Abstract
The carcinoembryonic antigen (CEA) was visualized in vitro in tissue from patients with colorectal cancer with trivalent bispecific antibody TF2 and two hapten molecules, [67/68Ga]Ga-IMP461 and [67/68Ga]Ga-IMP485 by means of pretargeting. Colorectal cancer tissue samples obtained from surgery at Uppsala University Hospital, were frozen fresh and cryosectioned. The two hapten molecules comprising 1,4,7-triazacyclononanetriacetic acid chelate moiety (NOTA) were labeled with 67Ga or 68Ga. The autoradiography was conducted by incubating the tissue samples with the bispecific antibody TF2, followed by washing and incubation with one of the radiolabeled hapten molecules. After washing, drying and exposure to phosphor imager plates, the autoradiograms were analyzed and compared to standard histochemistry (hematoxylin-eosin). Pronounced binding was found in the tissue from colorectal cancer using the bispecific antibody TF2 and either of the haptens [67/68Ga]Ga-IMP461 and [67/68Ga]Ga-IMP485. Distinct binding was also detected in the epithelium of most samples of neighboring tissue, taken at a minimum of 10 cm from the site of the tumor. It is concluded that pretargeting CEA with the bispecific antibody TF2 followed by the addition of 67/68Ga-labeled hapten is extremely sensitive for visualizing this marker for colorectal cancer. This methodology is therefore a very specific complement to other histochemical techniques in the diagnosis of biopsies or in samples taken from surgery. Use of the pretargeting technique in vivo may also be an advance in diagnosing patients with colorectal cancer, either using 67Ga and SPECT or 68Ga and PET.
Keywords: Autoradiography, carcinoembryonic antigen, CEA, colorectal cancer, Ga-67, Ga-68, pretargeting
Introduction
The family of carcinoembryonic antigen (CEA) constitutes glycoproteins that are involved in cell adhesion, thus referred to as carcinoembryonic antigen cell adhesion molecules (CEACAMs) and are produced during fetal development [1]. Many CEACAMs are overly-expressed in a variety of carcinomas, can therefore be detected both in tissue and in blood in those patients used as a serum marker for the prognosis of colorectal cancer, especially CEACAM5, or CD66e [2-4]. Elevation of serum CEA has also been seen in symptom-free patients, where endoscopic examinations have verified the presence of tumors [5]. Blood levels of CEA can also be used as a preoperative prognostic marker for colorectal and breast cancers [6,7] and may also be elevated in other cancer types, such as lung [8,9], prostate [10], and adrenocortical cancer [11]. Its usefulness as a general marker is, however, limited in these diseases, and more for monitoring than diagnosis.
Positron emission tomography (PET) is a sensitive tool for visualization of various diseases. This technique is based on the introduction of a positron emitting nuclide, such as 11C-, 18F or 68Ga, in molecules that bind to a target of interest. The most commonly used radiotracer is [18F]fluoro-deoxy-glucose ([18F]FDG), which accumulates in tissue with high glucose consumption such as in tumors. [18F]FDG has been widely used in the diagnosis and in management of colorectal cancer [12]. However, one drawback of [18F]FDG is that it is non-specific and may also accumulate in the inflamed tissues resulting in false-positive diagnosis as well as demonstrating negligible uptake in slowly-growing tumor cells resulting in false-negative diagnoses. A selective tool for a specific biomarker, like CEA, is therefore of interest; therefore, monoclonal antibodies against CEA have been radiolabeled with several different radionuclides for use in radioscintigraphy [13-18]. However, it is essential that the labeled radiotracer has a fast clearance and has low binding to other endogenous components, especially those in the proximity to the target of interest. Since most intact antibodies have a long biological half-life (days - weeks), this has hampered a wider use of radiolabeled antibodies in PET, where most radionuclides have a very short half-life [19]. One way to overcome this problem is to use methods of pretargeting, i.e., to pretarget the tissue with a bispecific antibody followed by visualization of the bound antibody in a second step using s smaller molecules with more appropriate kinetics some days later [20]. A recently developed pretargeting technique is to use complex antibodies or fragments of antibodies having multiple binding sites interacting with both antigen and reporter moiety carrying hapten molecules containing the radionuclide. In this report we have used a pretargeting system made by a trivalent, bispecific binding antibody (TF2), consisting of two identical Fab fragments reacting against CEACAM5, is covalently linked to a different Fab fragment capable of reacting with a divalent hapten peptide containing histamine-succinyl-glycine (HSG) residues and a chelate to be used for attaching the appropriate radionuclide [21-23]. The specificity of TF2 to CEA has been demonstrated in previous studies [21,24-26]. TF2 is pre-administered to allow the localization to the target and clearance from the blood within its pharmacokinetics time frame. The subsequent administration of the radiolabeled hapten peptide characterized with fast target (TF2) localization and blood clearance provides imaging of high contrast [21,24].
