Abstract
Quantum dots (QDs) have many intriguing properties suitable for biomedical imaging applications. The poor tissue penetration of optical imaging in general, including those using QDs, has motivated the development of various QD-based dual-modality imaging agents. In this issue of AJNMMI (http://www.ajnmmi.us), Sun et al. reported the synthesis and in vitro/in vivo characterization of intrinsically radio-labeled QDs (r-QDs), where 109Cd was incorporated into the core/shell of QDs of various compositions. These r-QDs emit in the near-infrared range, have long circulation half-life, are quite stable with low cytotoxicity, exhibit small size and low accumulation in the reticuloendothelial system, and can allow for accurate measurement of their biodistribution in mice. With these desirable features demonstrated in this study, future development and optimization will further enhance the biomedical potential of intrinsically radio-labeled QDs.
Keywords: Quantum-dots (QDs), nanoparticle, positron emission tomography (PET), single-photon emission computed tomography (SPECT), near-infrared (NIR), optical imaging
The first decade of the 21st century has witnessed an explosion of biomedical research based on various nanomaterials, which hold tremendous potential to revolutionize disease diagnosis and treatment [1-3]. In the second decade of this century, clinical translation is the key in this vibrant research area and it is expected that nanotechnology will advance into clinical trials and eventually the day-to-day clinical practice in the near future. In this blooming nanotechnology arena, one of the most extensively studied classes of nanomaterials is quantum dots (QDs) [4,5]. Due to the many intriguing properties that are more advantageous than traditional organic dyes, QDs are desirable fluorophores for biomedical imaging applications. Since the first demonstration of the biomedical potential of QDs in 1998 [6,7], QD-based research has increased exponentially and QDs have become powerful tools in an array of research disciplines such as molecular biology, cell biology, molecular imaging, and medical diagnostics. For imaging applications, QDs have been investigated in a wide variety of scenarios in both cells and live animals. Aside from applications based on non-specific distribution/accumulation of QDs such as vasculature imaging, lymph node mapping, etc. [4,5,8], active tumor targeting using QD-based probes has also been achieved by several research groups [9-13].
One of the major limitations for optical imaging in general is poor tissue penetration, even in the relatively optically clear near-infrared (NIR, 700-900 nm) window [14-16]. Since magnetic resonance imaging (MRI) has no limit in tissue penetration, a wide variety of QD-based dual-modality agents have been reported for both optical imaging and MRI [17,18]. However, the very low sensitivity of MRI severely limits the potential applications of these QD-based dual-modality agents. Furthermore, whether combination of optical imaging and MRI is a desirable approach is questionable, since neither imaging technique is highly quantitative. On the other hand, radionuclide-based imaging techniques, such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET) [19,20], are very sensitive and highly quantitative with virtually no limit in tissue penetration. Clinically, PET/SPECT imaging has been widely used in oncology for cancer staging and monitoring the therapeutic response [20-26]. Clearly, combination of QD-based optical imaging and SPECT/PET can offer synergistic advantages over either modality alone.
In this issue of the American Journal of Nuclear Medicine and Molecular Imaging, Sun et al. reported a novel approach to synthesize intrinsically radio-labeled QDs (r-QDs), which can allow for accurate quantitation of their biodistribution in mice through measurement of the radioactivity signal (Figure 1) [27]. In this work, the radioactive nuclide of cadmium (i.e. 109Cd) was incorporated into the core/shell of QDs of various core compositions such as CdSe and CdTeSe. The significance of this intriguing study lies in several aspects. First, NIR r-QDs were synthesized which is suitable for in vivo imaging applications. The peak emission of the NIR r-QDs generated in this report was at about 780 nm and within the most optimal wavelength range for in vivo imaging, where the combined absorbance of all biomolecules in living tissues is at the minimum. In addition, these NIR r-QDs are very bright, with quantum yields of 40% and 11% in chloroform and aqueous solution (after ligand exchange to render water solubility) respectively.
Figure 1.
