Abstract
In cardiac cells, Ca2+ release flux (Jrel) via ryanodine receptors (RyRs) from the sarcoplasmic reticulum (SR) has a complex effect on the action potential (AP). Coupling between Jrel and the AP occurs via L-type Ca2+ channels (ICa) and the Na+/Ca2+ exchanger (INCX). We used a combined experimental and modelling approach to study interactions between Jrel, ICa and INCX in porcine ventricular myocytes. We tested the hypothesis that during normal uniform Jrel, the interaction between these fluxes can be represented as occurring in two myoplasmic subcompartments for Ca2+ distribution, one (T-space) associated with RyR and enclosed by the junctional portion of the SR membrane and corresponding T-tubular portion of the sarcolemma, the other (M-space) encompassing the rest of the myoplasm. ICa and INCX were partitioned into subpopulations in the T-space and M-space sarcolemma. We denoted free Ca2+ concentrations in T-space and M-space Cat and Cam, respectively. Experiments were designed to allow separate measurements of ICa and INCX as a function of Jrel. Inclusion of T-space in the model allowed us to reproduce in silico the following important experimental results: (1) hysteresis of INCX dependence on Cam; (2) delay between peak INCX and peak Cam during caffeine application protocol; (3) delay between INCX and Cam during Ca2+-induced-Ca2+-release; (4) rapid ICa inactivation (within 2 ms) due to Jrel, with magnitude graded as a function of the SR Ca2+ content; (5) time delay between ICa inactivation due to Jrel and Cam. Partition of 25% NCX in T-space and 75% in M-space provided the best fit to the experimental data. Measured Cam and ICa or INCX were used as input to the model for estimating Cat. The actual model-computed Cat, obtained by simulating specific experimental protocols, was used as a gold standard for comparison. The model predicted peak Cat in the range of 6–25 μm, with time to equilibrium of Cat with Cam of ∼350 ms. These Cat values are in the range of LCC and RyR sensitivity to Ca2+. An increase of the SR Ca2+ load increased the time to equilibrium. The ICa-based estimation method was most accurate during the ascending phase of Cat. The INCX-based method provided a good estimate for the descending phase of Cat. Thus, application of both methods in combination provides the best estimate of the entire Cat time course.
Key points
Ca2+ release from intracellular stores affects the cardiac action potential via currents through L-type Ca2+ channels (ICa) and the sodium/calcium exchanger (INCX).
Dynamic interactions between released calcium, ICa and INCX occur in a restricted subcellular compartment, close to Ca2+ released sites, where calcium concentration (Cat) cannot be measured.
We used a computational model and experimental data to define this compartment and to provide a theoretical basis for estimating Cat.
We estimated Cat from recordings of ICa and INCX and optical recordings of whole-cell calcium concentration (Cam).
Estimated peak Cat ranged from 6 μm to 25 μm, depending on calcium load. Time to equilibrium between Cat and Cam was ∼350 ms. The Cat values are in the range of ICa and INCX sensitivity to calcium, implying that there is significant effect of Ca2+ in this restricted domain on their kinetics and on the action potential during cell excitation.
Introduction
Ca2+ release flux (Jrel) from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyRs) exerts a complex effect on the cardiac action potential (AP) via Ca2+-dependent inactivation of L-type Ca2+ channels (LCCs) (repolarizing effect) and activation of 3Na+/Ca2+ exchangers (NCXs) (depolarizing effect). It has been shown that abnormality of Jrel due to genetic defects in RyR, heart failure, or excessive phosphorylation during exercise and stress could cause abnormal cellular electrical activity and arrhythmic behaviour. For example (1) beat-to-beat alternans in Jrel is associated with arrhythmogenic AP alternans, with amplitude that depends on the balance between Ca2+ flux via LCCs (ICa) and NCX (INCX); this process is modulated by the Ca2+/calmodulin-dependent protein kinase II (CAMKII) (Sato et al. 2006, Livshitz & Rudy, 2007); (2) ICa recovery from Ca2+-dependent inactivation following Jrel is implicated in the development of early afterdepolarizations (EADs) (Antoons et al. 2007); and (3) diastolic Jrel underlies the formation of delayed afterdepolarizations (DADs) and triggered activity at high levels of β-adrenergic tone (e.g. Belevych et al. 2011; Priori & Chen, 2011).
The mechanism of coupling between Jrel, INCX, ICa and the AP is not completely understood, even under normal physiological conditions. Consensus exists that the interactions are mediated by the free Ca2+ sensed by these channels, and are modulated by the CaMKII signalling pathway. However, optical recordings of the whole-cell (global) Ca2+ transient (Cam) show dynamics which correlate poorly with the much faster kinetics of the ionic currents (ICa and INCX). This implies that these channels are experiencing different local Ca2+ transients (Cat) that cannot be measured with current experimental techniques with sufficient temporal and spatial resolution. Consequently, combined experimental/computational methods were developed for estimating local Ca2+ from measurements of whole-cell Ca2+ using fluorescence imaging, together with recorded INCX (Egan et al. 1989; Trafford et al. 1995; Weber et al. 2001) or ICa (Sipido et al. 1995; Sham, 1997; Zahradnikova et al. 2004; Antoons et al. 2007) as local Ca2+ sensors (reporters). Early work used Cam and mathematical modelling to calculate Jrel in skeletal (Melzer et al. 1987) and cardiac (Sipido & Wier, 1991) fibres. These indirect (inverse) methods for estimating local Ca2+ from INCX and ICa recordings rely heavily on the mathematical models employed and the quality of recorded INCX and ICa data.
Due to tight coupling between Ca2+ cycling and membrane voltage (V) in cardiac myocytes, it is difficult to control INCX and ICa independently and separate Ca2+-dependent from V-dependent processes in experiments. For example, it is extremely challenging to separate effects of Ca2+-dependent (CDI) and voltage-dependent (VDI) inactivation of ICa, or effects of Ca2+-dependent and voltage-dependent modulation of INCX. In addition, modulation of ICa by Ca2+ is due to both CDI and changes in the driving force. Moreover, there is strong interaction (mediated by Ca2+ changes) between ICa and INCX during an AP and therefore caution should be taken in extrapolating estimates of local Ca2+ from each individual current to the physiological environment of the myocyte during excitation. Ca2+ fluxes have been measured under experimental conditions (voltage clamp, dialysed whole cell, cocktails of blockers, non-physiological ion concentrations, artificial ion buffers, non-physiological levels of protein phosphorylation, etc.) which differ from those in intact functioning cardiac cells. Additional complexity arises from the fact that different cardiac cells (e.g. ventricular, atrial, purkinje) exhibit distinct structural organization, which is also age and species dependent (Bootman et al. 2006; Soeller et al. 2007, 2009; Li & Rudy, 2011).
In ventricular myocytes, RyRs, NCXs and LCCs are distributed non-uniformly (Fig. 1A) in the SR membrane (RyRs) and sarcolemma (NCXs and LCCs) (Pasek et al. 2008; Jayasinghe et al. 2009; Smyrniasa et al. 2010). Not all LCCs and only a fraction of NCXs sense the same Ca2+ as RyRs (Adachi-Akahane et al. 1996), underscoring the importance of considering subcellular compartments for Ca2+ dynamics in mathematical models of the cell. Experimental data and model simulations suggest that Ca2+ flux per unit time through LCCs and RyRs is several orders of magnitude greater than the diffusion flux towards the core of the cell, resulting in local subcellular Ca2+ gradients (Allbritton et al. 1992). In addition, local Ca2+ buffers contribute to a relatively slow (tens of milliseconds) dissipation of these Ca2+ gradients (Naraghi & Neher, 1997; Higgins et al. 2007).
Figure 1. Compartmental model of Ca2+ cycling, based on structural organization of a ventricular myocyte.
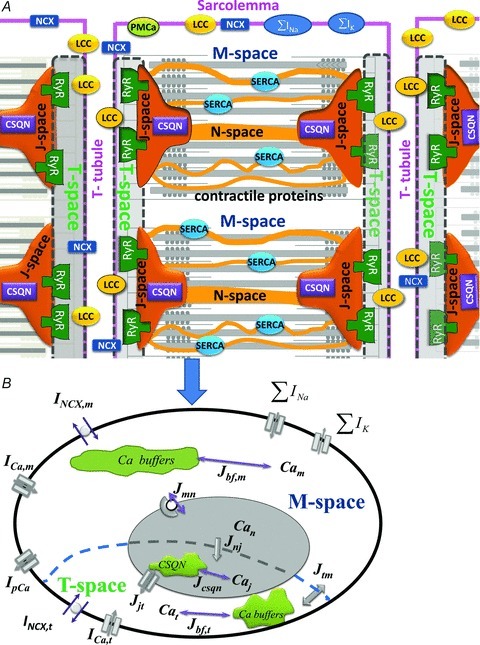
A, schematic diagram of the structural organization of a ventricular myocyte. Sarcolemmal Ca2+-ATPase (PMCa), L-type Ca2+ channels (LCC), 3Na+/Ca2+ exchanger (NCX) and Ca2+ binding proteins (buffers) are expressed throughout the sarcolemma. T-Tubules run radially and flank each sarcomere. Ca2+ release channels (RyRs) are expressed in the junctional portion of the SR membrane which faces the T-tubular portion of the sarcolemma and bounds the calsequestrin (CSQN) containing J-spaces. The junctional portion of the SR membrane and corresponding T-tubular portion of the sarcolemma form the T-spaces (grey spaces enclosed by dashed boundaries). During uniform activation of all RyR, identical Ca2+ distribution can be assumed in all J-spaces and in all T-spaces. Given the structural periodicity of the myocyte, a cell average Ca2+ concentration in J-space (Caj) and T-space (Cat) can be defined. SR Ca2+-ATPase (SERCA) is expressed in the network portion of the SR membrane which encloses the N-space. M-space is separated by the network portion of the SR membrane from the N-space and contains Ca2+ sensitive contractile (troponin) and regulatory (calmodulin) proteins. ∑IK and ∑INa are sum of trans-sarcolemmal K+ and Na+ fluxes, respectively. B, compartmental model of Ca2+ cycling based on the structural organization in panel A, when synchronized activation of RyR generates a uniform Ca2+ flux (Jjt) from J-space to T-space. The transsarcolemmal Ca2+ fluxes, which include currents via LCC (ICa) and NCX (INCX), are partitioned between T-space and M-space. The effects of the contractile and regulatory proteins on Ca2+ dynamics is accounted for by buffering fluxes in the M-space (Jbf,m), T-space (Jbf,t), and J-space (Jcsqn). Ca2+ flux between M-space and N-space (Jmn) is carried by SERCA. Transfer of Ca2+ between T-space and M-space (Jtm) is proportional to the difference between Cat and Cam. Similarly, Jnj is proportional to the difference between Can and Caj. Additional symbols are defined in the text. Computer code is available on the website http://rudylab.wustl.edu/research/cell/lrd2.htm.
Unfortunately, using numerical models of the reaction-diffusion system in the realistic, detailed geometry of the cell requires precise knowledge of the intracellular structure and of Ca2+ diffusion properties in this structure. It involves many parameters that cannot be constrained precisely and is computationally very demanding (e.g. Chen-Izu et al. 2006; Soeller et al. 2009).
The representation of spatial organization in a compartmental model is a simplification that makes computation possible (Atkins, 1969). It has been used extensively in modelling calcium cycling in cardiac myocytes (Standen & Stanfield, 1982; Adler et al. 1985; Luo & Rudy, 1994). Here, we use this approach to better define functional cellular compartments for Ca2+ interactions and to provide a theoretical basis for estimation of dynamic Ca2+ concentrations in these compartments. We hypothesize that under normal physiological conditions (i.e. uniform whole cell Jrel), the interaction between Jrel, ICa and INCX can be formulated, to a very good approximation, as occurring in four functional compartments of Ca2+ distribution, two in the myoplasm and two in the SR. One myoplasmic compartment (denoted T-space) is associated structurally with the RyRs and enclosed by the tubular portion of the sarcolemma and the junctional portion of the SR membrane (enclosed by the dashed curve in Fig. 1A). The other myoplasmic compartment (M-space) encompasses the rest of the myoplasm, enclosed by the non-tubular sarcolemma and non-junctional SR membrane. LCCs and NCXs are present in the sarcolemma of both T-space and M-space, but with different density in each compartment. We denote free Ca2+ concentrations in T and M spaces Cat and Cam, respectively. Similarly, the SR is also divided into two compartments of Ca2+ distribution. One compartment (denoted J-space) is associated structurally with the RyRs and the junctional portion of the SR membrane. The other compartment (N-space) encompasses the rest of SR (network SR) as we have done previously (Luo & Rudy, 1994). We employ mathematical modelling together with experimental measurements to explore the interactions of Jrel, ICa and INCX in the T-space and to provide a theoretical basis for estimating the Cat dynamic properties in this functional space, where Jrel, ICa and INCX interact.
The choice of compartments was dictated by the assumptions of a uniform Ca2+ distribution in each compartment and a spatially uniform Ca2+ flux between communicating compartments. It was guided by structural and functional considerations and differs from ‘subspace’ and ‘bulk myoplasm’ used in many previous compartmental models. In these models, ‘subspace’ has been used to define a continuous subsarcolemmal space in the entire cell. In certain models, it was subdivided to contain a ‘dyadic’ space (Shannon et al. 2004; Higgins et al. 2007; Mahajan et al. 2008); the dyadic space communicated with the subsarcolemmal subspace, which in turn communicated with the bulk myoplasm. This implies that dyads are distributed uniformly beneath the sarcolemma, and all NCXs are affected identically by Jrel via a Ca2+ diffusion flux from the dyadic space. Based on structural and functional properties, including the non-uniform distribution and partition of LCCs and NCXs between T-tubular and non-T-tubular portions of the sarcolemma, and of RyRs in the SR membrane, Ca2+ distribution cannot be assumed uniform in a subsarcolemmal ‘subspace’ compartment. Rather, RyRs interact with LCCs and NCXs in a functional compartment near the RyR, which we label T-space. Figure 1 depicts two populations of LCCs and NCXs, one interacting with RyRs in the T-space (via a common pool of Ca2+, Cat), and the other communicating directly with the M-space. Based on experimental data, we estimate the fraction of NCXs that are coupled functionally to RyRs via Cat.
Methods
Symbols and definitions are given in Table 1.
Table 1.