Non-invasive visualization of the distribution and density of CEA not only in tissue fluids, but also in the patient in order to locate the spread of the CEA containing cancer by using the pretargeting technique in combination with PET could be advantageous for staging of the disease. This sensitive technique for visualization of various endogenous binding sites should be suitable for prognostic investigations of the presence of CEA, and due to the high sensitivity of PET this also allows for the detection of small metastases that may be occult by other imaging modalities.
A considerable number of radionuclide-labeled antibodies is investigated currently for cancer therapy with radionuclides such as 90Y, 125/131I, 213Bi, 188Re, 177Lu, 211At where the radiation to healthy organs is high due to the slow pharmacokinetics and blood clearance, with high concentrations in the red marrow [27]. Thus, the introduction of the bispecific pretargeting concept contributes to the improvement of not only of diagnostic imaging but also possible therapy applications. The hapten molecule may contain various chelators (DTPA, DOTA, NOTA) for complexation with a variety of radionuclides, such as 111In, 99mTc, Al18F [28,29], 67Ga or 68Ga for the imaging in SPECT and PET studies of the distribution of CEA, or has been proven efficacious for therapy when labeled with 177Lu or 90Y [23].
The present in vitro study was undertaken to evaluate the binding of the trivalent bispecific antibody TF2 and the two haptens, Ga-IMP461 and Ga-IMP485 (Figure 1), in colorectal cancers removed from patients at surgery. Both haptens were labeled with 68Ga for PET and with 67Ga for SPECT for the in vitro evaluation.
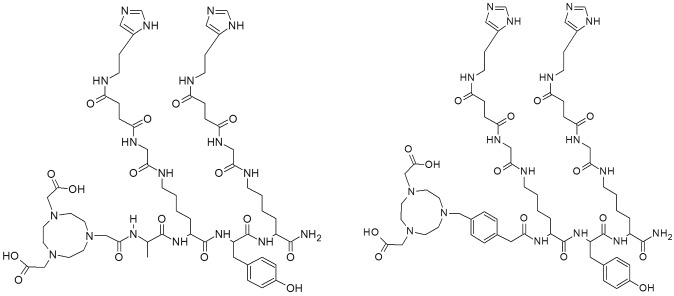
Figure 1.
Structural formula of IMP461 (left) and IMP485 (right).
Material and methods
Compounds and chemicals
The trivalent, bispecific binding antibody TF2 was prepared as described previously and stored at + 4°C until use [22]. Two haptens, IMP461(NOTA-D-Ala-D-Lys(HSG)-D-Tyr-D-Lys (HSG)-NH2) and IMP485 (NOTA-MPAA-D-Lys (HSG)-D-Tyr-D-Lys(HSG)-NH2) (Figure 1) [20,23], as well as TF2, were obtained from Immunomedics Inc., Morris Plains, NJ, USA. 68Ga was obtained from a 68Ge/68Ga generator system (iThemba, IDB Holland bv, The Netherlands). HEPES (4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, used as a powder), doubly distilled hydrochloric acid (Riedel de Haën), NaCl (38979), NaOH (306576, semiconductor grade) were obtained from Sigma-Aldrich Sweden (Stockholm, Sweden). Trifluoroacetic acid (TFA) was obtained from Merck (Darmstadt, Germany). Water (18.2 MΩ), produced with a Purelab Ultrapure system (Elga Bucks, the UK), was used in all reactions. The purchased chemicals were used without further purification. 67Ga (T½ = 3.2612 d, decay by electron capture (EC), gamma energy 93 keV 40%, 184 keV 20%, 300 keV 17%, 393 keV 5%) in 0.1M HCl solution was obtained from MDS Nordion Inc., Vancouver, BC, Canada and used without further purification. Strong anion exchange solid phase extraction cartridges (Chromafix, PS-HCO3) were purchased from Macherey-Nagel GmbH, Düren, Germany.
Tissues
The colorectal cancer tissue samples were obtained from surgeries performed at Uppsala University Hospital, and were immediately frozen and stored at -80°C until sectioned. Tissues from cancerous colorectals as well as from neighboring tissue taken at a minimum of 10 cm from the site of the tumor were used. The sections were also stained with hematoxylin-eosin.
CEA-expressing LS174T human colorectal cancer xenografts from one nude rat and one nude mouse were sectioned and used as positive control tissues. All tissues were cryosectioned using a Leica cryocut to 20 μm, and put on Superfrost glass slides. The sections were stored at -25°C before use within a few months.
Analytical methods for radiolabeling
HPLC analysis was performed on a Beckman Nouveau HPLC system equipped with a Beckman 166 variable-wavelength UV detector and a flow-count radio detector (Bioscan, Inc., USA) connected in series. The reverse-phase separation was conducted using a Grace Vydac C18 Protein and Peptide HPLC column (Vydac, USA,150 × 4.6 mm; 5μm). The gradient elution with 0.1% trifluoroacetic acid (TFA) aqua (A) and acetonitrile/water 70:30, containing 0.1% TFA (B), as mobile phase, was as follows: 5% B for 2 min, and then to 90% B in 8 min. The flow rate and UV wave length were 1 ml/min and 280 nm, respectively.