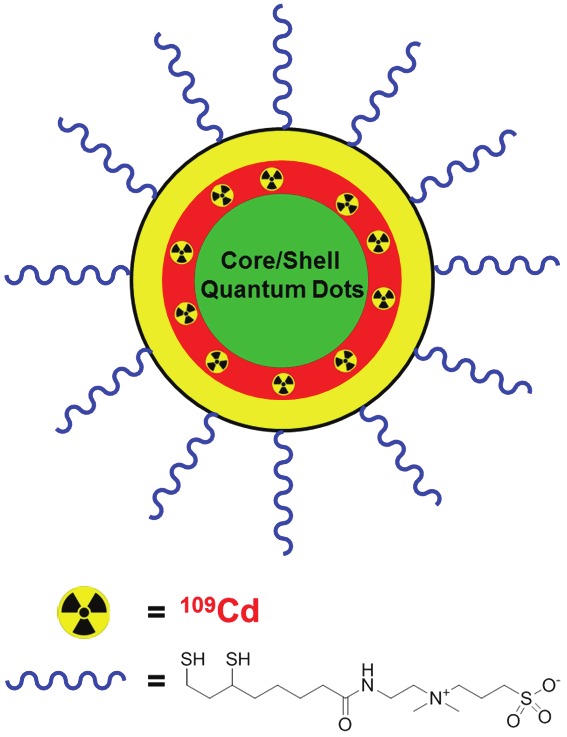
109Cd-containing quantum dots coated with zwitterionic ligands.
Second, these r-QDs have very long circulation half-life in mice (~20 h) because of the surface functionalization with zwitterionic ligands (Figure 1). In most literature reports, polyethylene glycol (PEG) was used for the coating of QDs, which indeed was able to increase the circulation lifetime of the resulting QDs [28]. However, PEG chains of molecular weight above 5,000 Da are typically needed to observe prolonged circulation half-life in mice as QDs coated with 2,000 Da PEG chains only exhibited very short circulation lifetime on the order of minutes [29]. Similar phenomenon was also observed in this study: PEG-coated r-QDs had a circulation half-life of a few minutes, which is several orders of magnitude shorter than the r-QDs coated with small zwitterionic ligands [27]. Through future engineering and exploration of the zwitterionic ligands, it is expected that the circulation half-life of these r-QDs can be adjusted to match specific applications. For in vivo imaging applications, circulation half-life in the range of a few hours is perhaps most desirable. Another advantage resulted from the use of small zwitterionic ligands is that the hydrodynamic size of functionalized QDs is significantly smaller than the PEG-coated r-QDs, which may lead to better extravasation from the vasculature and improve the targeting efficacy in future studies.
Third, the biodistribution of these r-QDs can be accurately and quantitatively measured because of the radioactive signal, which would not have been possible with optical imaging. Since the radioisotope 109Cd is inside the r-QDs (i.e. within the shell) instead of on the surface, in vivo stability of the radio-label is quite good. The commonly used approach to generate QD-based dual-modality agents for optical and PET/SEPCT imaging applications is through direct/indirect labeling of QDs with radioisotopes [29-35]. However, the stability of the radio-label in living animals is not very high. For example, a few hours after intravenous injection of 64Cu-labeled NIR QDs into mice, the PET and NIR fluorescence imaging data were in good agreement with each other [30,32]. However, distribution of 64Cu (measured by PET) and the QDs (detected by optical imaging) was substantially different at late time points. Incorporation of the radio-label inside the r-QDs eliminated the concerns regarding in vivo stability of the radio-label relative to the QDs, since it has been well documented that QDs do not undergo significant degradation unless they are subjected to very harsh conditions [36-38], which is largely irrelevant to the in vivo environment. Not only are these zwitterionic r-QDs quite stable in aqueous solution, they are also less toxic to cells than PEG-coated r-QDs. More comprehensive toxicology studies will be needed for future generations of r-QDs, and the radionuclide-based technique offered by these r-QDs will provide a robust quantitative platform for such studies.