Symbols and definitions
| V | Transmembrane potential | mV |
| Cat | Free Ca2+ concentration in T-space | mm |
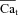 |
Inverse estimated Ca t | |
| Cam | Free Ca2+ concentration in M-space | |
| Caj | Free Ca2+ concentration in junctional SR (J-space) | mm |
| Can | Free Ca2+ concentration in network SR (N-space) | mm |
| Cacmdn | Ca2+ bound to calmodulin in M-space | mm |
| Cacsqn | Ca2+ bound to calsequestrine in J-space | mm |
| Catrpn | Ca2+ bound to troponin in M-space | mm |
| Cab,f | Ca2+ bound to fast buffer in T-space | mm |
| Cab,s | Ca2+ bound to slow buffer in T-space | mm |
| JCa,m | Trans-sarcolemmal Ca2+ flux in M-space | mm ms−1 |
| JCa,t | Trans-sarcolemmal Ca2+ flux in T-space | mm ms−1 |
| Jnj | Ca2+ between N-space to J-space | mm ms−1 s |
| τtr | Time constant of Jnj | ms |
| Jtm | Ca2+ flux between T-space and M-space | mm ms−1 s |
| τdiff | Time constant of Jtm | ms |
| Jmn | Ca2+ flux between M-space and N-space | mm ms−1 |
| AP | Action potential | |
| ICa | L-type Ca2+ current | μA μF−1 |
| fCa | Ca2+-dependent inactivation gate of ICa | |
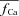 |
Inverse estimated fCa | |
| β | Fraction of NCX in T-space | |
| INCX | Na+-Ca2+ exchanger current | μA μF−1 |
| NCXmax | Maximal turnover rate of INCX | μA μF−1 |
| Kncx | INCX affinity to Ca2+ | mm |
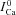 |
ICa in the absence of Jrel | μA μF−1 |
ΔICa=ICa–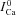
|
Difference current (presence-absence of Jrel) | |
| tmax | Time of maximal ΔICa | ms |
| IpCa | Ca2+ current via PMCA | μA μF−1 |
| ICab | Background Ca2+ current | μA μF−1 |
| Jrel | Ca2+ flux via RyRs | mm ms−1 |
| ∑INa | Sum of transsarcolemmal Na+ fluxes | mm ms−1 |
| ∑IK | Sum of transsarcolemmal K+ fluxes | mm ms−1 |
| JSERCA | Ca2+ flux via SERCA | mm ms−1 |
| Jleak | Ca2+ leak flux from J-space | mm ms−1 |
| Jb,x | Ca2+ flux from and to Ca2+ buffers ‘x’ | mm ms−1 |
| v,x | Volume of compartment ‘x’ | μl |
| Cm,Ct | M-space and T-space capacitances of sarcolemma, μF | |
| F | Faraday constant, 96,487 C mol−1 | |
| R | Gas constant, 8314 J mmol−1 K−1 | |
| T | Temperature, 310 K | |
| Nao | External Na+ concentration | |
| Cao | External Ca2+ concentration |
Ethical approval
All experiments were conducted at the University of Leuven, Belgium, according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (USA). Experimental protocols were approved by the ethical committee of the University of Leuven. The current study conforms to the ethical standards set by The Journal of Physiology (Drummond, 2009). Killing of the pigs was done under full anaesthesia (premedication with tiletamine and zolazepam, 4 mg kg−1 i.m. and xylazine 0.25 mg kg−1 i.m., maintenance anaesthesia with propofol i.v., 7 mg kg−1 h−1); pigs were intubated and ventilated with a 1:1 oxygen–air mixture. After an additional bolus of pentobarbital (100 mg kg−1 i.m.), hearts were excised quickly and placed in oxygenated Tyrode solution.
Experimental considerations
The experiments were conducted in porcine ventricular myocytes and described in detail in Acsai et al. (2011). Briefly, experiments were designed to allow separate measurements of ICa and INCX as a function of Jrel, which was controlled by regulation of SR Ca2+ load using caffeine and voltage-clamp protocols. Whole-cell Ca2+ was measured optically. The size of the T-space compartment is orders of magnitude smaller than that of the M-space. Therefore, contribution from Cat to the optically measured Ca2+ signal can be regarded as negligible and the cell-average Ca2+ signal reflects Cam. The experimental protocols were conducted under voltage clamp conditions, with all ion concentrations, except Ca2+, maintained constant. In addition, we utilized a depolarizing step to +10 mV which ensures maximal spatially uniform activation of Jrel to reflect normal conditions in non-failing myocytes (see Fig. 2A in Acsai et al. 2011). In addition, large concentrations of caffeine were used (10 mm) to ensure spatial uniformity of caffeine-evoked Jrel. Also, the state of the cell (SR Ca2+ load, depolarizing V pulses) in both the ICa and INCX experiments was identical to yield identical estimations of Cat. Experimental recordings of ICa, Cam and INCX were used as an input to the model for estimating Cat; the mathematical formulation and simulation procedures are described below.
Figure 2. Cam and ICa inactivation increase with increasing SR load.
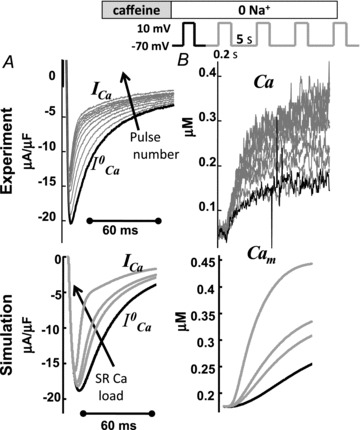
A, superimposed traces of ICa evoked by depolarizing pulses to 10 mV for 200 ms (inset on top show the experimental protocol). B, corresponding traces of the global Ca2+ transient (Cam). Experimental recordings are shown at the top, model simulations at the bottom. The first trace of ICa and of Cam after caffeine application (i.e. for depleted SR) is depicted in black. Arrows indicate increasing number of depolarizing pulses.
Myocyte isolation
In the current study we used ventricular myocytes isolated from the mid-myocardial layer of the posterior wall of healthy pigs. Animals (n= 12, weight 35–45 kg) were housed and treated according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, USA) and experimental protocols were approved by the in-house ethical committee at the University of Leuven. Killing of the pigs was done under full anaesthesia, hearts were excised quickly and placed in oxygenated Tyrode solution. The circumflex coronary artery was cannulated and the myocardium was perfused with Ca2+-free Tyrode solution and subsequently enzyme solution. Isolated cells were stored at room temperature and used within 12 h. The isolation procedure was as described before (Heinzel et al. 2002).
Chemical solutions
Chemicals were purchased from Sigma, fluorescent probes from Invitrogen, Belgium. Tyrode solution for cell storage contained (in mm) NaCl 130, KCl 5.4, Hepes 11.8, MgCl2 0.5, CaCl2 1.8, and glucose 10, pH 7.40 with NaOH. For recording ICaL and INCX, the extracellular solution contained (in mm): NaCl 130, CsCl 10, Hepes 11.8, MgCl2 0.5, CaCl2 1.8, glucose 10, pH 7.4 adjusted with NaOH. To block the fast Na+ current, 200 μm lidocaine was included in the external solution. In order to increase the amplitude of the ICaL and facilitate study of Ca2+-dependent inactivation and recovery by increasing SR Ca2+ content, 5 μm forskolin was added to the external solution. The pipette solution contained (in mm): caesium aspartate 120, TEACl 10, NaCl 5, Hepes 10, MgCl2 0.5, MgATP 5, pH 7.2 adjusted with CsOH with 50 μm K5Fluo-3 was added as a fluorescent Ca2+ indicator for measurement of the global cytosolic Ca2+ concentration. In the experiments carried out in Na+ free conditions, the external solution contained N-methyl-d-glucamine 120, TEACl 20, Hepes 11, MgCl2 0.5, CaCl2 1.8, glucose 10, pH 7.4 with HCl.
Cardiac myocytes were placed in a perfusion chamber on an inverted microscope (Nikon Diaphot). The set-up for epifluorescence recording, calibration of the Ca2+-dependent fluorescence and measurement of membrane currents were as described previously (Acsai et al. 2011). Fluorescence signals were recorded from the entire cell and calibrated to [Ca2+] after obtaining Fmax at the end of the experiments. All experiments were performed at 36°C.
Model geometry and calcium compartments
Typical spatial organization of a non-failing ventricular myocyte is shown in Fig. 1A. The cell membrane (sarcolemma) invaginates to form transverse tubules (T-tubule) which run predominantly radially across the muscle fibre. These tubules are situated between the sarcomeres, which consist of a regular array of Ca2+ binding myofilaments, forming the basic unit of the contractile machinery (Adrian, 1983; Bootman et al. 2006). Given the structural periodicity of the myocyte, only two sarcomeres in the radial direction (out of many) are shown. Approximately half of the sarcolemmal surface area is associated with the T-tubular system, where LCCs and NCXs are expressed at higher density. Ca2+-binding calsequestrin (CSQN) and RyRs are expressed in the J-space membrane and lumen, respectively. SR Ca2+-ATPase (SERCA) is expressed in the N-space membrane, in close proximity to the contractile proteins in the myoplasm.
When all RyRs are activated in synchrony to generate Jrel flux from J-spaces to T-spaces, uniform Ca2+ distributions can be assumed in these compartments and represented in the model by average Caj and Cat (Fig. 1A and B).
Compartmental model based on structural organization of a ventricular myocyte
A schematic diagram of the model is shown in Fig. 1B. The Luo–Rudy dynamic (LRd) model (Luo & Rudy, 1994) was used as the template for modelling Ca2+ cycling; it was modified to fit porcine data, as experiments were conducted in porcine myocytes. The LRd model includes three intracellular Ca2+ compartments (myoplasm, network SR and junctional SR). Each compartment is characterized functionally by the bound and free Ca2+ concentrations, and Ca2+ fluxes in and out of the compartment (Hund & Rudy, 2004; Faber et al. 2007). Bound Ca2+ in each compartment is associated with endogenenous Ca2+ buffers. For the purpose of this study (and to better represent the structural organization of a ventricular myocyte), the original myoplasmic Ca2+ compartment of the LRd model was subdivided into two compartments: T-space and M-space (Fig. 1B). Following Winka et al. (1999), we assumed that SR calcium is divided between two functionally discrete pools, one is available for release during normal ICa-triggered Jrel (J-space), while the other is releasable by caffeine application but unavailable for release during normal triggered release (N-space).
Also, note that the T-space is not separated by a membrane from the M-space (nor is J-space from N-space); rather, they are separated functionally by Ca2+ diffusion barriers which result in faster and larger Ca2+ transients in the T-space (J-space) than in the M-space (N-space). Ca2+ diffusion barriers between T-space and M-space and between J-space and N-space (dashed curve, Fig. 1B) are accounted for by diffusion fluxes Jtm and Jnj, respectively.
The mathematical model
Free Ca2+ concentration (mm) in T-space, M-space, N-space and J-space are denoted Cat, Cam, Can and Caj, respectively. Ca2+ efflux from compartment ‘x’ to compartment ‘y’ (Jxy) has units of millimolar of x volume (vx) per ms. Consequently, to ensure Ca2+ conservation, Ca2+ influx into compartment y is normalized by the volume fractions vxy=vx/vy. The balance of fluxes for each compartment in terms of free Ca2+ concentrations is as follows:
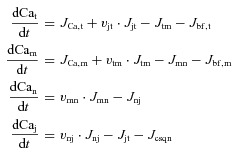 |
(1) |
where subscripts t, m, n, and j indicate the corresponding quantities for T-space, M-space, N-space and J-space, respectively. Trans-sarcolemmal Ca2+ fluxes into M-space and T-space are denoted JCa,m=ψ m(ICa,m+ICab,m+IpCa,m− 2INCX,m) and JCa,t=ψ t(ICa,t+ICab,t+IpCa,t− 2INCX,t), respectively. ψ m=−Cm/(2vmF) and ψ t=−Ct/(2vtF) are Ca2+ current to flux conversion factors for T-space and M-space, respectively. F= 96,485 is the Faraday constant. Membrane capacitance in M-space (Cm) and T-space (Ct) is the same. IpCa is current via PMCA (plasma membrane ATPase) and ICab is background leak current (Livshitz & Rudy, 2009).
M-space and T-space, and N-space and J-space communicate via diffusion fluxes, Jtm= (Cat– Cam)/τdiff (vtm= vt/vm≍ 0.0152) and Jnj= (Can−Caj)/τtr (vnj=vn/vj≍ 11.5), respectively. Ca2+ flux between J-space and T-space (Jjt) has two components, Jrel and Jleak. Formulations of these fluxes are provided in the Appendix eqn (A2).
Ca2+ binding sites (buffers) are defined in terms of Ca2+ fluxes between a buffer and its corresponding compartment (Jbf,m, Jbf,t and Jcsqn in Fig. 1B). In M-space, Jbf,m includes a fast component (calmodulin) and a slow component (troponin). Similarly, Jbf,m in T-space has fast (Jb,f) and slow (Jb,s) Ca2+ anionic binding sites. Jmn is carried by SERCA. Formulations of fluxes are provided in the Appendix eqns (A1)–(A3).
Parametrization of the model
Experimental data were used to fit the model parameters, including the activation and inactivation kinetics of ICa, partition of NCX between the T and M spaces, density of Ca2+ binding sites on the SR membrane and sarcolemma, calmodulin partition between the T and M compartments, and intra-compartmental Ca2+ diffusion rates. Whenever possible, we used different sets of experimental data for the forward model validation and for inverse model estimation. The parameter τtr (200–600 ms) was set to reproduce experimentally measured Cam restitution (Luo & Rudy, 1994). The time constant of Ca2+ flux between the T-space and M-space was fitted to reproduce the experimentally observed delay between peak Cam and the onset of ICa inactivation due to Jrel. The parameter τdiff (0.1–0.2 ms) and T-space buffering parameters were set to reproduce the experimentally measured rate of rise of Cam (4–8 mm ms−1, as in Sipido & Wier (1991)) and its time to peak. Several model parameters, such as compartment volumes and total concentrations of Ca2+ buffers, were constrained by conservation of total Ca2+ in the cell.
The state variables of the model are membrane voltage, free ion concentrations, and ion channels gating variables. Computation of ionic currents (e.g. ICa and INCX) from these state variables constitutes a forward problem. Estimation of Cat (the estimate is denoted 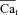 ) from Cam together with ICa or INCX constitutes an inverse problem (Keller, 1976). Note that Cam, ICa or INCX can be either measured experimentally or generated by the model in a forward simulation. In the approach presented here, the forward-calculated Cat (obtained by simulating specific experimental protocols in silico) is used as a standard for comparison and evaluation of
) from Cam together with ICa or INCX constitutes an inverse problem (Keller, 1976). Note that Cam, ICa or INCX can be either measured experimentally or generated by the model in a forward simulation. In the approach presented here, the forward-calculated Cat (obtained by simulating specific experimental protocols in silico) is used as a standard for comparison and evaluation of 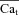 . This allows us to critically validate and evaluate different experimental methods and protocols that have been used to estimate Cat.