Pre-concentration and purification of 68Ga
68Ga (T½ = 68 min, β+ = 89% and EC = 11%) was available from a 68Ge/68Ga generator system (iThemba, IDB Holland bv, The Netherlands) in which 68Ge (T½= 270 d) was attached to a column of an inorganic matrix based on stannous oxide. A pre-elution using 6 ml of 1 M HCl was performed four hours prior to the synthesis. 68Ga was eluted with 6 ml of 1 M HCl into a vial containing 1.4 g NaCl and stirred until dissolved, giving a chloride concentration of 5 M. The solution was passed through the anion exchange cartridge (Chromafix, PS-HCO3), and afterwards the 68GaCl3 was eluted with 300 μl of water.
Preparation of [68Ga]Ga-IMP461 and [68Ga]Ga-IMP485
HEPES (0.048 g, 0.20 mmol) was dissolved in the 300 μl of preconcentrated 68Ga in a 2 ml Eppendorf tube. The pH was 4.2-4.6 (adjusted by the addition of 30% HCl (aq) or 10 M NaOH (aq) if necessary). Thereafter, an aqueous solution of IMP461 or IMP485 (1-6 nmol) was added. The reaction took place by using conventional heating at 90°C for 10-15 min. The purity of the labeled product was analyzed using HPLC and the pH of the reaction mixture was adjusted to ~7 by addition of 10 M NaOH (aq) prior to use in the biological experiments.
Preparation of [67Ga]Ga-IMP461 and [67Ga]Ga-IMP485
67Ga obtained as GaCl3 solution in 0.1M HCl (106 μL) was used without further purification. The amount of 67Ga (9 ± 1 MBq) used for the labeling was adjusted to the required specific radioactivity. The corresponding volume of the stock solution was added to the HEPES solution (0.3 M, 130 μl) in 0.1 H HCl. The pH of the solution was adjusted to 3.5-4.5, if needed, with NaOH. Then 3 nmol of IMP461 or IMP485 were added, and the reaction mixture was incubated either at room temperature for 10 minutes or 90°C for 5 min. The radiochemical purity of [67Ga]Ga-IMP461 and [67Ga]Ga-IMP485 was assessed by UV-radio-HPLC, and the concentration of the conjugate and the tracer was determined from UV-HPLC standard plots.
Synthesis of [NatGa]Ga-IMP461 and [NatGa]Ga-IMP485
NatGa of natural isotope composition (69Ga and 71Ga) was complexed with IMP461 and IMP485. Twenty mM aqua solution of NatGaCl3 (5 μl) were added to the HEPES solution (0.3 M) of 0.1 M HCl (200 μl) and the pH was adjusted with NaOH to 3.5-4.5. Then 10 nmol of either IMP461 or IMP485 were added and the reaction mixture was incubated at room temperature for 10 min or 90°C for 5 min. [NatGa]Ga-IMP461 and [NatGa]Ga-IMP485 were used as authentic references for the confirmation of the radio-HPLC chromatogram signals.
Stability study
The stability of formulated [67/68/NatGa]Ga-IMP461 and [67/68/NatGa]Ga-IMP485 counterparts in phosphate-buffered saline (PBS) at pH 7.4 was monitored at room temperature by UV-radio-HPLC during 4h, 17h and 3d, respectively, for 68Ga, 67Ga and NatGa. Appearance of radiosignals was followed for the compounds comprising 68Ga and 67Ga. The stability of the molecules containing NatGa was followed by UV-HPLC and determinations of the corresponding absorbance and concentration were made.
Autoradiography
The assay conditions were optimized by varying concentrations of the bispecific binding antibody and of the radioligand on rat and human tumors and control tissue. Both [67Ga]Ga-IMP461 and [68Ga]Ga-IMP461 were used in the optimization studies.
The optimal assay conditions for in vitro autoradiography were found to be as follows. The thawed sections were preincubated for 60 min at 37°C in Tris-HCl buffer (Tris-HCl 0.05 M, pH7.4, with BSA 0.2%, MgCl2 5 mM, bacitracin 50 mg/l ) without and with bispecific antibody diluted 1:10000 (“pretargeting”). The sections were then washed in buffer twice for two min in ice-cold buffer to remove unbound antibody. The incubation was conducted for 60 min at 37°C with [67/68Ga]Ga-IMP461. Unbound radiotracer was then removed by washing twice for three min in ice-cold buffer. The sections were dried at 37°C for 7-10 min and exposed to phosphor imager plates (Molecular Dynamics, Amersham Biosciences, UK) over night before analyzing in a PhosphorImager SI (Molecular Dynamics, Amersham Biosciences, UK) and quantification using ImageQuant TL (GE Healthcare).