Fourth, the accumulation of zwitterionic r-QDs in the liver and spleen is many fold lower than PEG-coated r-QDs. One of the major hurdles for all nanomaterial-based research in living subjects is the prominent accumulation in the reticuloendothelial system (e.g. liver, spleen, bone marrow, lymph nodes, etc.) [39,40]. Lower accumulation in these organs/tissues is undoubtedly beneficial for future applications of these r-QDs.
With the many desirable features of the r-QDs reported in this study [27], future development and optimization will further enhance the biomedical potential of this class of intrinsically radio-labeled QDs. Of note, a few previous studies have investigated r-QDs generated via a different approach with 125mTe as the radio-label. In one report, antibody-conjugated Cd125mTe/ZnS QDs were used for targeting the mouse lung endothelium, where the targeting efficacy was evaluated with biodistribution studies and SPECT imaging (no optical imaging was reported) [41]. Subsequently, the competition between vascular targeting and interaction of functionalized Cd125mTe/ZnS QDs with the reticuloendothelial system was investigated [42]. Antigen-specific uptake of antibody-conjugated Cd125mTe/ZnS QDs was demonstrated by the uniform distribution of radioactivity in the mouse lungs and significantly less accumulation in the liver and spleen than non-targeted Cd125mTe/ZnS QDs. Since QDs can be engulfed by phagocytic cells and removed from the circulation, biodistribution studies were also carried out in mice that had been depleted of phagocytic cells by the use of clodronate-loaded liposomes, and significantly reduced liver accumulation of the QDs was observed.
The main focus of these reports [27,41,42] was on the quantitative biodistribution of r-QDs, based on measurement of the radioactivity signal, rather than optical/SPECT imaging with the dual-modality agents. r-QDs represent an emerging class of dual-modality probes with promising potential for clinical applications and much research effort should be devoted to this area in the future. For SPECT imaging, different isotopes that emit different energy gamma rays can be differentiated based on the energy [43]. Since QDs are ideal agents for multiplexed fluorescence imaging [44], combination of the multiplexing capabilities of both SPECT and QDs can enable the interrogation of a number of biological events simultaneously using molecularly-targeted r-QDs, which should be explored in the future.
The recent trend for generating more biocompatible NIR QDs is in the use of other less toxic materials such as InAs and InP [45,46], which can alleviate the concerns regarding the long term toxicity of Cd, Se, and Te. Incorporation of radionuclides of these more biocompatible elements should be investigated in future work. For example, 111In has been widely used for SPECT imaging and arsenic isotopes (e.g. 72As and 74As) have been reported for PET imaging [47]. It is likely that we will see reports on r-QDs that contain clinically relevant radionuclides in the near future, such as 111In-containing r-QDs.
Although the efficiency of incorporating 109Cd into the shell of these r-QDs is almost 100%, the total radioactivity injected into each mouse was only about 1 μCi in this study [27]. Thanks to the high sensitivity of gamma counting, such a low level of radioactivity was sufficient for accurate quantification of the biodistribution of r-QDs in mice. Much more radioactivity will need to be injected into each mouse for in vivo imaging. Since SPECT has significantly lower sensitivity than PET [48], it will be more desirable to incorporate PET isotopes into future generations of r-QDs if feasible. Lastly, the decay half-life of 109Cd is 464 days, which is too long for biomedical applications. Future incorporation of other radioisotopes with suitable half-lives on the order of several hours to a few days is preferred. Given the proof-of-principle demonstrated in this study for generating r-QDs that emit in the NIR range, have long circulation half-life, are quite stable with low cytotoxicity, exhibit small size and low accumulation in the reticuloendothelial system, and can enable accurate measurement of their biodistribution in mice, it is expected that more interesting studies in this area will continue to emerge in the near future.