. This allows us to critically validate and evaluate different experimental methods and protocols that have been used to estimate Cat.
It should be noted that the sensitivity of the inverse model to variations in parameter values is much greater than that of the forward model, especially at saturating levels of Ca2+.
Current through L-type Ca2+ channels, ICa
LCCs were partitioned between the T-space and M-space, with a large fraction (85–95%) in T-space; total ICa=ICa,m+ ICa,t. We assumed that the intrinsic LCC properties are independent of their location and dropped the subscripts m and t for notational convenience. The L-type channel current is as in Luo & Rudy (1994):
| (2) |
where PCa is the maximal conductance, d is a V-dependent activation gate, f is a V- and Ca2+-dependent inactivation gate, and fCa is a Ca2+-dependent inactivation gate. ECa denotes the driving force for Ca2+ flux through the LCC as a function of trans-sarcolemmal Ca2+ and V gradients, given by the Goldman–Hodgkin–Katz relation (GHK) (Hille, 2001),
| (3) |
where ϕ=F/R/T= 0.0374 (V−1), R= 8314 (J mol−1 K−1) is the universal gas constant, T= 310 is the absolute temperature (K), and γCa and γCao are activity coefficients for free intracellular (Ca) and extracellular (Cao) concentrations, respectively. By definition γCai > γCao if Ca < Cao. γCaCai can be viewed as an effective concentration of Ca2+ (Hille, 2001). ICa inactivation is represented by the product of two time-dependent processes: (1) voltage-dependent inactivation modulated by free Ca2+ ions, f-gate, and (2) a Ca2+-dependent process, fCa-gate.
Steady-state voltage dependence of ICa activation (d-gate)
Formulation of the voltage-dependent activation d-gate was derived using peak values of ICa measured in porcine ventricular myocytes (Stengl et al. 2010). ICa elicited by test voltage pulses was normalized by the driving force ECa (eqn (3)) at each voltage step. A best-fit analytical expression for steady-state d∞ was obtained using the Matlab (The Mathworks, Natick, MA, USA) subroutine ‘lsqcurvefit’ and is as follows:
 |
(4) |
Supplemental Fig. S1A shows simulated (continuous trace) and measured (circles) I–V curves. Supplemental Fig. S1B shows model simulated d∞ (eqn (4), continuous black curve). Ca2+ was set to 0.1 μm in these simulations. The time constant of ICa activation, τd, is fast and was set to its average value of 1 ms (Luo & Rudy, 1994).
Steady-state dependence of f-gate on V and Ca2+
Following Morad & Soldatov (2005) we assumed that ICa inactivation is V-dependent and is not complete in the absence of Ca2+
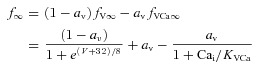 |
(5) |
0 < av < 1 is a factor that defines the contribution of Ca2+ (fVCa∞) and voltage (fV∞) to steady-state f∞. Typically, av was set to 0.25. fV∞ and fVCa∞ are saturating functions of V and Ca2+, respectively. KVCa= 3–5 μm is a half-saturation coefficient. Supplemental Fig. S1B shows measured (circles) and simulated (grey curve) fV∞ (Stengl et al. 2010). Note that av was set to 0 in this simulation.
Time-constant of ICa inactivation (τf) with Ca2+-dependent acceleration
We assumed that ICa inactivation is V dependent and is accelerated in the presence of Ca2+:
| (6) |
τfVca is a Ca2+-dependent acceleration factor; its maximal value equals CaVτ= 55 ms, and half-saturation constant KVτca= 1 μm. The best fit of τf (V-dependent term in eqn (6)) to experimental data is shown in Supplemental Fig. S1C. In the simulations, τf values beyond experimentally recorded voltages (i.e. more than 40 mV and less than −10 mV) were set to 143 ms and 95 ms, respectively. CaVτ was set to zero in the simulation of Fig. S1C.
Ca2+-dependent inactivation gate fCa
Steady-state (fCa∞) and time constant (τfCa) of Ca2+-dependent inactivation were formulated as follows:
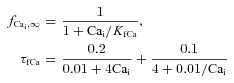 |
(7) |
where KfCa= 4 μm is the half-saturation constant. Parameters in eqns (6) and (7) were fitted to reproduce the time course of ICa with and without Jrel. Supplemental Fig. S1D shows a measured trace of ICa just after caffeine application (i.e. depleted SR Ca2+) (dotted grey trace) and for a Ca2+ loaded SR (dotted black trace). Comparison of the ICa traces shows significant acceleration of inactivation and decrease of peak ICa due to Jrel. In addition, a long lasting effect of Jrel on the ICa time course is observed even after 60 ms. These features are well replicated by the ICa model (see continuous traces in Supplemental Fig. S1D).
Cat estimation using Jrel modulation of ICa
Estimation of free Ca2+ in the T-space requires formulation of its estimate, 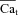 , as a function of Jrel-dependent inactivation (RDI) of ICa. Assuming that Jrel is the major Ca2+ flux that contributes to Cat, the kinetics of ICa inactivation in the presence of different RDI levels could be used to estimate Cat. We assume that RDI is a saturating function of Ca2+, denoted
, as a function of Jrel-dependent inactivation (RDI) of ICa. Assuming that Jrel is the major Ca2+ flux that contributes to Cat, the kinetics of ICa inactivation in the presence of different RDI levels could be used to estimate Cat. We assume that RDI is a saturating function of Ca2+, denoted 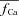 , with a half-saturation constant KΔfca. With this assumption,
, with a half-saturation constant KΔfca. With this assumption, 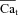 is given by:
is given by:
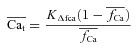 |
(8) |
RDI is a very fast process, with a time constant less than 4 ms (Antoons et al. 2007); thus, an instantaneous relation between  and
and 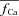 was assumed. Note that the estimation of Cat using eqn (8) does not depend on the protocol used for estimating
was assumed. Note that the estimation of Cat using eqn (8) does not depend on the protocol used for estimating 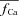 . To estimate
. To estimate  , we utilized the protocol of Acsai et al. 2011, shown in Fig. 2. Briefly, after depletion of SR Ca2+ with caffeine, the cell was repeatedly stimulated with a voltage pulse of duration 200 ms (i.e. typical AP duration, APD), from a holding potential Vh (−70 mV) to a depolarized potential Vd (10 mV). The magnitude and time course of the resulting ICa were influenced by VDI and CDI. However, the difference between the first ICa trace immediately after caffeine application (black trace, denoted
, we utilized the protocol of Acsai et al. 2011, shown in Fig. 2. Briefly, after depletion of SR Ca2+ with caffeine, the cell was repeatedly stimulated with a voltage pulse of duration 200 ms (i.e. typical AP duration, APD), from a holding potential Vh (−70 mV) to a depolarized potential Vd (10 mV). The magnitude and time course of the resulting ICa were influenced by VDI and CDI. However, the difference between the first ICa trace immediately after caffeine application (black trace, denoted 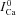 ) and the following traces of ICa (ΔICa=ICa–
) and the following traces of ICa (ΔICa=ICa–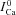 ) reflects ICa inactivation due to Jrel only. Note that due to the long (>4 s) interpulse interval, no accumulation of diastolic Ca2+ was detected, i.e. the initial phases of Cam (<10 ms) were almost identical for all pulses. This implies that most of the Ca2+ that entered through LCCs during the 200 ms depolarizing voltage pulses was sequestered by the SR and did not contribute directly to ICa inactivation. The decline of ICa was therefore attributed to an augmented Jrel due to increased SR Ca2+ load with each consecutive pulse. Thus, in forward model simulations (Fig. 2, bottom panels) we generated the data using different (graded) initial values of SR Ca2+ content (Caj= Can) to modulate Jrel and, in turn, ICa. Note that zero SR Ca2+ content (Can= Caj= 0) corresponds to experimental conditions after caffeine application. Other Caj values were varied from 0.62 to 1.3 mm. As was shown previously (e.g. Shannon et al. 2004) and modelled in this study (eqn (A2)), Jrel is a steep non-linear function of the SR Ca2+ content. Therefore, relatively small changes in Caj result in large changes of Jrel. The time course of Ca2+ in the J-space and N-space is shown in Supplemental Fig. S2.
) reflects ICa inactivation due to Jrel only. Note that due to the long (>4 s) interpulse interval, no accumulation of diastolic Ca2+ was detected, i.e. the initial phases of Cam (<10 ms) were almost identical for all pulses. This implies that most of the Ca2+ that entered through LCCs during the 200 ms depolarizing voltage pulses was sequestered by the SR and did not contribute directly to ICa inactivation. The decline of ICa was therefore attributed to an augmented Jrel due to increased SR Ca2+ load with each consecutive pulse. Thus, in forward model simulations (Fig. 2, bottom panels) we generated the data using different (graded) initial values of SR Ca2+ content (Caj= Can) to modulate Jrel and, in turn, ICa. Note that zero SR Ca2+ content (Can= Caj= 0) corresponds to experimental conditions after caffeine application. Other Caj values were varied from 0.62 to 1.3 mm. As was shown previously (e.g. Shannon et al. 2004) and modelled in this study (eqn (A2)), Jrel is a steep non-linear function of the SR Ca2+ content. Therefore, relatively small changes in Caj result in large changes of Jrel. The time course of Ca2+ in the J-space and N-space is shown in Supplemental Fig. S2.
In the protocol of Fig. 2, any effect due to a small fraction of Ca2+ carried via LCCs in the M-space was eliminated by the 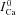 subtraction procedure. Because Jrel is much larger (10 times; see Fig. 3B) than JCa,t, ΔICa is a good surrogate for the time-dependent Cat transient. To convert ΔICa into the dimensionless units of
subtraction procedure. Because Jrel is much larger (10 times; see Fig. 3B) than JCa,t, ΔICa is a good surrogate for the time-dependent Cat transient. To convert ΔICa into the dimensionless units of 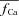 , we normalized it by the value of
, we normalized it by the value of 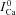 when ΔICa was maximal:
when ΔICa was maximal:
Figure 3. The relative contributions of different Ca2+ fluxes to Cat is SR-load dependent.
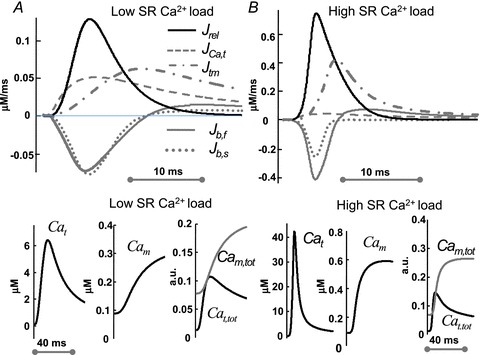
The time course of Ca2+ fluxes in the T-space (top) and Ca2+ redistribution between Cat and Cam (bottom) as a function of Jrel (SR Ca2+ load). Top: Jrel (black trace), trans-sarcolemmal Ca2+ influx, JCa,t (dashed grey trace), Ca2+ efflux from T-space to M-space, Jtm (grey dashed-dot trace) and fast (Jb,f, continuous grey trace) and slow (Jb,t, dotted grey trace) buffering fluxes. Bottom (left to-right): corresponding free Cat, free Cam and total (free and buffered) Ca2+ content in M-space (Cam,tot, grey) and T-space (Cat,tot, black) as fractions of the total cell Ca2+ content. A, at low SR Ca2+ load, Caj= Can= 0.875 (small Jrel). B, at a large SR Ca2+ load, Caj= Can= 1.167 mm (large Jrel). Note that panels A and B use different scales and Jrel is normalized by vjt (eqn (1)).
| (9) |
where 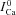 (tΔmax) is the value of
(tΔmax) is the value of 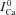 at the time (tΔmax) when the effect of Jrel on the ICa time course is maximal. This normalization procedure eliminates a possible effect of the time onset of Jrel on the relative contribution of Ca2+ and V to ICa inactivation. Previously, ΔICa was normalized by peak
at the time (tΔmax) when the effect of Jrel on the ICa time course is maximal. This normalization procedure eliminates a possible effect of the time onset of Jrel on the relative contribution of Ca2+ and V to ICa inactivation. Previously, ΔICa was normalized by peak  ; we will discuss the benefits of the new normalization procedure in connection with Fig. 7. It is clear from eqn (8) that in order to accurately estimate
; we will discuss the benefits of the new normalization procedure in connection with Fig. 7. It is clear from eqn (8) that in order to accurately estimate 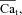 KΔfca must be known with sufficient accuracy. Values of fCa affinity to Ca2+ (which is largely dependent on calmodulin affinity to Ca2+) were reported in the range 2–10 μm (Saucerman & Bers, 2012); we set the value of KΔfca=KfCa= 4 μm.
KΔfca must be known with sufficient accuracy. Values of fCa affinity to Ca2+ (which is largely dependent on calmodulin affinity to Ca2+) were reported in the range 2–10 μm (Saucerman & Bers, 2012); we set the value of KΔfca=KfCa= 4 μm.
Figure 7. Model-based validation of the ΔICa method for estimating Cat.
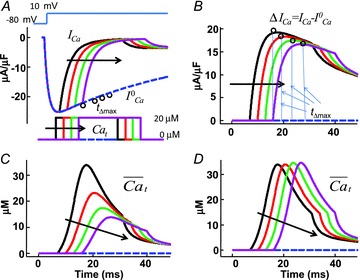
A, model simulated traces of ICa evoked by a depolarizing step to 10 mV (inset on top) in the presence of controlled (calcium clamp) Cat pulses of 20 μm amplitude and 25 ms duration, with progressively delayed onset by 5 ms (arrows; inset on bottom). 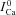 , blue dashed trace, is subjected to V-dependent inactivation only (Cat set to zero). Circles indicate the values of
, blue dashed trace, is subjected to V-dependent inactivation only (Cat set to zero). Circles indicate the values of 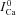 at time tΔmax. Note that all traces coincide prior to application of the Cat pulse. B, difference current, ΔICav=ICa–
at time tΔmax. Note that all traces coincide prior to application of the Cat pulse. B, difference current, ΔICav=ICa–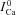 . Circles indicate the maximal values of ΔICa, occurring at time tΔmax. C,
. Circles indicate the maximal values of ΔICa, occurring at time tΔmax. C, 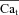 estimated with ΔICa normalized by peak
estimated with ΔICa normalized by peak 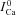 (old method). D,
(old method). D, 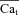 estimated by normalizing each ΔICa trace by the corresponding value of
estimated by normalizing each ΔICa trace by the corresponding value of  at time tΔmax (eqn (8); new method).
at time tΔmax (eqn (8); new method).