Results
Synthesis of [67/68/NatGa]Ga-IMP461 and [67/68/NatGa]Ga-IMP485
The optimization of the 68Ga-labeling synthesis was conducted using IMP461. The lowest amount of the hapten molecule in the protocol providing radioactivity incorporation (RAI) of >95% was 3 nmol. The nearly quantitative RAI and biological compatibility of HEPES buffer allowed for the omission of the purification step. This amount was used throughout the study in order to provide highest possible specific radioactivity and resulting in relevant comparison of the biological results. SRA values varied, dependent on the age of the generator, from 41 to 117 MBq/nmol. The pre-concentration of the generator eluate was conducted using methods described earlier [30].
The amount of 67Ga used in the labeling reactions was defined by the product specific radioactivity determined by the biological experiment design. It was below (3-10 pmol) the amount (200 pmol) when the concentration of metal impurities starts to interfere with the labeling complexation [31]. The amount of the hapten molecules used for the synthesis was defined by the values optimized for the 68Ga-labeling in order to keep the same amount and ensure relevant comparison. The specific radioactivity was 3.1 ± 0.1 MBq/nmol.
The hapten counterparts were complexed with stable isotopes of gallium, 69,71Ga (NatGa). NatGa -IMP461 and NatGa -IMP485 were synthesized for the authentic reference purpose and in order to follow the stability of the compounds for longer time periods. The reaction mixture was spiked with the reference and monitored by UV-Radio-HPLC. The radiosignals of 67/68Ga-IMP461 (Rt = 5.12 ± 0.04) and 67/68Ga-IMP485 (Rt = 5.35 ± 0.14), and the UV-signals, respectively, from NatGa-IMP461 (Rt = 5.25 ± 0.04) or NatGa-IMP485 (Rt = 5.10 ± 0.05) co-eluted with slight delay due to in-line configuration of the detectors. The original compounds, IMP461 (Rt = 5.41 ± 0.01) and IMP485 (Rt = 5.68 ± 0.01), were well resolved from their respective complexes with stable gallium and allowed unambiguous determination during the stability tests. All compounds demonstrated high stability in the buffer at room temperature for at least 4, 17 and 72 h, respectively, containing 67Ga, 68Ga and NatGa. The detected signals corresponded only to the compounds of interest.
Optimization and validation for autoradiography
In the optimization studies using [67/68Ga]Ga-IMP461, the concentration of the bispecific binding TF2 antibody varied between 1:1000 and 1:100000 (Figures 2 and 3). Maximum binding was obtained at a dilution of approximately 1:5000, but sufficient binding contrast was obtained with a dilution of 1:10000, which therefore was used in subsequent studies. Varying the concentrations of [68Ga]Ga-IMP461, based on radioactivity (from 0.05 MBq/ml to 0.5 MBq/ml), showed that high intensity of binding was obtained already at the lowest concentration, although the maximum binding was increased with increasing hapten concentrations. In subsequent studies concentrations close to 0.25 MBq/ml (corresponding to 2-6 nM, depending upon specific radioactivity obtained) were used. A lower concentration of [67Ga]Ga-IMP461 was found to be optimal at lower concentrations, due to the longer nuclide half-life (0.01 MBq/ml, corresponding to approximately 3 nM) (Figure not shown). Similar conditions and concentrations were subsequently used for [67/68Ga]Ga-IMP485.
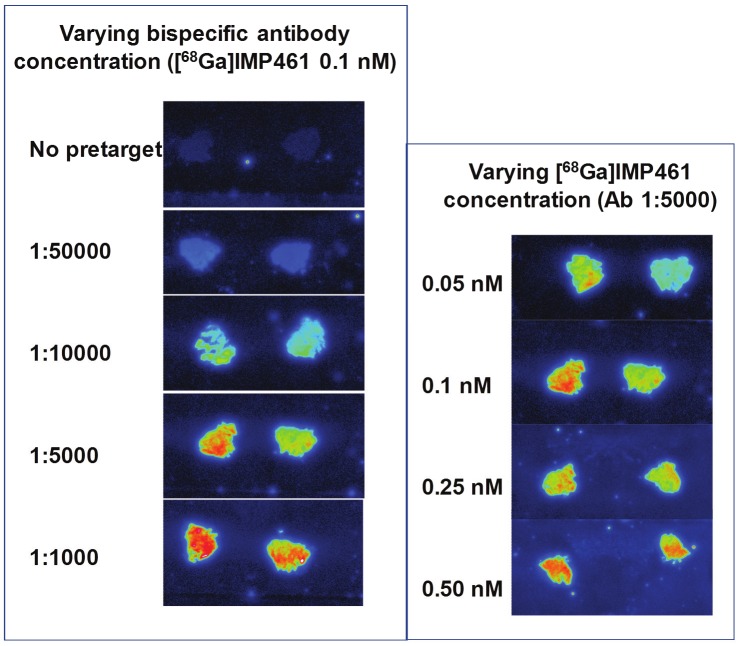
Figure 2.