References
- 1.Thayer AM. Building up nanotech research. Chem Eng News. 2007;85:15–21. [Google Scholar]
- 2.Farrell D, Alper J, Ptak K, Panaro NJ, Grodzinski P, Barker AD. Recent advances from the National Cancer Institute Alliance for Nanotechnology in Cancer. ACS Nano. 2010;4:589–594. doi: 10.1021/nn100073g. [DOI] [PubMed] [Google Scholar]
- 3.Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-based cancer therapeutics--promise and challenge--lessons learned through the NCI Alliance for Nanotechnology in Cancer. Pharm Res. 2011;28:273–278. doi: 10.1007/s11095-010-0214-7. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, Hsu AR, Li ZB, Chen X. Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Res Lett. 2007;2:265–281. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 7.Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 8.Li ZB, Cai W, Chen X. Semiconductor quantum dots for in vivo imaging. J Nanosci Nanotechnol. 2007;7:2567–2581. doi: 10.1166/jnn.2007.628. [DOI] [PubMed] [Google Scholar]
- 9.Akerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo . Proc Natl Acad Sci USA. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 11.Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007;67:1138–1144. doi: 10.1158/0008-5472.CAN-06-1185. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 13.Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett. 2008;8:2599–2606. doi: 10.1021/nl080141f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Lee S, Chen X. Design of “smart” probes for optical imaging of apoptosis. Am J Nucl Med Mol Imaging. 2011;1:3–17. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Hong H, Engle JW, Yang Y, Barnhart TE, Cai W. Positron emission tomography and near-infrared fluorescence imaging of vascular endothelial growth factor with dual-labeled bevacizumab. Am J Nucl Med Mol Imaging. 2012;2:1–13. [PMC free article] [PubMed] [Google Scholar]
- 17.Koole R, Mulder WJ, van Schooneveld MM, Strijkers GJ, Meijerink A, Nicolay K. Magnetic quantum dots for multimodal imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:475–491. doi: 10.1002/wnan.14. [DOI] [PubMed] [Google Scholar]
- 18.Mulder WJ, Griffioen AW, Strijkers GJ, Cormode DP, Nicolay K, Fayad ZA. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomedicine (Lond) 2007;2:307–324. doi: 10.2217/17435889.2.3.307. [DOI] [PubMed] [Google Scholar]
- 19.Buckle T, van den Berg NS, Kuil J, Bunschoten A, Oldenburg J, Borowsky AD, Wesseling J, Masada R, Oishi S, Fujii N, van Leeuwen FWB. Non-invasive longitudinal imaging of tumor progression using an 111indium labeled CXCR4 peptide antagonist. Am J Nucl Med Mol Imaging. 2012;2:99–109. [PMC free article] [PubMed] [Google Scholar]
- 20.Alauddin MM. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am J Nucl Med Mol Imaging. 2012;2:55–76. [PMC free article] [PubMed] [Google Scholar]
- 21.Eary JF, Hawkins DS, Rodler ET, Conrad EUI. 18F-FDG PET in sarcoma treatment response imaging. Am J Nucl Med Mol Imaging. 2011;1:47–53. [PMC free article] [PubMed] [Google Scholar]
- 22.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 23.Iagaru A. 18F-FDG PET/CT: timing for evaluation of response to therapy remains a clinical challenge. Am J Nucl Med Mol Imaging. 2011;1:63–64. [PMC free article] [PubMed] [Google Scholar]
- 24.Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The clinical use of PET with 11C-acetate. Am J Nucl Med Mol Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- 25.Vach W, Høilund-Carlsen PF, Fischer BM, Gerke O, Weber W. How to study optimal timing of PET/CT for monitoring of cancer treatment. Am J Nucl Med Mol Imaging. 2011;1:54–62. [PMC free article] [PubMed] [Google Scholar]