Cat estimation using INCX and Cam: forward and inverse relations between INCX, Cam and Cat
Experimental data suggest that INCX is a saturating function of the intracellular Ca2+ concentration, at given values of V and other ion concentrations (Trafford et al. 1995; Ottolia et al. 2009); it is expressed as follows:
| (10) |
where the Ca2+-dissociation constant and maximal turnover rate are denoted Kncx and NCXmax, respectively. Kncx is estimated to be around 3.6 μm (e.g. Weber et al. 2001). This value indicates that NCX responds to Ca2+ in T-space dynamically, without saturation. For forward model simulations, NCXmax and Kncx in eqn (10) were determined by best fit to data simulated using eqn (1) in Weber et al. 2001 (Supplemental Fig. S3A). NCXmax and Kncx depend on V and intracellular sodium concentration (Nai), respectively. Our simulation shows that Kncx increases from 3 to 11 μm as Nai increases from 4 to 16 mm (Supplemental Fig. S3B) and is not sensitive to V in the range from −90 to −50 mV. NCXmax magnitude decreases from 7.5 to 5 μA μF−1 when V increases from −90 to −50 mV (Supplemental Fig. S3C) and is not sensitive to Nai in the range from 4 to 16 mm.
Unlike the original formulation (Weber et al. 2001), eqn (10) can be readily inverted to calculate Ca2+ in terms of INCX, as required for inverse Ca2+ estimation. Assuming that total INCX is the sum of two populations (in T-space and M-space), eqn (10) is represented as follows:
| (11) |
where β is the fraction of INCX in the T-space (sensing Cat). Manipulating eqn (11) leads to the following expression for 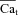 :
:
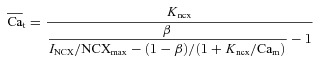 |
(12) |
The value of β can be estimated directly using immunohistochemical methods. Here, the parameter β was determined from the best fit of simulated Cam and INCX to those measured experimentally using the caffeine induced Jrel protocol. NCXmax was calibrated during the phase of Cam when both INCX populations sense the same Ca2+ (i.e. Cat= Cam) and eqn (11) reduces to eqn (10). Time to equilibrium of Cat with Cam was estimated using model simulations.
Cat estimation using INCX and Cam: Cat estimation using INCX and Cam during Jrel induced by a caffeine pulse
A commonly used intervention to study INCX activity is the caffeine pulse experiment, where rapid application of caffeine depletes the SR Ca2+ content and produces a Cam rise that activates INCX (e.g. Trafford et al. 1995). We simulated the application of caffeine by increasing the sensitivity of Jrel to Cat, reducing its refractoriness and decreasing its regulation by SR Ca2+ eqn (A2) in Appendix) (Kong et al. 2008). In addition, SERCA activity was inhibited (Blinks et al. 1972). The time course of caffeine-induced Jrel and consequently of Cam and INCX were different from those induced by voltage and ICa. During caffeine induced Jrel, both the J-space and N-space Ca2+ pools were depleted. Taking advantage of the fully controlled in silico conditions, we utilized a Jrel-clamp protocol to simulate caffeine application; the Jrel waveform was represented by a Gaussian function, Jrel=Aexp[–(t–μ)2/σ], where the parameters A, μ, and σ were fitted to match the INCX and Cam measured in the experiment, while keeping the total amount of Ca2+ released (∫Jreldt) constant and equal to the initial total SR Ca2+ content.
Cat estimation using INCX and Cam: Cat estimation using INCX tail current and Cam
We also simulated an INCX-tail current experiment, which activates Jrel by the CICR mechanism via ICa (Egan et al. 1989). Square depolarizing pulses were applied from holding (Vh) to depolarizing (Vd) potentials to ensure cell-wide Jrel. The INCX tail current, measured a few milliseconds after return to Vh, allows for separation of INCX from all other Na+- and Ca2+-dependent currents, which inactivate very rapidly at negative voltages (Vh) and do not contribute significantly to the total current. Optically assessed Cam was sampled simultaneously. This procedure was repeated with a progressively increased duration of depolarizing pulses (with increments of Δt ms), which were allowed to cover the entire duration of the Ca2+ transient elicited by Jrel. The values of the INCX and Cam were used as input to eqn (12). This method relies on the assumption that Jrel is independent of the depolarizing pulse duration.
Results
Relative contributions of Ca2+ fluxes to Cat are SR-load dependent
Model simulations were conducted to test the assumptions made with regard to contributions of different Ca2+ fluxes to the time course of Cat. The top panels in Fig. 3 show the time course of Ca2+ fluxes in T-space during depolarization to 10 mV for 200 ms, for a small (Fig. 3A) or large (Fig. 3B) Jrel (note that Jrel in Fig. 3 is normalized by vjt; eqn (1)). Ca2+ contribution to Cat from trans-sarcolemmal Ca2+ flux into the T-space, JCa,t (dominated by ICa, dashed grey) is almost 50% that of Jrel (black trace) when Jrel is small (due to a small SR Ca2+ load, Caj= Can= 0.875 mm). In contrast, the contribution from JCa,t is negligible when Jrel is large (large SR Ca2+ load, Caj= Can= 1.167 mm). This verifies that Jrel is a good surrogate for Cat at larger SR Ca2+ loads. The time course of Ca2+ in the J-space and N-space is shown in Supplemental Fig. S2. Note that small changes in Caj result in large changes of Jrel. Jrel lags slightly behind JCa,t, reflecting the typical CICR sequence. Contributions of the fast (Jb,f dotted grey) and slow (Jb,s continuous grey) buffers are also Jrel dependent. The relative contribution of Jb,f increases with increased Jrel. Note that initially Jb,f and Jb,s act as Ca2+ sinks (negative fluxes), decreasing Cat; later, the buffering fluxes reverse direction, increasing Cat. The buffering fluxes reverse direction earlier (by 5 ms) when Jrel is large. The peak of the Ca2+ flux from T-space to M-space (Jtm, dashed-dot grey curve) coincides with the reversal of the buffering fluxes, demonstrating the importance of including Ca2+ buffering sites in the model.
Magnitude and time course of Cat and Cam are different and SR load dependent
The bottom panels in Fig. 3A show, for small Jrel (small SR Ca2+ load), Cat, Cam and the total Ca2+ content (free and bound) in M-space (Cam,tot= Cam+ Catrpn+ Cacmdn, grey) and T-space (Cat,tot= Cat+ Cab,f+ Cab,s, black), normalized by the total cell Ca2+ content. Figure 3B shows similar data, but for a large Jrel (large SR Ca2+ load). Importantly, the results show that even Ca2+ flux via ICa alone has sufficient capacity to increase Cat up to ∼5 μm and thus to induce inactivation of LCCs in T-space. LCCs in M-space are exposed to smaller Ca2+ values and the relative contribution of CDI to their inactivation is smaller.
The time course of Cam,tot and Cat,tot indicates that the total amount of Ca2+ in the T-space can reach similar values to those in the M-space for a brief period of time, during both small and large Jrel, in spite of a large (∼70 fold) difference in the compartments’ volumes. This indicates that buffering capacitance is more important in determining the balance of Ca2+ fluxes during dynamic Ca2+ transients than the compartmental volumes per se.
Partition of NCX between T-space and M-space
To investigate the effect of INCX localization on Cai and to estimate the fraction of NCX in T-space (β), we simulated a caffeine pulse protocol and compared the results to experimental data. The amplitude of the simulated caffeine-induced intracellular calcium transient agrees well with the measurements (compare Fig. 4A right and left panels).
Figure 4. An NCX partition of 25% in T-space and 75% in M-space provides the best fit to experimental data.
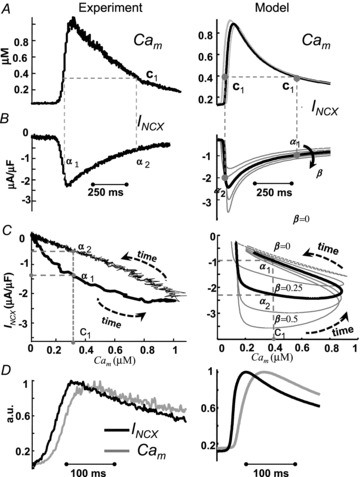
Peak Cam lags by 25 ms relative to peak INCX, reflecting the NCX partition. The fraction of NCX in T-space, β (right panels), estimated from best fit to experimental data (left panels). Experimentally recorded Cam (A, left) and INCX (B, left) evoked by caffeine pulse; V was held at −80 mV. Phase plots of INCX vs. Cai are constructed in C from the data in A and B. It is clear from panel C that INCX is a bi-valued function of Cam; that is, one value of Cam (e.g. c1) is associated with two values on INCX (α1 and α2). In panel C, right, β (fraction of NCX in T-space) is varied from 0 to 0.5. The continuous black trace is for 25% of INCX (β= 0.25) in the T-space, which provides the best fit for the experimental data. Note that for β= 0 (grey dashed trace), INCX is a single-valued function of Cam. D, INCX (black) and Cam (grey), corresponding to the traces in panels A and B, are shown on an expanded time scale. Cam, normalized to its peak value, is superimposed on normalized and inverted INCX. Note that peak INCX precedes peak Cam by about 25 ms in both simulation and experiment.
The left panels in Fig. 4 show experimentally recorded Ca2+ transient (Cam) (Fig. 4A) and INCX (Fig. 4B) after caffeine induced Jrel. A phase plot of INCX vs. Cam is provided in Fig. 4C (same data as in Fig. 4A and Fig. 4B). INCX is a bi-valued function of Cam; that is, one value of Cam (e.g. c1) is associated with two values of INCX (a1 and a2). Figure 4D shows that INCX precedes global Cam by 25 ms. The right panels in Fig. 4 show the corresponding simulated Cam (Fig. 4A) and INCX (Fig. 4B) as a function of INCX fractional population in T-space, β (arrow in Fig. 4B indicates increasing β). The corresponding simulated phase plots of INCX vs. Cam are shown in Fig. 4C (right). As in the experiment, INCX is a bi-valued function of Cam; for example Cam= 0.4 μm (c1) is associated with two values of INCX (α1=−1 μA μF−1 and α2=−2.3 μA μF−1). Note that for β= 0 (all INCX in M-space), INCX is a single-valued function of Cam (dashed grey trace). Thus, the hysteresis and delay between the peaks of Cam and INCX are due to the existence of a population of INCX that senses Cat, which rises faster and reaches higher values than the cell-average Ca2+. The area of the hysteresis loop was chosen as a criterion for determining the best fit to experimental data; it reflects the difference between the ascending (due to increase of Cam) and descending (due to decrease of Cam) limbs of the INCX curves and the relative contribution of Cat to whole-cell INCX. The continuous black curve is for β = 0.25; it provides the best fit for the experimental data and a similar delay (Fig. 4D) to that measured experimentally. Based on this fit, all estimations of Cat were conducted for 25%INCX in T-space (75% in M-space; β= 0.25).
Estimation of Cat using INCX and Cam due to caffeine induced Jrel
Figure 5 shows estimation of Cat (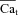 ) during a caffeine pulse experiment similar to that in Fig. 4, but from a different cell. V was held constant throughout at −70 mV. The top panels in Fig. 5 show experimentally recorded Cam (Fig. 5A) and INCX (Fig. 5B) after caffeine induced Jrel. A phase plot of INCX
vs. Cam is provided in Fig. 5C (same data as in Fig. 5A and Fig. 5B). Figure 5D shows measured Cam (grey trace) and inverse-estimated
) during a caffeine pulse experiment similar to that in Fig. 4, but from a different cell. V was held constant throughout at −70 mV. The top panels in Fig. 5 show experimentally recorded Cam (Fig. 5A) and INCX (Fig. 5B) after caffeine induced Jrel. A phase plot of INCX
vs. Cam is provided in Fig. 5C (same data as in Fig. 5A and Fig. 5B). Figure 5D shows measured Cam (grey trace) and inverse-estimated 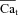 (black trace) using Cam and INCX as input to eqn (12). Na+-sensitive Kncx was set to 3.6 mm and NCXmax was estimated to be 11 μA μF−1. These results predict a peak Cat in the range of 10 μm, with time to equilibrium with global Cam of 250–350 ms. The Cat peak value is an order of magnitude larger than global Cam (1 μm).
(black trace) using Cam and INCX as input to eqn (12). Na+-sensitive Kncx was set to 3.6 mm and NCXmax was estimated to be 11 μA μF−1. These results predict a peak Cat in the range of 10 μm, with time to equilibrium with global Cam of 250–350 ms. The Cat peak value is an order of magnitude larger than global Cam (1 μm).
Figure 5. The time course of Cat can be estimated from caffeine-induced INCX and Cam; it shows different dynamics from Cat generated by CICR.
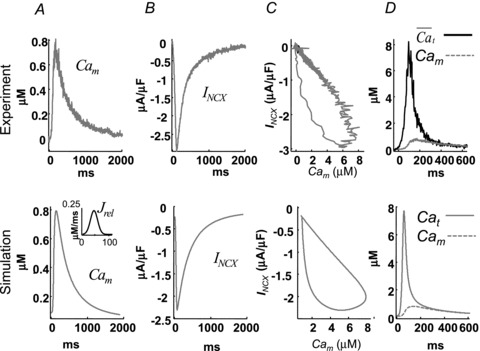
Experimentally recorded Cam (A, top) and INCX (B, top) evoked by a caffeine pulse; V was held at −70 mV. Phase plots of INCX
vs. Cam are constructed in C from the data in A and B. D, estimated Cat, 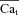 , using eqn (12) with INCX from A and Cam from B as input. For the model-generated data (bottom panels), the Jrel waveform shown in the inset to panel A, bottom, was used as input. Model generated (D, bottom) Cat (black) and Cam (grey) are shown for comparison with those estimated from experimental data (D, top).
, using eqn (12) with INCX from A and Cam from B as input. For the model-generated data (bottom panels), the Jrel waveform shown in the inset to panel A, bottom, was used as input. Model generated (D, bottom) Cat (black) and Cam (grey) are shown for comparison with those estimated from experimental data (D, top).