Autoradiography using [68Ga]Ga-IMP461 and bispecific antibody TF2 on a mouse tumor. Effects of varying antibody concentration and hapten concentration, respectively.
Figure 3.
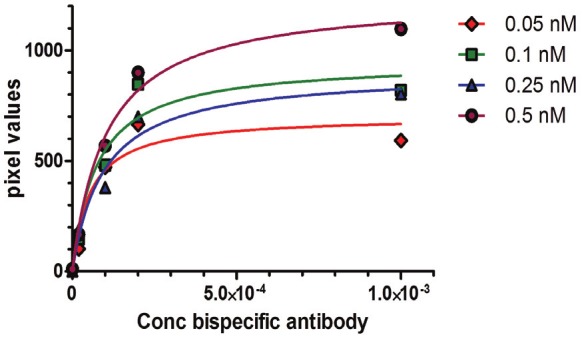
Effects of varying antibody concentrationand hapten concentration on autoradiography using [68Ga]Ga-IMP461 and bispecific antibody TF2. Halfmaximum was reached at antibody concentrations of approximately 1:10000 and 1:50000 with haptenconcentration of 0.5 and 0.05 nM, respectively.
In the initial studies we used rat and mouse tumor xenografts and single human colorectal cancers from surgical specimens. All four ligands, regardless radionuclide or hapten, showed strong binding to pretargeted tissue. The addition of [67/68Ga]Ga-IMP461 to sections without in vitro pretargeting, i.e., without prior pre-treatment with the bispecific antibody TF2, resulted in absence of, or very low binding of the radioligand to either xenografts (Figure 2) or primary human tumors. Using the optimal conditions as mentioned above very distinct images were seen in the murine xenografted tumor (Figure 2).
Patient tissue study
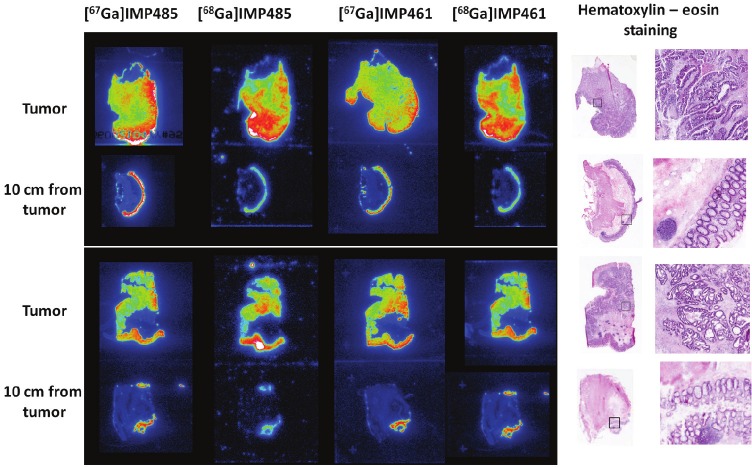
The bispecific antibody, TF2, and the haptens [67/68Ga]Ga-IMP461 and [67/68Ga]Ga-IMP485 were used for the study of specimens from colorectal cancers obtained at surgery. The tissue sections were subsequently analyzed using hematoxylin-eosin staining in order to verify the autoradiographic localization. In all tissue judged from the staining to be part of the tumor, extensive and strong labeling was found (Figure 4). Moreover, distinct labeling was also found in the epithelium of most samples of tissue obtained at least 10 cm from the cancer. However, in one of the samples, considered to be taken from the ileum, much lower labeling was found.
Figure 4.
Autoradiograhy using [67/68Ga]Ga-IMP461 and [67/68Ga]Ga-IMP485 and bispecific antibody TF2 on two samples from two colorectal cancer patients.
No marked differences in the radioactivity uptake, dependent on the radionuclide (67Ga vs. 68Ga) or hapten molecule (IMP461 vs. IMP485), were found. All four radioligands showed strong binding to the tumor tissue as well as to the epithelial tissue in the surgical specimen to a similar extent.
Discussion
These in vitro autoradiographical studies confirm previous in vivo studies on animal xenografts of human tumors and primary human tumor specimens that the TF2 bispecific antibody, labeled with [67/68Ga]Ga-IMP461 or [67/68Ga]Ga-IMP485, can be used for the visualization of CEA-containing cancers. Moreover, the in vitro imaging allows for higher resolution and thus more detailed information and more precise distribution of the target in the tissue providing valuable information on cancer cell extension. The signal-to-noise ratio in the colorectal cancer tissues was excellent, and could be considered specific as the radioligand did not bind to the tissue in the absence of pretargeting with the bispecific antibody. The labeling of CEA in the epithelial cells could be easily distinguished from the labeling of the tumor, because the localization of the epithelium resulted in a thin, more localized image than what was achieved in the tumor tissue. The good specificity versus binding to non-cancerous tissue thus makes this pretargeting in vitro autoradiography very reliable.
