromal tumors. Oncologist. 2008;13(Suppl 2):22–6. doi: 10.1634/theoncologist.13-S2-22. Review. [DOI] [PubMed] [Google Scholar]
- 28.Schuetze SM, Baker LH, Benjamin RS, Canetta R. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist. 2008;13(Suppl 2):32–40. doi: 10.1634/theoncologist.13-S2-32. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Conrad EU rd, Eary JF. [18F] Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J. Clin. Oncol. 2005;23(34):8828–34. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 30.Schulte M, Brecht-Krauss D, Werner M, Hartwig E, Sarkar MR, Keppler P, Kotzerke J, Guhlmann A, Delling G, Reske SN. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J Nucl Med. 1999;40(10):1637–43. [PubMed] [Google Scholar]
- 31.Sato J, Yanagawa T, Dobashi Y, Yamaji T, Takagishi K, Watanabe H. Prognostic significance of 18F-FDG uptake in primary osteosarcoma after but not before chemotherapy: a possible association with autocrine motility factor/phosphoglucose isomerase expression. Clin Exp Metastasis. 2008;25(4):427–35. doi: 10.1007/s10585-008-9147-5. [DOI] [PubMed] [Google Scholar]
- 32.Ye Z, Zhu J, Tian M, Zhang H, Zhan H, Zhao C, Yang D, Li W, Lin N. Response of osteogenic sarcoma to neoadjuvant therapy: evaluated by 18F-FDG-PET. Ann Nucl Med. 2008;22(6):475–80. doi: 10.1007/s12149-008-0147-y. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins DS, Rajendran JG, Conrad EU rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94(12):3277–84. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 34.Franzius C, Schober O. Assessment of therapy response by FDG PET in pediatric patients. Q J of Nucl Med. 2003;47(1):41–5. [PubMed] [Google Scholar]
- 35.McCarville MB, Christie R, Daw NC, Spunt SL, Kaste SC. PET/CT in the evaluation of childhood sarcomas. AJR Am J Roentgenol. 2005;184(4):1293–304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 36.Peng F, Rabkin G, Muzik O. Use of 2-deoxy-2-[F-18]-fluoro-D-glucose positron emission tomography to monitor therapeutic response by rhabdomyosarcoma in children: report of a retrospective case study. Clin Nucl Med. 2006;31(7):394–7. doi: 10.1097/01.rlu.0000222954.38724.be. [DOI] [PubMed] [Google Scholar]