- 26.Aparici CM, Carlson D, Nguyen N, Hawkins RA, Seo Y. Combined SPECT and Multidetector CT for Prostate Cancer Evaluations. Am J Nucl Med Mol Imaging. 2012;2:48–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M, Hoffman D, Sundaresan G, Yang L, Lamichhane N, Zweit J. Synthesis and characterization of intrinsically radio-labeled quantum dots for bimodal detection. Am J Nucl Med Mol Imaging. 2012;2:122–135. [PMC free article] [PubMed] [Google Scholar]
- 28.Ballou B. Quantum dot surfaces for use in vivo and in vitro. Curr Top Dev Biol. 2005;70:103–120. doi: 10.1016/S0070-2153(05)70005-3. [DOI] [PubMed] [Google Scholar]
- 29.Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J, Chen X, Wu AM, Weiss S, Gambhir SS. microPET-based biodistribution of quantum dots in living mice. J Nucl Med. 2007;48:1511–1518. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 31.Tu C, Ma X, House A, Kauzlarich SM, Louie AY. PET Imaging and Biodistribution of Silicon Quantum Dots in Mice. ACS Med Chem Lett. 2011;2:285–288. doi: 10.1021/ml1002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Li ZB, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35:2235–2244. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 33.Duconge F, Pons T, Pestourie C, Herin L, Theze B, Gombert K, Mahler B, Hinnen F, Kuhnast B, Dolle F, Dubertret B, Tavitian B. Fluorine-18-labeled phospholipid quantum dot micelles for in vivo multimodal imaging from whole body to cellular scales. Bioconjug Chem. 2008;19:1921–1926. doi: 10.1021/bc800179j. [DOI] [PubMed] [Google Scholar]
- 34.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, Chen X, Banin U, Wu AM, Sinclair R, Weiss S, Gambhir SS. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier AM, Gaub HE, Stoelzle S, Fertig N, Parak WJ. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 38.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 40.Hong H, Zhang Y, Sun J, Cai W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodward JD, Kennel SJ, Mirzadeh S, Dai S, Wall JS, Richey T, Avenell J, Rondinone AJ. In vivo SPECT/CT imaging and biodistribution using radioactive Cd125mTe/ZnS nanoparticles. Nanotechnology. 2007;18:175103. [Google Scholar]
- 42.Kennel SJ, Woodward JD, Rondinone AJ, Wall J, Huang Y, Mirzadeh S. The fate of MAb-targeted Cd125mTe/ZnS nanoparticles in vivo. Nucl Med Biol. 2008;35:501–514. doi: 10.1016/j.nucmedbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Berman DS, Kiat H, Van Train K, Friedman JD, Wang FP, Germano G. Dual-isotope myocardial perfusion SPECT with rest thallium-201 and stress Tc-99m sestamibi. Cardiol Clin. 1994;12:261–270. [PubMed] [Google Scholar]
- 44.Kobayashi H, Hama Y, Koyama Y, Barrett T, Regino CA, Urano Y, Choyke PL. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007;7:1711–1716. doi: 10.1021/nl0707003. [DOI] [PubMed] [Google Scholar]
- 45.Pradhan N, Battaglia DM, Liu Y, Peng X. Efficient, stable, small, and water-soluble doped ZnSe nanocrystal emitters as non-cadmium biomedical labels. Nano Lett. 2007;7:312–317. doi: 10.1021/nl062336y. [DOI] [PubMed] [Google Scholar]
- 46.Xie R, Chen K, Chen X, Peng X. InAs/InP/ ZnSe core /shell /shell quantum dots as near-infrared emitters: bright, narrow-band, non-cadmium containing, and biocompatible. Nano Res. 2008;1:457–464. doi: 10.1007/s12274-008-8048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jennewein M, Lewis MA, Zhao D, Tsyganov E, Slavine N, He J, Watkins L, Kodibagkar VD, O'Kelly S, Kulkarni P, Antich PP, Hermanne A, Rosch F, Mason RP, Thorpe PE. Vascular imaging of solid tumors in rats with a radioactive arsenic-labeled antibody that binds exposed phosphatidylserine. Clin Cancer Res. 2008;14:1377–1385. doi: 10.1158/1078-0432.CCR-07-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]