A pre-concentration procedure was performed for 68Ga by anion exchange using sodium chloride instead of hydrochloric acid [30]. To obtain >95% radioactivity incorporation in the 68Ga-labeling reaction it was necessary to use 3 nmol of the IMP461 precursor. Using this protocol for radioactivity incorporation no further purification was needed prior to the biological experiments. This is important because purification can lead to a decrease in specific radioactivity by means of losses by time and also by the procedure itself.
In the previous published studies a large number of variants of the hapten molecules have been used. The haptens can be labeled with several different radionuclides, e.g. 18F [20,24,29,32], 111In [20,33], 99mTc [20,34] for diagnosis, as well as with other radionuclides for radiotherapy (90Y [20], 177Lu [35]). However, the use of 68Ga [32] is not common for these studies, in spite of obvious advantages such as availability and suitable half-life. In this study, we have used and compared two haptens, IMP485 and IMP461, both with NOTA as a chelator, labeled with 67Ga or 68Ga. We have also labeled both haptens with the SPECT radionuclide, 67Ga, for comparison. It is obvious from the figures that the two haptens result in identical in vitro images, and that any of the two radionuclides may be used.
In surgery of colorectal cancer patients, the surgeon resected the cancerous tissue, as well as neighboring tissue at a minimum of 10 cm from the site of the tumor. In this study the tissue obtained 10 cm from the tumor was used as control. However, previous immunohistological studies have shown that the epithelial cells of the normal colon also contain CEA, although to a lower extent than tissue with cancer cells [36-38]. Thus, this very sensitive pretargeting technique can also visualize CEA in putatively normal tissue adjacent to cancer. However, as the epithelial cells have a very distinct localization in comparison to the cancer cells, the difference between the two cell types is easily recognized in the autoradiograms. It must be appreciated, however, that normal-appearing epithelium adjacent to cancers of the colon and rectum may have elevated CEA expression, as compared to intestinal tissues from individuals free of cancer in this organ [36-38].
It can be concluded that pretargeting the CEA with the bispecific antibody TF2 followed by the addition of Ga-labeled hapten is extremely sensitive in visualizing CEA. This methodology can therefore be a very specific method that is complementary to other histochemical techniques in the diagnosis of colorectal cancer in biopsies or in samples taken from surgery. These tissue findings support the view that the pretargeting technique may also be a step forward in diagnosing patients with colorectal cancer, either using 67Ga and SPECT or 68Ga and PET for imaging. Furthermore the use of high specific radioactivity might be valuable if repeated PET studies using 68Ga can be undertaken in order to optimize the therapeutic protocol, as was discussed previously [23].
Acknowledgements
We are grateful to Simin Tahmasebpoor for sectioning of the colorectal tissue samples. We also appreciate the assistance of Victoria Trindade, Department of the Nuclear Research Center (CIN) of the Faculty of Sciences, Montevideo, Uruguay, and Soledad Fernández, Faculty of Chemistry of Uruguay, The Republic University, Montevideo, Uruguay. This work was performed when the authors HH, AM, JU and IV were employed at Uppsala Applied Science Lab, GE Healthcare, Uppsala, Sweden.
Competing interests
Dr David M Goldenberg and Dr William McBride have financial interests in Immunomedics, Inc. The other authors declare no competing interests.
Authors' contributions
HH was responsible for the in vitro binding studies and was responsible for the writing of the manuscript. IV, EB, JU, WMB and BL adapted and performed all radiochemistry work. AM performed all animal in vivo work with the xenografted animals from which the animal tumors were taken. LP was responsible for surgery and for obtaining suitable tissue from the colorectal cancer. PM and AW prepared the samples for sectioning, were responsible for the sectioning and performed the pathological examinations of the human tissue samples. BL and DMG were the initiators of the project. All authors have contributed to the writing of the manuscript.