The bottom panels of Fig. 5 show model-generated data. As explained in Methods, a Jrel-clamp protocol was used (waveform in inset to panel A). The duration of Jrel was set so that the time course of Cat estimated using experimental data matched that of Cat generated by the model. The best fit was achieved with Jrel= 400exp[–(t– 45)2/200]. Model generated Cat (black) and Cam (dashed grey) are shown in Fig. 5D, bottom; both are very similar to experimental Cam and  (Fig. 5D, top). The model predicts that the time course of Cam and INCX during caffeine-pulse experiment is V dependent (Supplemental Fig. S4). Peak Cam (Supplemental Fig. S4A) increases and peak INCX decreases (Supplemental Fig. S4A) with depolarizing voltage. Time to equilibrium between Cat and Cam shows a moderate V-dependence (Supplemental Fig. S4D); it is shorter for negative V. This is important, because to calibrate NCXmax, Cam and Cat should reach equilibrium.
(Fig. 5D, top). The model predicts that the time course of Cam and INCX during caffeine-pulse experiment is V dependent (Supplemental Fig. S4). Peak Cam (Supplemental Fig. S4A) increases and peak INCX decreases (Supplemental Fig. S4A) with depolarizing voltage. Time to equilibrium between Cat and Cam shows a moderate V-dependence (Supplemental Fig. S4D); it is shorter for negative V. This is important, because to calibrate NCXmax, Cam and Cat should reach equilibrium.
Cat estimation using INCX tail current and Cam due to CICR
Figure 6 shows experimental (top) and simulated (bottom) results of the INCX tail current protocol. During the simulations, similar to experimental protocols, V pulses were applied from a holding (−70 mV) to a depolarizing (10 mV) potential. The depolarizing step was interrupted at different time intervals (increments of 10 ms in the experiment), stepping back to −70 mV (see inset in Fig. 6A). In the simulations (bottom panels of Fig. 6), the time increments were decreased to 5 ms in order to increase sampling and thus the quality of the estimation; traces for the first and last V pulses are shown as continuous and dashed black lines, respectively. In Fig. 6A changes of INCX tail current amplitude with pulse duration (circles) reflect changes of Cat sensed by INCX. Corresponding values of Cam were sampled at the same time and are indicated by circles in Fig. 6B; note that the locus of sampled Cam values coincides with the Cam transient during the last (dashed black) pulse. This indicates that the same Jrel (and consequently Cat) is evoked by each pulse, ensuring that the estimation of  is unique. Figure 6C shows estimated
is unique. Figure 6C shows estimated  from experimental (top) and model generated (bottom) INCX and Cam data. In the bottom panel, the actual model-computed Cat traces (grey) are also shown as a gold standard for comparison. Importantly, the INCX-based method is able to recover both the ascending and descending limbs of Cat. However, it is difficult to estimate the value of peak Cat using this method with sufficient accuracy due to the limited number of sampling points, which limits its temporal resolution.
from experimental (top) and model generated (bottom) INCX and Cam data. In the bottom panel, the actual model-computed Cat traces (grey) are also shown as a gold standard for comparison. Importantly, the INCX-based method is able to recover both the ascending and descending limbs of Cat. However, it is difficult to estimate the value of peak Cat using this method with sufficient accuracy due to the limited number of sampling points, which limits its temporal resolution.
Figure 6. The time course of Cat, estimated from the INCX tail current.
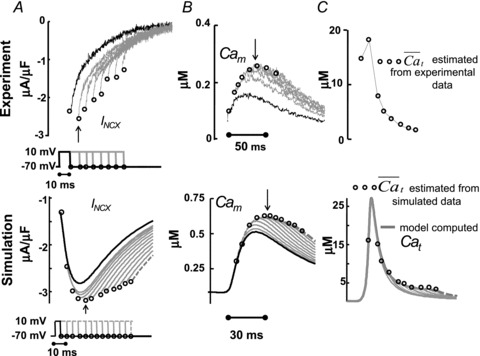
Estimation of free Ca2+ in T-space,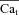 , using experimentally measured (top) and model generated (bottom) INCX and Cam, generated by V pulses as shown in the inset. A, INCX tail current traces. Raw current traces were truncated before the voltage was returned to the holding potential. Simulated traces, corresponding to the first and last V pulses, are shown in continuous and dashed black lines, respectively. B, corresponding optically measured (top) or model simulated (bottom) Cam. Circles in panels A and B indicate peaks of INCX tail currents and corresponding Cam values. Arrows in A and B indicate peak INCX and Cam, respectively. C, estimated Cat,
, using experimentally measured (top) and model generated (bottom) INCX and Cam, generated by V pulses as shown in the inset. A, INCX tail current traces. Raw current traces were truncated before the voltage was returned to the holding potential. Simulated traces, corresponding to the first and last V pulses, are shown in continuous and dashed black lines, respectively. B, corresponding optically measured (top) or model simulated (bottom) Cam. Circles in panels A and B indicate peaks of INCX tail currents and corresponding Cam values. Arrows in A and B indicate peak INCX and Cam, respectively. C, estimated Cat, 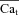 , using eqn (12) with INCX from A and Cam from B as input. For the model-generated data (bottom panel), the actual model computed Cat is shown (grey traces) together with the estimated
, using eqn (12) with INCX from A and Cam from B as input. For the model-generated data (bottom panel), the actual model computed Cat is shown (grey traces) together with the estimated 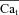 (circles).
(circles).
Note that the first three model simulated INCX tail current traces (bottom of Fig. 6A) are biphasic (increasing then decreasing in magnitude) and reach their peaks after the end of the V pulse. This non-monotonic behaviour is because Cat is still increasing during the first 30 ms of simulation (Fig. 6C). This early phase was not covered by the experimental protocol (top of Fig. 6A). Note that INCX reaches its maximum negative magnitude earlier than peak Cam, reflecting the existence of a T-space population of INCX which is activated by Cat. Cam reaches its peak value at 50 ms, 25 ms later than INCX (arrows in Fig. 6A and B), which corresponds well to the results of the caffeine pulse simulations in Fig. 4D.
Estimation of Cat using ICa inactivation as reporter (ΔICa -based method)
Model-based validation of the ΔICa method
To elucidate the effect of the normalization procedure in eqn (9) on 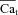 , we simulated V and Cat clamp protocols in Fig. 7. The model cell was depolarized to 10 mV for 200 ms (Fig. 7A top, inset) and Cat was also clamped to 20 μm for 20 ms, with a progressive 5 ms shift of Cat pulse onset (Fig. 7A bottom, inset) as indicated by arrow. Figure 7A shows five ICa traces elicited by the V step, with four Cat pulses following the ICa descending limb. The first ICa trace (
, we simulated V and Cat clamp protocols in Fig. 7. The model cell was depolarized to 10 mV for 200 ms (Fig. 7A top, inset) and Cat was also clamped to 20 μm for 20 ms, with a progressive 5 ms shift of Cat pulse onset (Fig. 7A bottom, inset) as indicated by arrow. Figure 7A shows five ICa traces elicited by the V step, with four Cat pulses following the ICa descending limb. The first ICa trace (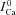 , blue dashed curve) was simulated without applying a Cat pulse, and thus shows inactivation by only a V-dependent mechanism. Figure 7B shows the difference current ΔICa=ICa–
, blue dashed curve) was simulated without applying a Cat pulse, and thus shows inactivation by only a V-dependent mechanism. Figure 7B shows the difference current ΔICa=ICa–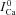 ; note that peak ΔICa is progressively decreased as Cat pulses are progressively delayed relative to the V pulse (circles in Fig. 7B). This indicates that the contribution of Cat to ICa inactivation decreases with the delay, despite the identical waveforms of Cat pulses. Consequently, calibration of ΔICa by peak
; note that peak ΔICa is progressively decreased as Cat pulses are progressively delayed relative to the V pulse (circles in Fig. 7B). This indicates that the contribution of Cat to ICa inactivation decreases with the delay, despite the identical waveforms of Cat pulses. Consequently, calibration of ΔICa by peak 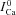 results in the
results in the 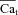 shown in Fig. 7C, which is progressively underestimated as the onset of the Cat pulse is increasingly delayed relative to the onset of the V step. This is because ICa is subjected to V-dependent inactivation immediately after onset of the depolarizing pulse; the longer delay between the V and Cat pulses results in greater ICa inactivation by VDI (not CDI, which is the basis of the ΔICa method), smaller ΔICa, and consequently a smaller estimate of
shown in Fig. 7C, which is progressively underestimated as the onset of the Cat pulse is increasingly delayed relative to the onset of the V step. This is because ICa is subjected to V-dependent inactivation immediately after onset of the depolarizing pulse; the longer delay between the V and Cat pulses results in greater ICa inactivation by VDI (not CDI, which is the basis of the ΔICa method), smaller ΔICa, and consequently a smaller estimate of 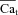 . To correct for this artifact of pulse delay, we calibrated each ΔICa trace by
. To correct for this artifact of pulse delay, we calibrated each ΔICa trace by 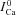 values at the time when ΔICa was maximal (i.e. contribution of Cat to ICa inactivation was maximal). The resulting
values at the time when ΔICa was maximal (i.e. contribution of Cat to ICa inactivation was maximal). The resulting 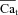 traces are shown in Fig. 7D; note that with this calibration, the peaks of
traces are shown in Fig. 7D; note that with this calibration, the peaks of 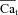 are identical (time-invariant), independent of the Cat pulse time delay.
are identical (time-invariant), independent of the Cat pulse time delay.
Estimation of Cat from experimental data and comparison to model simulation
Figure 8 shows estimation of 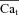 by the ΔICa-based method, using experimental (top panels) and model simulated (bottom panels) data. The experimental data set was from a different cell from the one in Fig. 2; however the V-clamp protocol was identical (inset in Fig. 2). Both experimental and simulated ICa show similar dependence on Jrel. That is, both peak amplitude and residual current decrease as Jrel increases. In Fig. 8A the current trace evoked by the first pulse after SR depletion by caffeine is denoted by
by the ΔICa-based method, using experimental (top panels) and model simulated (bottom panels) data. The experimental data set was from a different cell from the one in Fig. 2; however the V-clamp protocol was identical (inset in Fig. 2). Both experimental and simulated ICa show similar dependence on Jrel. That is, both peak amplitude and residual current decrease as Jrel increases. In Fig. 8A the current trace evoked by the first pulse after SR depletion by caffeine is denoted by 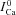 (black trace). Jrel significantly inactivates and decreases ICa immediately (∼2 ms) after Jrel initiation, while maximal inactivation of ICa is reached after 5–10 ms. The model data were generated using graded initial values of Caj= Can from 0 to 1.5 mm.
(black trace). Jrel significantly inactivates and decreases ICa immediately (∼2 ms) after Jrel initiation, while maximal inactivation of ICa is reached after 5–10 ms. The model data were generated using graded initial values of Caj= Can from 0 to 1.5 mm.
Figure 8. Estimation of Cat from Jrel-dependent inactivation of ICa using the ΔICa method.
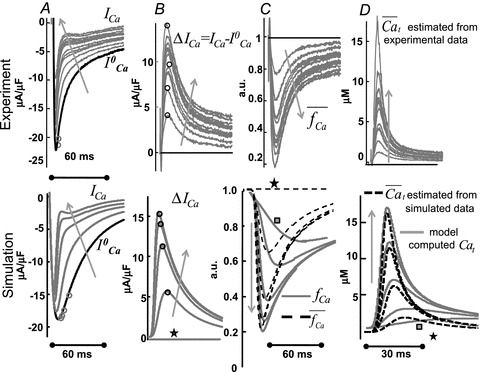
Estimation of free Ca2+ in T-space (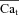 ) using experimentally measured (top) and model simulated (bottom) ICa. A, superimposed traces of ICa evoked by depolarizing pulses to 10 mV (V-clamp protocol is identical to that in the inset of Fig. 2); levels of SR Ca2+ load increase with the number pulses (arrows).
) using experimentally measured (top) and model simulated (bottom) ICa. A, superimposed traces of ICa evoked by depolarizing pulses to 10 mV (V-clamp protocol is identical to that in the inset of Fig. 2); levels of SR Ca2+ load increase with the number pulses (arrows). 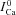 is the current trace evoked by the first pulse after SR depletion by caffeine (black trace). B, difference current, ΔICa=ICa–
is the current trace evoked by the first pulse after SR depletion by caffeine (black trace). B, difference current, ΔICa=ICa–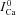 ; the ΔICa trace which corresponds to
; the ΔICa trace which corresponds to 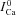 (depleted SR; Jrel= 0) (continuous black) is equal to zero. Circles indicate the maximal values of ΔICa, occurring at time tΔmax. C, estimate of RDI,
(depleted SR; Jrel= 0) (continuous black) is equal to zero. Circles indicate the maximal values of ΔICa, occurring at time tΔmax. C, estimate of RDI, 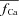 . Each ΔICa trace was normalized by the
. Each ΔICa trace was normalized by the 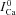 value at the time of maximal ΔICa (tΔmax, indicated by circles in panel A). In the bottom panel, model computed CDI curves, fCa (grey traces), are also shown for comparison.
value at the time of maximal ΔICa (tΔmax, indicated by circles in panel A). In the bottom panel, model computed CDI curves, fCa (grey traces), are also shown for comparison.  and fCa traces which correspond to
and fCa traces which correspond to 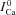 are indicated by a star and a square, respectively. The fCa trace indicated by a square shows contribution to CDI from Ca2+ fluxes other than Jrel. D,
are indicated by a star and a square, respectively. The fCa trace indicated by a square shows contribution to CDI from Ca2+ fluxes other than Jrel. D, 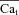 estimated via eqn (8) using
estimated via eqn (8) using 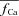 as input. In the bottom panel, model computed Cat (grey traces) are also shown for comparison.
as input. In the bottom panel, model computed Cat (grey traces) are also shown for comparison. 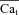 and Cat traces which correspond to
and Cat traces which correspond to  are indicated by a star and a square, respectively. The trace indicated by a square shows contribution to Cat from Ca2+ fluxes other than Jrel (via LCC and NCX). Arrows indicate increasing number of depolarizing pulses (SR load).
are indicated by a star and a square, respectively. The trace indicated by a square shows contribution to Cat from Ca2+ fluxes other than Jrel (via LCC and NCX). Arrows indicate increasing number of depolarizing pulses (SR load).
Figure 8B shows the difference current ΔICa=ICa–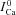 . The traces exhibit a biphasic behaviour, with a fast initial increase reaching a maximum at around 10 ms, followed by a slow decrease which lasts for more than 60 ms. Model-based analysis of these data clarifies that the first phase of ΔICa is dominated by an increase of Cat due to Jrel, while the second phase is dominated by Ca2+ diffusion from T-space to M-space, Jtm.
. The traces exhibit a biphasic behaviour, with a fast initial increase reaching a maximum at around 10 ms, followed by a slow decrease which lasts for more than 60 ms. Model-based analysis of these data clarifies that the first phase of ΔICa is dominated by an increase of Cat due to Jrel, while the second phase is dominated by Ca2+ diffusion from T-space to M-space, Jtm.
Figure 8C shows estimated 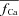 using experimental (top) and simulated (bottom, dashed black) data as input to eqn (9). Circles in Figure 8A and B indicate
using experimental (top) and simulated (bottom, dashed black) data as input to eqn (9). Circles in Figure 8A and B indicate 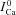 at tΔmax and maximal ΔICa, respectively. Model computed fCa traces (continuous grey) are shown in (8) (top panel is from experimental data; bottom panel from model generated data). In addition, Fig. 8C as a gold standard for comparison. The trace of fCa which corresponds to CDI in the absence of RDI (Jrel= 0) is denoted by a square symbol. Note that the
at tΔmax and maximal ΔICa, respectively. Model computed fCa traces (continuous grey) are shown in (8) (top panel is from experimental data; bottom panel from model generated data). In addition, Fig. 8C as a gold standard for comparison. The trace of fCa which corresponds to CDI in the absence of RDI (Jrel= 0) is denoted by a square symbol. Note that the 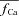 trace which corresponds to
trace which corresponds to 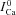 (indicated by a star) is equal to unity, i.e. it predicts correctly the absence of ICa inactivation due to RDI. In contrast, the model computed fCa trace, indicated by a square, is different from unity. Unlike
(indicated by a star) is equal to unity, i.e. it predicts correctly the absence of ICa inactivation due to RDI. In contrast, the model computed fCa trace, indicated by a square, is different from unity. Unlike 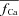 , which is estimated via RDI (due to Jrel only), fCa accounts for CDI due to all Ca2+ fluxes into the T-space. Fluxes other than Jrel (via LCC and NCX) contribute to CDI (compare traces indicated by square and star symbols). Clearly,
, which is estimated via RDI (due to Jrel only), fCa accounts for CDI due to all Ca2+ fluxes into the T-space. Fluxes other than Jrel (via LCC and NCX) contribute to CDI (compare traces indicated by square and star symbols). Clearly, 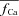 underestimates CDI of ICa because it does not consider Ca2+ fluxes other than Jrel. However, as SR load increases (arrow), Jrel increases and the accuracy of
underestimates CDI of ICa because it does not consider Ca2+ fluxes other than Jrel. However, as SR load increases (arrow), Jrel increases and the accuracy of 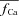 estimation improves, especially during the descending limb of fCa. Figure 8D shows
estimation improves, especially during the descending limb of fCa. Figure 8D shows 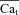 (dashed black traces) estimated using
(dashed black traces) estimated using 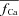 as an input to eqn (8) (top panel is from experimental data; bottom panel from model generated data). In addition, Fig. 8D (bottom) shows the actual model computed Cat (grey traces) for comparison. Note that the
as an input to eqn (8) (top panel is from experimental data; bottom panel from model generated data). In addition, Fig. 8D (bottom) shows the actual model computed Cat (grey traces) for comparison. Note that the 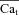 trace which corresponds to
trace which corresponds to 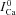 (star) is equal to zero (depleted SR, Jrel= 0), while the corresponding Cat (square) is not (it reaches peak values of 2–3 μm) due to contribution from LCC and reverse-mode NCX. As SR load increases, Jrel increases and the accuracy of Cat estimation (assessed as the ratio of peak Cat to peak
(star) is equal to zero (depleted SR, Jrel= 0), while the corresponding Cat (square) is not (it reaches peak values of 2–3 μm) due to contribution from LCC and reverse-mode NCX. As SR load increases, Jrel increases and the accuracy of Cat estimation (assessed as the ratio of peak Cat to peak 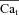 ) improves. This is due to an increased contribution of Jrel-dependent RDI to total CDI. Using normalized peak differences of the corresponding Cat and
) improves. This is due to an increased contribution of Jrel-dependent RDI to total CDI. Using normalized peak differences of the corresponding Cat and 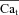 in Fig. 8D (bottom), the estimation error decreases from 47% to 7% as SR load increases.
in Fig. 8D (bottom), the estimation error decreases from 47% to 7% as SR load increases.
The ascending phase of Cat is estimated with a greater accuracy than the descending phase. This is because the method is based on the Jrel component of inactivation and peak Jrel coincides with the steepest ascending portion of Cat. In addition, a large Jrel accelerates inactivation, which makes the instantaneous approximation between Cat and inactivation more accurate. However, the ICa-based method tends to underestimate the descending portion of Cat when the relative contribution of Jrel to total inactivation is smaller.
Discussion
In this study, we verified that the minimal model for interactions between Jrel, ICa and INCX requires two myoplasmic functional compartments for Ca2+ distribution, T-space and M-space. This compartmental organization differs from the compartmentalization used in previous models (Hund & Rudy, 2004; Shannon et al. 2004; Faber et al. 2007; Mahajan et al. 2008) primarily due to a different partition of LCCs, NCXs and Ca2+ buffers. Inclusion of the T-space, which is structurally associated with RyRs, in the compartmental model of Ca2+ cycling allowed us to reproduce in silico the following important experimental results. (1) Hysteresis of INCX dependence on Cam during a caffeine application protocol (Fig. 4C). (2) A delay between peak INCX and peak Cam during a caffeine application protocol (Fig. 4D). (3) A delay between INCX and Cam during CICR (Fig. 6). (4) A rapid ICa inactivation (∼2 ms) due to Jrel, with a graded inactivation magnitude as a function of the SR Ca2+ content (Fig. 8). (5) A delay between ICa inactivation due to Jrel and due to Cam (Fig. 8).
A rapid ICa inactivation due to Jrel indicates a strong functional coupling between LCCs and RyRs in ventricular myocytes, which is not surprising because structurally most of the LCCs and RYRs are located in close proximity to each other. NCXs are usually considered to be expressed outside the Ca2+ microdomain of interaction between LCCs and RyRs. However, using the experimental results from the non-failing ventricular myocytes as an input to the compartmental model, we found that a small but significant (∼25%) fraction of NCXs is functionally coupled to RyRs via a common pool of Ca2+ (Cat).
The study provides a theoretical basis for estimating the dynamic properties of Cat using measured ICa, INCX and Cam as input to the compartmental model. The model was used to generate Cat transients, simulating specific experimental protocols, and the simulated Cat was used as a gold standard for evaluation of inverse-estimated Cat using ICa, Cam and INCX. The simulations confirmed estimations by previous investigators (Egan et al. 1989; Trafford et al. 1995; Weber et al. 2002; Acsai et al. 2011), demonstrating that peak Cat is an order of magnitude larger than the corresponding peak Cam and that Ca2+ gradients between T-space and M-space could persist into the late phase of the AP. Previous procedures to estimate Cat from Ca2+-dependent currents used a reductionist approach by employing each current in isolation. Here we used data from both ICa and INCX, where Jrel was induced by either CICR or by caffeine application. The results revealed that the ICa-based method is more accurate during the rising phase of Cat, while the INCX-based method is more accurate during its slow descending phase. Combined application of both methods provides a close estimate of both phases and a more accurate estimate of the entire Cat than the application of each method alone.
Characteristics of free Ca2+ in T-space (Cat)
The model predicts peak Cat in the range of 6–25 μm; these values are similar to computed Ca2+ transients averaged over many dyadic spaces (15 μm, Bondarenko et al. 2004; Greenstein et al. 2006), but significantly lower than the maximal values computed for a single dyad (>50 μm, Stern, 1992; Langer & Peskoff, 1998; Higgins et al. 2007; Gaur & Rudy, 2011). These results are consistent with our definition of T-space, which assumes a uniform whole-cell Jrel and an average Cat over many dyads. We also found that the time to equilibrium between Cat and Cam was strongly dependent on the experimental conditions, including the amount of Ca2+ released via RyRs (SR Ca2+ load) and the magnitude of the depolarizing V pulses (the driving force for INCX) (Supplemental Fig. S4). An increase of Jrel and/or of V increased the time to equilibrium, which was in the range of 50–350 ms, depending on Jrel and V. The lower bound of these values are consistent with those previously computed in models for CICR (Shannon et al. 2004; Greenstein et al. 2006; Faber et al. 2007). The caffeine pulse protocol shows significant prolongation of Cat duration (Fig. 5).
We found that the time course of Cat is strongly regulated by fast and slow Ca2+ buffers (Fig. 3). We also found that Jrel significantly inactivates and decreases ICa immediately (∼2 ms) after Jrel initiation, while maximal inactivation of ICa due to Jrel is reached after 5–10 ms.
The compartmental model of Ca2+ cycling
The success of inverse Cat estimation depends on the accuracy of the mathematical model used, and how well constrained is the model by experimental data. The model should describe quantitatively the relationship between the time course of Ca2+-dependent currents and the time course of Ca2+ which modulates them. Below we discuss modelling considerations for ICa and INCX.
Accuracy of the ICa model
In the ICa model development, we utilized experimental data from porcine ventricular myocytes. Using the Hodgkin–Huxley formalism, model activation and inactivation gates were fitted to reproduce the recorded ICa I–V curve (Supplemental Fig. S1). Note that the I–V curve is SR Ca2+ load dependent (Sham, 1997). ICa inactivation was assessed by the conventional two-step voltage-clamp protocol (Stengl et al. 2010) (Supplemental Fig. S1). Following (Kass & Sanguinetti, 1984; Lee et al. 1985; Hadley & Hume, 1987) we assumed that ICa inactivation is V dependent and is not complete in the absence of Ca2+. In the model, ICa inactivation was characterized by the product of two time-dependent processes: (1) voltage-dependent inactivation modulated by Ca2+, and (2) a pure free Ca2+-dependent process. Ca2+-dependent parameters were fitted to reproduce the magnitude and time course of ICa and its dependence on Jrel (variable SR Ca2+ load) (Fig. 2). To validate the model in the context of whole cell Ca2+ cycling, we verified that it reproduces experimentally recorded whole cell Ca2+ transients and ICa at different levels of SR Ca2+ load. Model simulated results match simultaneously the experimentally recorded ICa (Fig. 2A) and Cam (Fig. 2B) at different levels of Jrel (SR Ca2+ load).
Accuracy of the INCX model
Bidirectional flux of Ca2+ through NCX depends on V, intracellular concentration (Nai) and extracellular concentration (Nao) of Na+ and extracellular concentration of Ca2+ (Cao), which complicates the analysis. To keep INCX operating in the forward (Ca2+ extrusion) mode, experimental protocols were conducted at constant V close to the resting values (∼−80 mV) and at low Nai (<5 mm). Since Nao and Cao can be controlled in experiments, measured INCX can be regarded as a function of Cam and Cat only. An independent measurement of Cam (whole-cell optical signal) allows for a unique estimate of Cat. The formulation proposed by Weber et al. (2001) was used as a starting point for INCX modelling here. The slow allosteric dependence of INCX on intracellular Ca2+ was eliminated based on the recent study of Reeves & Condrescu (2003). We show that the resulting INCX model (eqn (10)) can be approximated by a Hill equation with a variable intracellular Ca2+ at fixed Nao, Cao, Nai and V, conditions that can be realized experimentally. This formulation (eqn (10)) was inverted to calculate Cat in terms of INCX, as required for inverse  estimation (eqn (12)).
estimation (eqn (12)).
Spatial constraints
In ventricular myocytes, Ca2+ concentration gradients exist between SR dyads, a subsarcolemmal space (including caveolae) and the bulk myoplasm (Trafford et al. 1995; Weber et al. 2002; Higgins et al. 2007). The model used here does not consider the dynamics of Ca2+ in individual dyadic spaces (Shannon et al. 2004; Gaur & Rudy, 2011); instead all dyads are incorporated into the T-space and their Ca2+ concentration is assumed to be in rapid equilibrium. This implies that no Ca2+ gradients exist between dyads. Consistently, the experimental protocols evaluated here rely on the cell-average Ca2+ and are not designed to estimate Ca2+ in a single dyad. Therefore, we implemented protocols for which RyRs are activated uniformly throughout the cell, either by CICR (via LCC flux) or by caffeine-induced-Ca2+ release.
The physical characteristics of T-space
Unlike structurally defined, membrane delimited organelles such as the myoplasm, sarcoplasmic reticulum, nucleus, or mitochondrion, subsarcolemmal compartments have been considered as functional, with little correspondence to the actual structural organization of the cell (e.g. Standen & Stanfield, 1982; Shiferaw et al. 2003; Shannon et al. 2004). Here, T-space and M-space are functional compartments; however, their physical characteristics, i.e. size, localization of Ca2+ sensitive proteins (LCC, NCX, RyR), buffers, and Ca2+ fluxes into and out of the compartments were determined based on the structural organization of the porcine ventricular myocyte and using best fit to experimental data. T-space is enclosed by the T-tubules portion of the sarcolemma and the junctional portion of the SR membrane. M-space is enclosed by the non-tubular sarcolemma and the non-junctional SR membrane. LCCs and NCXs reside with different densities in the sarcolemma of T-space and M-space. Note (Fig. 1A) that a portion of the T-tubular membrane can be outside the T-space and LCCs and NCXs there sense M-space Ca2+.
The effects of the spatial localization of INCX on Cam were investigated with a simulated caffeine induced Jrel (Fig. 4). Experiments show that the time course of global Ca2+ lags (>30 ms) behind INCX. Interpretation of this result is not unique and is model dependent. For example, Weber et al. (2002) and Trafford et al. (1995) showed that the existence of a single diffusion barrier between the subsarcolemmal space (where all INCX were located in their models) and the bulk myoplasm is sufficient to account for this phenomenon; the time constant of Ca2+ diffusion flux between the subsarcolemmal space and global myoplasm was used as a fitted parameter in these studies. We used a different approach in this study; instead of fitting the time constant of Jtm, we used the fraction of INCX in the T-space, β, as a parameter. Best fit to the experimental data was obtained for 25% of INCX in the T-space, 75% of INCX in the M-space.
The quantity of sarcolemmal and SR membrane anionic Ca2+ binding sites (‘buffers’) in the T-space defines the functional ‘size’ of this compartment. Note that, the effective buffering capacity depends strongly on the kinetics of the buffers (dissociation constants) and not only on the number of available binding sites per se. This effective size depends on the membrane surface area that encloses this compartment (which is large) and not on the myoplasmic volume that it encloses (which is small). The effective size of the T-space compartment depends on the Ca2+ sensitivity (half-saturation constants) of Jrel, ICa and INCX; it is estimated to be in the range of 0.5–10 μm (units of concentration; Hofer et al. 1997; Bers, 2001; Ottolia et al. 2009). An increase of sensitivity (i.e. a decrease of half-saturation constants) will increase the effective size of the T-space. In fact the T-space merges with the M-space when the half-saturation constants drop below the average Cam. A similar merger occurs if Jtm is large and time to equilibrium between Cat and Cam is short.