References
- 1.Hammarströ S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 2.Watine J, Miedouge M, Friedberg B. Carcinoembryonic antigen as an independent prognostic factor of recurrence and survival in patients resected for colorectal liver metastases: a systematic review. Dis Colon Rectum. 2001;44:1791–1799. doi: 10.1007/BF02234457. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338–351. doi: 10.1081/cnv-58878. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita K, Watanabe M. Clinical significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009;100:195–199. doi: 10.1111/j.1349-7006.2008.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim YK, Kam MH, Eu KW. Carcinoembryonic antigen screening: how far should we go? Singapore Med J. 2009;50:862–865. [PubMed] [Google Scholar]
- 6.Carriquiry LA, Pineyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer? Dis Colon Rectum. 1999;42:921–929. doi: 10.1007/BF02237104. [DOI] [PubMed] [Google Scholar]
- 7.Robertson JF, Ellis IO, Bell J, Todd JH, Robins A, Elston CW, Blamey RW. Carcinoembryonic antigen immunocytochemistry in primary breast cancer. Cancer. 1989;64:1638–1645. doi: 10.1002/1097-0142(19891015)64:8<1638::aid-cncr2820640814>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Hsu WH, Huang CS, Hsu HS, Huang WJ, Lee HC, Huang BS, Huang MH. Preoperative serum carcinoembryonic antigen level is a prognostic factor in women with early non-small-cell lung cancer. Ann Thorac Surg. 2007;83:419–424. doi: 10.1016/j.athoracsur.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 9.Shi HZ, Liang QL, Jiang J, Qin XJ, Yang HB. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology. 2008;13:518–527. doi: 10.1111/j.1440-1843.2008.01291.x. [DOI] [PubMed] [Google Scholar]
- 10.Kodama S, Itoh H, Ide H, Kataoka H, Takehara T, Nagano M, Hamasuna R, Koono M, Osada Y. Carcinoembryonic antigen and carbohydrate antigen 19-9-producing adenocarcinoma of the prostate: report of an autopsy case. Urol Int. 1999;63:193–197. doi: 10.1159/000030446. [DOI] [PubMed] [Google Scholar]
- 11.Ballesta AM, Molina R, Filella X, Jo J, Gimenez N. Carcinoembryonic antigen in staging and follow-up of patients with solid tumors. Tumour Biol. 1995;16:32–41. doi: 10.1159/000217926. [DOI] [PubMed] [Google Scholar]
- 12.de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med. 2009;50(Suppl 1):43S–54S. doi: 10.2967/jnumed.108.057224. [DOI] [PubMed] [Google Scholar]
- 13.Curtet C, Vuillez JP, Daniel G, Aillet G, Chetanneau A, Visset J, Kremer M, Thedrez P, Chatal JF. Feasibility study of radioimmunoguided surgery of colorectal carcinomas using indium-111 CEA-specific monoclonal antibody. Eur J Nucl Med. 1990;17:299–304. doi: 10.1007/BF01268019. [DOI] [PubMed] [Google Scholar]
- 14.Delaloye AB, Delaloye B, Buchegger F, Vogel CA, Gillet M, Mach JP, Smith A, Schubiger PA. Comparison of copper-67- and iodine-125-labeled anti-CEA monoclonal antibody biodistribution in patients with colorectal tumors. J Nucl Med. 1997;38:847–853. [PubMed] [Google Scholar]
- 15.Goldenberg DM, Wlodkowski TJ, Sharkey RM, Silberstein EB, Serafini AN, Garty II, Van Heertum RL, Higginbotham-Ford EA, Kotler JA, Balasubramanian N, Swayne LC, Hansen HJ, Pinsky CM. Colorectal cancer imaging with iodine-123-labeled CEA monoclonal antibody fragments. J Nucl Med. 1993;34:61–70. [PubMed] [Google Scholar]
- 16.Lind P, Lechner P, Arian-Schad K, Klimpfinger M, Cesnik H, Kammerhuber F, Eber O. Anti-carcinoembryonic antigen immunoscintigraphy (technetium-99m-monoclonal antibody BW 431/26) and serum CEA levels in patients with suspected primary and recurrent colorectal carcinoma. J Nucl Med. 1991;32:1319–1325. [PubMed] [Google Scholar]
- 17.Ychou M, Pelegrin A, Faurous P, Robert B, Saccavini JC, Guerreau D, Rossi JF, Fabbro M, Buchegger F, Mach JP, Artus JC. Phase-I/II radio-immunotherapy study with Iodine-131-labeled anti-CEA monoclonal antibody F6 F(ab') 2 in patients with non-resectable liver metastases from colorectal cancer. Int J Cancer. 1998;75:615–619. doi: 10.1002/(sici)1097-0215(19980209)75:4<615::aid-ijc20>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg DM. Carcinoembryonic antigen as a target cancer antigen for radiolabeled antibodies: prospects for cancer imaging and therapy. Tumour Biol. 1995;16:62–73. doi: 10.1159/000217930. [DOI] [PubMed] [Google Scholar]
- 19.Hong H, Sun J, Cai W. Radionuclide-based cancer imaging targeting the carcinoembryonic antigen. Biomark Insights. 2008;3:435–451. doi: 10.4137/bmi.s1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McBride WJ, Zanzonico P, Sharkey RM, Noren C, Karacay H, Rossi EA, Losman MJ, Brard PY, Chang CH, Larson SM, Goldenberg DM. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med. 2006;47:1678–1688. [PubMed] [Google Scholar]