The use of INCX and ICa as Ca2+ sensors requires that their sensitivity to Ca2+ (half-saturation constants, Kd) is in the range of expected values of the local Ca2+ concentration. If the local Ca2+ concentration is too low (≪Kd) its ability to affect these currents is blunted. Similarly, if it is too high (≫Kd) the dependence of these currents on Ca2+ reaches saturation and sensitivity is lost.
Number of compartments in the model
The representation of the structure in Fig. 1A by the compartmental model in Fig. 1B is not unique. Obviously, during the transition from a 3D structure to a compartmental model certain assumptions must be made. Several studies used three-compartment myoplasmic models (dyads, subsarcolemmal space and bulk myoplasm) or two-compartment models (subsarcolemmal space and bulk myoplasm). Inter-compartmental fluxes and partition of major Ca2+-sensitive proteins vary greatly between models. We assumed that regardless of the number of compartments in the model, the total amount of Ca2+ and total trans-sarcolemmal Ca2+ flux should be conserved. This conservation relation greatly decreases the parameter space of the model (Livshitz & Rudy, 2009). The strategy to include only two Ca2+ myoplasmic subcompartments was motivated by the need to ensure that the model is tractable and its parameters are experimentally verifiable. Importantly, it allowed us to formulate an analytic inverse relation between the Ca2+-dependent currents (ICa and INCX) and Cat.
Evaluation of methods for estimating Cat
We evaluated the validity of the experimental methods for Cat estimation by simulating the experimental protocols and comparing the inverse-estimated 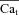 with the model-computed Cat. The results show that under ideal and fully controlled in silico conditions, all methods that use a V-clamp protocol to activate Jrel result in similar estimates of Cat, providing that the SR Ca2+ load and the depolarizing pulses are the same. Not surprisingly, the amplitude and duration of Cat induced by caffeine varies greatly from that induced by depolarization (compare Figs 4–5 and 7–8). Typically, Cat induced by caffeine has a significantly longer duration than its depolarization-induced counterpart.
with the model-computed Cat. The results show that under ideal and fully controlled in silico conditions, all methods that use a V-clamp protocol to activate Jrel result in similar estimates of Cat, providing that the SR Ca2+ load and the depolarizing pulses are the same. Not surprisingly, the amplitude and duration of Cat induced by caffeine varies greatly from that induced by depolarization (compare Figs 4–5 and 7–8). Typically, Cat induced by caffeine has a significantly longer duration than its depolarization-induced counterpart.
Estimation of Cat using tail INCX current and Cam
We show (Fig. 6) that estimation of Cat using the INCX-based method provides a good estimate for the descending phase of Cat. This method is less sensitive to the SR Ca2+ load than the ICa-based method, as it does not require modulation of the SR Ca2+ load. The quality of the estimation increases when the number of sampling points increases (duration of testing pulses decreases), a condition that can be difficult to achieve in experiments. An advantage of the INCX-based method is that pharmacological interventions are not required. However, in this method a greater number of parameters must be fitted. Parameter sensitivity analysis indicates that the accuracy is strongly dependent on the specified fraction of INCX in the T-space. In addition, the model used for the estimation should be re-calibrated for each set of ion concentrations (Nao, Cao, Nai) and test potential V.
Estimation of Cat using ICa
To derive an analytical expression for Cat as a function of ICa, several assumptions were made. Since Jrel provides the dominant contribution to Cat in ventricular myocytes, its contribution to ICa inactivation (denoted RDI) was used as a surrogate for Cat (Sham, 1997). To isolate the Jrel-dependent component from ICa traces, the ICa trace in the absence of Jrel (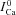 ) was subtracted from those in the presence of Jrel (Acsai et al. 2011). The ΔICa-based method does not depend on the partition of LCCs between the T-space and M-space, because the effects of M-space LCCs is eliminated by the subtraction procedure. We formulated the Ca2+-dependent inactivation of ICa as a function of free Ca2+ concentration assuming that the contributions of Ca2+ fluxes to changes in the Ca2+ concentration are additive (eqn (1)). To convert the resulting difference current, ΔICa (μA μF−1), into the dimensionless units of Ca2+-dependent inactivation, fCa, we calibrated ΔICa by the value of
) was subtracted from those in the presence of Jrel (Acsai et al. 2011). The ΔICa-based method does not depend on the partition of LCCs between the T-space and M-space, because the effects of M-space LCCs is eliminated by the subtraction procedure. We formulated the Ca2+-dependent inactivation of ICa as a function of free Ca2+ concentration assuming that the contributions of Ca2+ fluxes to changes in the Ca2+ concentration are additive (eqn (1)). To convert the resulting difference current, ΔICa (μA μF−1), into the dimensionless units of Ca2+-dependent inactivation, fCa, we calibrated ΔICa by the value of 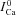 at the time when ΔICa reached its maximum (i.e. contribution of Cat to ICa inactivation was maximal, Fig. 7B). Taking advantage of the completely controlled in silico conditions, we simulated a double (V and Ca2+) clamp protocol (Fig. 7) to study the effect of the delay between depolarization (V step) and CICR (Ca2+ pulse) on the relative dynamics of VDI and CDI of ICa. We found that an increased delay between the onset of the V step and the Ca2+ step (which can happen under pathophysiological condition that impair Jrel; Bito et al. 2008) reduced the relative contribution of CDI to total ICa inactivation, even when the Ca2+ pulse shape and duration were held constant. Thus, calibrating ΔICa by peak
at the time when ΔICa reached its maximum (i.e. contribution of Cat to ICa inactivation was maximal, Fig. 7B). Taking advantage of the completely controlled in silico conditions, we simulated a double (V and Ca2+) clamp protocol (Fig. 7) to study the effect of the delay between depolarization (V step) and CICR (Ca2+ pulse) on the relative dynamics of VDI and CDI of ICa. We found that an increased delay between the onset of the V step and the Ca2+ step (which can happen under pathophysiological condition that impair Jrel; Bito et al. 2008) reduced the relative contribution of CDI to total ICa inactivation, even when the Ca2+ pulse shape and duration were held constant. Thus, calibrating ΔICa by peak 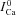 (eqn (9)) and using the resulting
(eqn (9)) and using the resulting 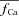 as an input to eqn (8) underestimates
as an input to eqn (8) underestimates 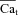 . The proposed new calibration procedure does not suffer from this artifact (compare Fig. 7C and D).
. The proposed new calibration procedure does not suffer from this artifact (compare Fig. 7C and D).
The method presented here is based on steady state (time-independent) approximations of Ca2+-dependent inactivation of ICa and Ca2+-dependent activation of INCX. It was shown that inactivation of ICa induced by Jrel is a fast process (e.g. Antoons et al. 2007; Puglisi et al. 1999), especially at high SR Ca2+ loads (i.e. large Jrel and Cat) and therefore this assumption is well justified. The steady-state approximation allowed us to use an analytic expression for Cat as a function of fCa. During a voltage pulse, the inverse estimation of Cat using the ΔICa-based method provided the best accuracy at high SR Ca2+ load, when ICa is sufficiently small relative to Jrel and does not contribute substantially to Cat, i.e. when the gain of CICR (i.e. Jrel/JCa,t ratio) is high, which is true under normal physiological conditions.
With the ICa-based method, the ascending phase of Cat is estimated with a greater accuracy than the descending phase (Fig. 8D) (the opposite is true for the INCX-based method). This is an expected result, because this method is based on the Jrel-component of inactivation and peak Jrel coincides with the steepest ascending portion of Cat. However, during the descending portion of Cat the contribution of Jrel to inactivation is decreased, resulting in a less accurate estimate. Therefore, combination of the ICa and INCX-based methods provides an estimate for the entire Cat time course.
New insights and potential future applications of the model
Beyond estimation of Cat, the compartmental model presented here provides a conceptual framework for studying the interactions between dynamic Ca2+, ICa and INCX in the functional microdomain where they interact physiologically (T-space). The functional compartmentalization is different from that in previous models; it does not employ a uniform subsarcolemmal ‘subspace’, but a functional volume near RyR (Acsai et al. 2011), where subpopulations of ICa and INCX (estimated here at 90%ICa and 25%INCX) interact with RyR through a common Ca2+ pool. The compartmentalization and partition are based on an interpretation of experimental data obtained under voltage clamp conditions and controlled SR Ca2+ load. For this compartmentalization, we estimated a peak Cat in the range of 6–25 μm, with time to equilibrium between Cat and Cam of about 350 ms. The Cat values are in the range of LCC and RyR sensitivity to Ca2+, which implies that there is a significant effect of Ca2+ in this domain on their kinetics and, in turn, on the action potential during physiological cell excitation. A natural continuation of the work presented here will be an implementation and application of the model to study cellular excitation and Ca2+ cycling under physiological conditions (i.e. an expansion from the controlled, clamped protocols implemented here) and during disease states associated with various pathologies.
Acknowledgments
This research was supported by the NIH–National Heart, Lung and Blood Institute Grants R01-HL049054-19 and R01-HL033343-27 (to Y.R.). This material is based upon work supported in part by the National Science Foundation under Grant No. CBET-0929633. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation (NSF). The work was also supported in part by the Fondation Leducq Award to the Alliance for Calmodulin Kinase Signalling in Heart Disease (to K.R.S. and Y.R.). Y.R. is the Fred Saigh Distinguished Professor at Washington University. This study was funded by grants from the Belgian Science Policy Program P6/31 and European Union grant Health-F2-2009-241526 EUTrigTreat (to K.R.S.).
Glossary
- AP
action potential
- CDI
Ca2+-dependent inactivation
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- LCC
L-type Ca2+ channel
- NCX
3Na+/Ca2+ exchanger
- PMCA
plasmalemmal Ca2+-ATPase pump
- RDI
Jrel-dependent inactivation of ICa
- RyR
ryanodine receptor
- SERCA
SR Ca2+-ATPase pump
- SR
sarcoplasmic reticulum
- VDI
voltage-dependent inactivation of ICa
Appendix
Units and equations
Physical units are as in the original LRd paper (Luo & Rudy, 1994): time (t) in milliseconds (ms), membrane voltage (V) in millivolts (mV), current through channel X (IX) in microamperes per cell capacitance (μA μF−1), channel conductance (GX) and maximal channel conductance ( ) in millisiemens per microfarad (mS μF−1), intracellular and extracellular ion concentrations (Xi, Xo) in millimoles per liter (mm). Inter-compartmental Ca2+ fluxes are defined in terms of millimoles per liter (of the compartment indicated by the first subscript) per milisecond (mm ms−1). Gating variables are given by their steady state and time constant expressions x∞ and τx (ms), or by forward αx and backward βx rate constants (ms−1) as follows: d x/d t= (x∞−x)/τx=αx(1 −x) −βx
x. Model equations and computer code can be found in the research section of http://rudylab.wustl.edu. The notations ϕ=F/R/T= 0.0374 (V−1) and ψ=Vϕ are used in equations, where R= 8314 (J mol−1 K−1) is the universal gas constant, F= 96,485 is the Faraday constant (C mol−1) and T= 310 is the absolute temperature (K); zCa= 2 is the valence of Ca2+ ions.
) in millisiemens per microfarad (mS μF−1), intracellular and extracellular ion concentrations (Xi, Xo) in millimoles per liter (mm). Inter-compartmental Ca2+ fluxes are defined in terms of millimoles per liter (of the compartment indicated by the first subscript) per milisecond (mm ms−1). Gating variables are given by their steady state and time constant expressions x∞ and τx (ms), or by forward αx and backward βx rate constants (ms−1) as follows: d x/d t= (x∞−x)/τx=αx(1 −x) −βx
x. Model equations and computer code can be found in the research section of http://rudylab.wustl.edu. The notations ϕ=F/R/T= 0.0374 (V−1) and ψ=Vϕ are used in equations, where R= 8314 (J mol−1 K−1) is the universal gas constant, F= 96,485 is the Faraday constant (C mol−1) and T= 310 is the absolute temperature (K); zCa= 2 is the valence of Ca2+ ions.
Geometrical considerations
Following the LRd model structure (Luo & Rudy, 1994), the M-space volume was set to vm= 25 × 10−6μl, leaving 30% of the total cell volume (vcell= 38e–6 μl) inaccessible to ions due to the presence of mitochondria and other organelles. The SR volume (6% of vcell) was subdivided into J-space (vj= 0.138e–6 μl) and N-space (vn= 2.1 × 10−6μl), vt was set conservatively to 0.38 × 10−6μl, 1% of vcell. To ensure Ca2+ conservation, any change in a compartment volume should be accompanied by a change in the total Ca2+ concentration. Thus, a decrease of vt should be accompanied by a corresponding increase in concentration of Ca2+ binding sites (buffers) to preserve the total amount of cell Ca2+.
Due to cell size variability, experimental recordings of ICa and INCX (μA μF−1) were normalized by the total cell capacitance Ccell (μF), which varied from 0.09 to 0.2 μF. To convert Ca2+ currents into units of Ca2+ fluxes, an average value of Ccell= 0.15 μF was assumed, which is very close to the value used in LRd (0.1534 μF). Note that the capacitive areas of the T-tubular and non-tubular portions of the sarcolemma are assumed equal Cm=Ct= 0.075 μF. Extracellular ion concentrations were set to the values in corresponding experiments.
Ca2+ buffering fluxes
The Ca2+ flux to and from a buffer protein X (Jb,x) is formulated as a first order kinetic equation:
| (A1) |
where Cax is bound Ca2+ to buffer x; Cay is free Ca2+ in compartment y. The buffering fluxes in M-space (Jbf,m) include binding–unbinding to troponin and calmodulin Ca2+ binding sites. The buffering flux in T-space involves fast and slow components (Jbf,t=Jb,f+Jb,s). These are composed of sarcolemmal anionic binding sites (Post & Langer, 1992), and Ca2+/calmodulin binding sites to LCC, RyR and CaMKII (Saucerman & Bers, 2012). Kinetics of the fast and slow buffers were adjusted to best fit ICa inactivation. Volumes of functional Ca2+ compartments, their buffering capacity, and Ca2+ fluxes between compartments are interdependent. Therefore, conservation of total cell Ca2+ was imposed as an additional constraint. Calsequestrin (Jcsqn) is located in J-space. Forward ( ) and backward
) and backward  rate constants and total concentration (
rate constants and total concentration ( ) of protein X binding sites are summarized in Table 2.
) of protein X binding sites are summarized in Table 2.
Table 2.
Ca2+ buffers (Bers, 2001)
| Name of buffer | kd=k−/k+ (mm) | k− (1/ms) | k+ (1/mm ms−1) |
 Maximal concentration (mm) Maximal concentration (mm) |
|---|---|---|---|---|
| M-space troponin | 0.5e–3 | 0.02 | 40 | 50e–3 |
| M-space calmodulin | 2.4e–3 | 0.24 | 100 | 70e–3 |
| T-space fast | 0.5e–3 | 0.05 | 100 | 0.7 |
| T-space slow | 10e–3 | 0.1 | 100 | 1.32 |
| J-space calsequestrin | 0.8 | 80 | 100 | 10 |
Ca2+ flux from J-space to T-space (Jjt)
Jjt has two components, Jrel and Jleak. Jleak is an intrinsic RyR Ca2+ leak, which depends on the Ca2+ gradient: Jleak=kleak(Caj–Cat). The Jrel model is based on a previously published model (Livshitz & Rudy, 2007). Following Faber & Rudy (2000), Jrel is activated by the flux of free Ca2+ into the T-space, regardless of its origin (ICa, INCX, Jtm). In addition, to simulate caffeine-induced Jrel, we included luminal (SR) activation of RyR in the model. The differential equation that describes Jrel is of the form:
| (A2) |
Jrel,∞=αrel(Arel+Brel)Drel represents the steady-state value of Jrel; τrel=βτ/(1+Krel,τ/Caj) is the time constant, Krel,τ= 0.0123, βτ= 4.75 is the maximal value of τrel, αrel= 12 βτ is the amplitude coefficient. Jrel,∞ is expressed as a product of the sum of cytosolic (Arel) and luminal (Brel) Ca2+-dependent activation with luminal deactivation (Drel). Arel= dCat/dt when dCat/dt is positive, and Arel= 0 otherwise. Similarly, Brel is not zero only above a certain level of Caj (threshold). This formulation involves logical expressions that introduce singularities which can cause problems for implementation in a problem solving environment such as MATLAB and for numerical analysis. To remove singularity, we follow the method in Livshitz & Rudy (2009) and introduce an auxiliary function λ(x) = 1/[1+exp(–xγ)], where γ is a small (e.g. 1 × 10−8) number. λ(x) is equal to unity for a positive argument and zero otherwise. Formulations of Arel and Brel in terms of λ(x) are Arel= (dCat/dt)·λ(dCat/dt) and Brel=λ (Threshold – Caj). Caj-dependent deactivation was modelled as a steep saturating function of Caj: Drel= 1/[1+(Krel,∞/Caj)h], where Krel,∞= 1 mm is the half-saturation coefficient and h= 9. Application of caffeine was simulated by a gradual decrease of Krel,∞ to zero, and by simultaneous increase of αrel and kleak.
Jmn (bidirectional) flux between the M-space and the N-space
Jmn is described by a phenomenological equation, as proposed in Shannon et al. (2004)
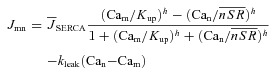 |
(A3) |
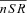 (maximal affinity to SR Ca) = 15 mm,
(maximal affinity to SR Ca) = 15 mm, 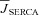 = 0.875 × 10−3 mm ms−1, Kup=0.8 × 10−3 mm, h= 1.7.
= 0.875 × 10−3 mm ms−1, Kup=0.8 × 10−3 mm, h= 1.7.
Author contributions
L.L. participated in data analysis, provided the theoretical basis for the computations, and contributed to manuscript writing. K.A. performed experiments and data analysis and participated in manuscript writing. K.A. developed the computational methods together with L.L. and Y.R. G.A. performed experiments and data analysis and participated in manuscript writing. K.S. contributed to the experimental study design and the final manuscript writing. Y.R. participated in data analysis, provided the theoretical basis for the computations, and participated in manuscript writing. All authors approved the final version of the manuscript. The experimental work was conducted at the laboratory in Leuven, Belgium, with additional analysis performed at the University of Szeged and at Washington University in St Louis.
Supplementary material
Supplemental Figure S1
Supplemental Figure S2
Supplemental Figure S3
Supplemental Figure S4
References
- Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler D, Wong AY, Mahler Y. Model of mechanical alternans in the mammalian myocardium. J Theor Biol. 1985;117:563–577. doi: 10.1016/s0022-5193(85)80238-1. [DOI] [PubMed] [Google Scholar]
- Adrian RH. Electrical properties of striated muscle. In: Peachy L, editor. Handbook of Physiology, section 10, Skeletal Muscle. Bethesda, MD: American Physiological Society; 1983. pp. 275–300. [Google Scholar]
- Acsai K, Antoons G, Livshitz L, Rudy Y, Sipido KR. Assessment of [Ca2+] near ryanodine receptors during sarcoplasmic reticulum Ca2+ release with L-type Ca2+ and Na/Ca exchange currents as [Ca2+] reporter. J Physiol. 2011;589:2569–2583. doi: 10.1113/jphysiol.2010.202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Antoons G, Volders PGA, Stankovicova T, Bito V, Stengl M, Vos MA, Sipido KR. Window Ca2+ current and its modulation by Ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block. J Physiol. 2007;579:147–160. doi: 10.1113/jphysiol.2006.124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GL. Multicompartment Models for Biological Systems. London: Methuen & Co. Ltd; 1969. [Google Scholar]
- Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Györke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation–Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers, Dordrecht; 2001. [Google Scholar]
- Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodeling. Cardiovasc Res. 2008;77:315–324. doi: 10.1093/cvr/cvm063. [DOI] [PubMed] [Google Scholar]
- Blinks JR, Olson CB, Jewell BR, Braveny P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Circ Res. 1972;30:367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Higazi DR, Coombes S, Roderick HL. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci. 2006;119:3915–3925. doi: 10.1242/jcs.03223. [DOI] [PubMed] [Google Scholar]
- Bondarenko VE, Szigeti GP, Bett GCL, Kim S-J, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1378–H1403. doi: 10.1152/ajpheart.00185.2003. [DOI] [PubMed] [Google Scholar]
- Chen-Izu Y, McCulle SL, Ward CW, Soeller C, Allen BM, Rabang C, Cannell MB, Balke CW, Izu LT. Three dimensional distribution of ryanodine receptor clusters in cardiac myocytes. Biophys J. 2006;91:1–13. doi: 10.1529/biophysj.105.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, Noble D, Noble SJ, Powell T, Spindler AJ, Twist VW. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic properties of the cardiac L-type Ca channel and its role in myocyte electrophysiology: A theoretical investigation. Biophys J. 2007;92:1522–1543. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78:2392–2404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein JL, Hinch R, Winslow RL. Mechanisms of excitation-contraction coupling in an integrative model of the cardiac ventricular myocyte. Biophys J. 2006;90:77–91. doi: 10.1529/biophysj.105.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur N, Rudy Y. Multiscale modeling of calcium cycling in cardiac ventricular myocyte: macroscopic consequences of microscopic dyadic function. Biophys J. 2011;100:2904–2912. doi: 10.1016/j.bpj.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RW, Hume JR. An intrinsic potential dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol. 1987;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA, USA: Sinauer Associates, Inc; 2001. [Google Scholar]
- Heinzel FR, Bito V, Volders PG, Antoons G, Mubagwa K, Sipido KR. Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ Res. 2002;91:1023–1030. doi: 10.1161/01.res.0000045940.67060.dd. [DOI] [PubMed] [Google Scholar]
- Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110:3168–3174. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ER, Goel P, Puglisi JL, Bers DM, Cannell M, Sneyd J. Modelling calcium microdomains using homogenisation. J Theor Biol. 2007;247:623–644. doi: 10.1016/j.jtbi.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. J Gen Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer GF, Hohenthanner K, Baumgartner W, Groschner K, Klugbauer N, Hofmann F, Romanin C. Intracellular Ca2+ inactivates L-type Ca2+ channels with a Hill coefficient of approximately 1 and an inhibition constant of approximately 4 microM by reducing channel's open probability. Biophys J. 1997;73:1857–1865. doi: 10.1016/S0006-3495(97)78216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JB. Inverse problems. Am Math Monthly. 1976;83:107–118. [Google Scholar]
- Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SRW. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe ID, Cannell MB, Soeller C. Organization of ryanodine receptors, transverse tubules, and sodium-calcium exchanger in rat myocytes. Biophys J. 2009;97:2664–2673. doi: 10.1016/j.bpj.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer GA, Peskoff A. Calcium concentration and movement in the ventricular cardiac cell during an excitation-contraction cycle. Biophys J. 1998;74:153–174. doi: 10.1016/S0006-3495(98)77776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Marban E, Tsien RW. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Rudy Y. A model of canine purkinje cell electrophysiology and Ca2+ cycling. Circ Res. 2011;109:71–79. doi: 10.1161/CIRCRESAHA.111.246512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshitz LM, Rudy Y. Regulation of Ca and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am J Physiol Heart Circ Physiol. 2007;292:H2854–H2866. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshitz LM, Rudy Y. Uniqueness and stability of action potential models during rest, pacing and conduction using problem solving environment. Biophys J. 2009;97:1265–1276. doi: 10.1016/j.bpj.2009.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–96. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie L-H, Yang M-J, Chen P-S, Restrepo JG, Karma A, Garfinkel A, Qu Z, Weiss JN. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2008;94:392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Rios E, Schneider MF. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys J. 1987;51:849–863. doi: 10.1016/S0006-3495(87)83413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M, Soldatov N. Calcium channel inactivation: Possible role in signal transduction and Ca2+ signaling. Cell Calcium. 2005;38:223–231. doi: 10.1016/j.ceca.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neuroscience. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolia M, Nicoll DA, Philipson KD. Roles of two Ca2+-binding domains in regulation of the cardiac Na+−Ca2+exchanger. J Biol Chem. 2009;284:32735–32741. doi: 10.1074/jbc.M109.055434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M, Brette F, Nelson A, Pearce C, Qaiser A, Christe G, Orchard CH. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Prog Biophys Mol Biol. 2008;96:244–257. doi: 10.1016/j.pbiomolbio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi J, Yuan W, Bassani J, Bers DM. Ca2+ influx through Ca2+ channels in rabbit ventricular myocytes during action potential clamp -Influence of temperature. Circ Res. 1999;85:E7–E16. doi: 10.1161/01.res.85.6.e7. [DOI] [PubMed] [Google Scholar]
- Post JA, Langer GA. Sarcolemmal calcium binding sites in heart. I. Molecular origin in “gas-dissected” sarcolemma. J Membr Biol. 1992;129:49–57. doi: 10.1007/BF00232054. [DOI] [PubMed] [Google Scholar]
- Reeves JP, Condrescu M. Allosteric activation of sodium–calcium exchange activity by calcium: persistence at low calcium concentrations. J Gen Physiol. 2003;122:621–639. doi: 10.1085/jgp.200308915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D, Shiferaw Y, Garfinkel A, Weiss JN, Qu Z, Karma A. Spatially discordant alternans in cardiac tissue: Role of calcium cycling. Circ Res. 2006;99:520–527. doi: 10.1161/01.RES.0000240542.03986.e7. [DOI] [PubMed] [Google Scholar]
- Saucerman, Bers Calmodulin binding proteins provide domains of local Ca2+ signaling in cardiac myocytes. J Mol Cell Cardiol. 2012;52:312–316. doi: 10.1016/j.yjmcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Wier WG. Flux of Ca2+ across the sarcoplasmic guinea-pig cardiac cells during excitation–coupling. J Physiol. 1991;435:605–663. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido KR, Callewaert G, Carmeliet E. Inhibition and rapid recovery of ICa during calcium release from the sarcoplasmic reticulum in guinea-pig ventricular myocytes. Circ Res. 1995;76:102–109. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J. 2003;85:3666–3686. doi: 10.1016/S0006-3495(03)74784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyrniasa I, Maira W, Harzheima D, Walkera SAHL, Bootman MD. Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation-contraction coupling and inotropic stimulation. Cell Calcium. 2010;47:210–223. doi: 10.1016/j.ceca.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Soeller C, Crossman D, Gilbert R, Cannell MB. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc Natl Acad Sci U S A. 2007;104:14958–14963. doi: 10.1073/pnas.0703016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C, Jayasinghe ID, Li P, Holden AV, Cannell MB. Three-dimensional high-resolution imaging of cardiac proteins to construct models of intracellular Ca2+ signalling in rat ventricular myocytes. Exp Physiol. 2009;94:496–508. doi: 10.1113/expphysiol.2008.043976. [DOI] [PubMed] [Google Scholar]
- Sham JSK. Ca2+ release-induced inactivation of Ca2+ current in rat ventricular myocytes: Evidence for local Ca2+ signalling. J Physiol. 1997;500:285–295. doi: 10.1113/jphysiol.1997.sp022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Wang F, Puglisi JL, Weber C, Bers DM. A mathematical treatment of integrated Ca2+ dynamics within the ventricular myocyte. Biophy J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc R Soc Lond B Biol Sci. 1982;217:101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Stengl M, Bartak F, Sykora R, Chvojka J, Benes J, Krouzecky A, Novak I, Sviglerova J, Kuncova J, Matejovic M. Reduced L-type calcium current in ventricular myocytes from pigs with hyperdynamic septic shock. Crit Care Med. 2010;38:579–587. doi: 10.1097/CCM.0b013e3181cb0f61. [DOI] [PubMed] [Google Scholar]
- Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, O’Neill SC, Eisner DA. Comparison of subsarcolemmal and bulk calcium-concentration during spontaneous calcium-release in rat ventricular myocytes. J Physiol. 1995;488:577–586. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Piacentino V, Ginsburg K, Houser S, Bers DM. Na+-Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- Weber C, Ginsburg K, Philipson K, Shannon T, Bers DM. Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes. J Gen Physiol. 2001;117:119–131. doi: 10.1085/jgp.117.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winka LL, Sheng-Yong Wang, Langer GA. Subcellular Ca2+distribution with varying Ca2+load in neonatal cardiac cell culture. Biophys J. 1999;76:2649–2663. doi: 10.1016/S0006-3495(99)77417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradnikova A, Kubalova Z, Pavelkova J, Gyorke S, Zahradnik I. Activation of calcium release assessed by calcium release-induced inactivation of calcium current in rat cardiac myocytes. Am J Physiol Cell Physiol. 2004;286:C330–C341. doi: 10.1152/ajpcell.00272.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