- 21.Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, Hansen HJ, Horak ID, Goldenberg DM. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg DM, Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734–3744. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang CH. Multifunctional antibodies by the Dock-and-Lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med. 2008;49:158–163. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 24.Sharkey RM, Karacay H, Vallabhajosula S, McBride WJ, Rossi EA, Chang CH, Goldsmith SJ, Goldenberg DM. Metastatic human colonic carcinoma: molecular imaging with pretargeted SPECT and PET in a mouse model. Radiology. 2008;246:497–507. doi: 10.1148/radiol.2462070229. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey RM, Rossi EA, McBride WJ, Chang CH, Goldenberg DM. Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin Nucl Med. 2010;40:190–203. doi: 10.1053/j.semnuclmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen HJ, Goldenberg DM, Newman ES, Grebenau R, Sharkey RM. Characterization of second-generation monoclonal antibodies against carcinoembryonic antigen. Cancer. 1993;71:3478–3485. doi: 10.1002/1097-0142(19930601)71:11<3478::aid-cncr2820711104>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 28.Gold DV, Goldenberg DM, Karacay H, Rossi EA, Chang CH, Cardillo TM, McBride WJ, Sharkey RM. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819–4826. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 29.McBride WJ, Sharkey RM, Karacay H, D'Souza CA, Rossi EA, Laverman P, Chang CH, Boerman OC, Goldenberg DM. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 30.Velikyan I, Beyer GJ, Lågströ B. Microwave-supported preparation of 68Ga bioconjugates with high specific radioactivity. Bioconjug Chem. 2004;15:554–560. doi: 10.1021/bc030078f. [DOI] [PubMed] [Google Scholar]
- 31.Velikyan I, Beyer GJ, Bergströ-Pettermann E, Johansen P, Bergströ M, Lågströ B. The importance of high specific radioactivity in the performance of 68Ga-labeled peptide. Nucl Med Biol. 2008;35:529–536. doi: 10.1016/j.nucmedbio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Schoffelen R, Sharkey RM, Goldenberg DM, Franssen G, McBride WJ, Rossi EA, Chang CH, Laverman P, Disselhorst JA, Eek A, van der Graaf WT, Oyen WJ, Boerman OC. Pretargeted immuno-positron emission tomography imaging of carcinoembryonic antigen-expressing tumors with a bispecific antibody and a 68Ga- and 18F-labeled hapten peptide in mice with human tumor xenografts. Mol Cancer Ther. 2010;9:1019–1027. doi: 10.1158/1535-7163.MCT-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindan SV, Griffiths GL, Michel RB, Andrews PM, Goldenberg DM, Mattes MJ. Use of galactosylated-streptavidin as a clearing agent with 111In-labeled, biotinylated antibodies to enhance tumor/non-tumor localization ratios. Cancer Biother Radiopharm. 2002;17:307–316. doi: 10.1089/10849780260179279. [DOI] [PubMed] [Google Scholar]
- 34.van Schaijk FG, Oosterwijk E, Soede AC, Broekema M, Frielink C, McBride WJ, Goldenberg DM, Corstens FH, Boerman OC. Pretargeting of carcinoembryonic antigen-expressing tumors with a biologically produced bispecific anticarcinoembryonic antigen x anti-indium-labeled diethylenetriaminepentaacetic acid antibody. Clin Cancer Res. 2005;11:7130s–7136s. doi: 10.1158/1078-0432.CCR-1004-0006. [DOI] [PubMed] [Google Scholar]
- 35.Schoffelen R, van der Graaf WT, Franssen G, Sharkey RM, Goldenberg DM, McBride WJ, Rossi EA, Eek A, Oyen WJ, Boerman OC. Pretargeted 177Lu radioimmunotherapy of carcinoembryonic antigen-expressing human colonic tumors in mice. J Nucl Med. 2010;51:1780–1787. doi: 10.2967/jnumed.110.079376. [DOI] [PubMed] [Google Scholar]
- 36.Ishii S, Steele G Jr, Ford R, Paliotti G, Thomas P, Andrews C, Hansen HJ, Goldenberg DM, Jessup JM. Normal colonic epithelium adheres to carcinoembryonic antigen and type IV collagen. Gastroenterology. 1994;106:1242–1250. doi: 10.1016/0016-5085(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 37.Ogura E, Senzaki H, Yoshizawa K, Hioki K, Tsubura A. Immunohistochemical localization of epithelial glycoprotein EGP-2 and carcinoembryonic antigen in normal colonic mucosa and colorectal tumors. Anticancer Res. 1998;18:3669–3675. [PubMed] [Google Scholar]
- 38.Goldenberg DM, Sharkey RM, Primus FJ. Carcinoembryonic antigen in histopathology: immunoperoxidase staining of conventional tissue sections. J Natl Cancer Inst. 1976;57:11–22. doi: 10.1093/jnci/57.1.11. [DOI] [PubMed] [Google Scholar]