Abstract
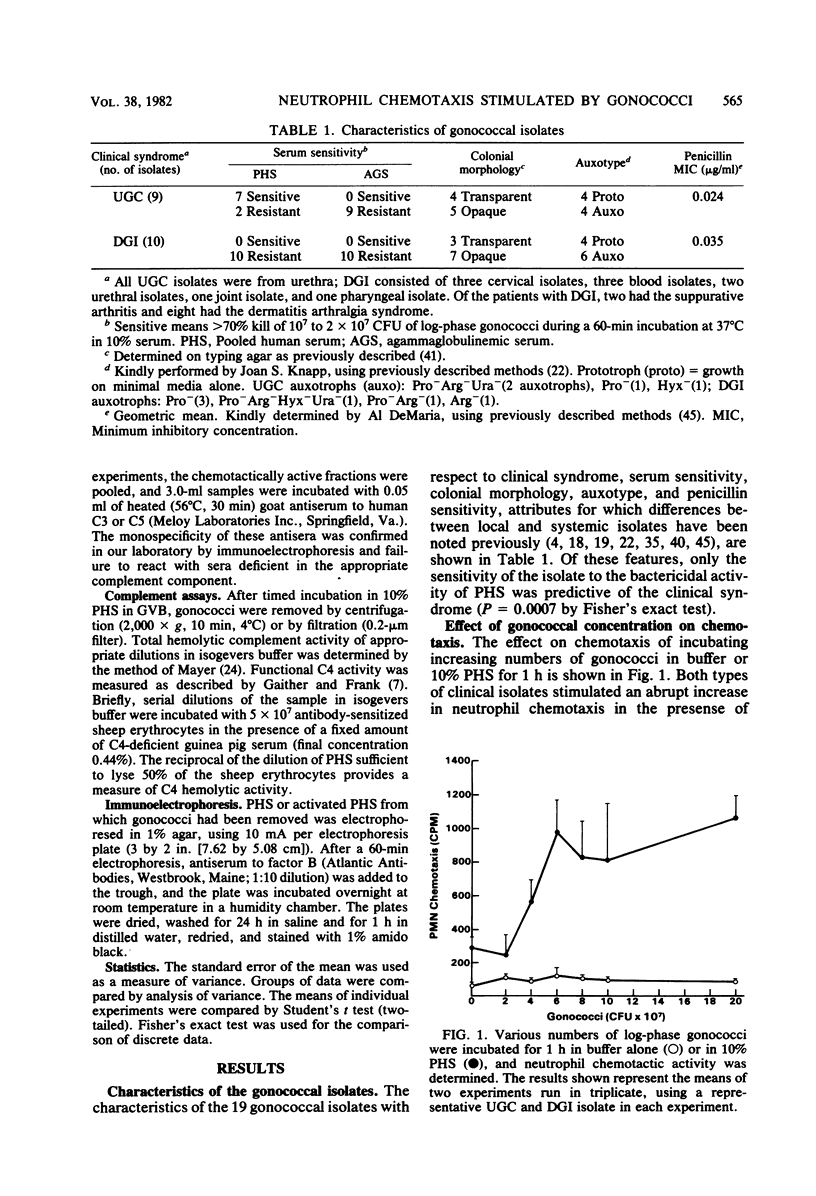
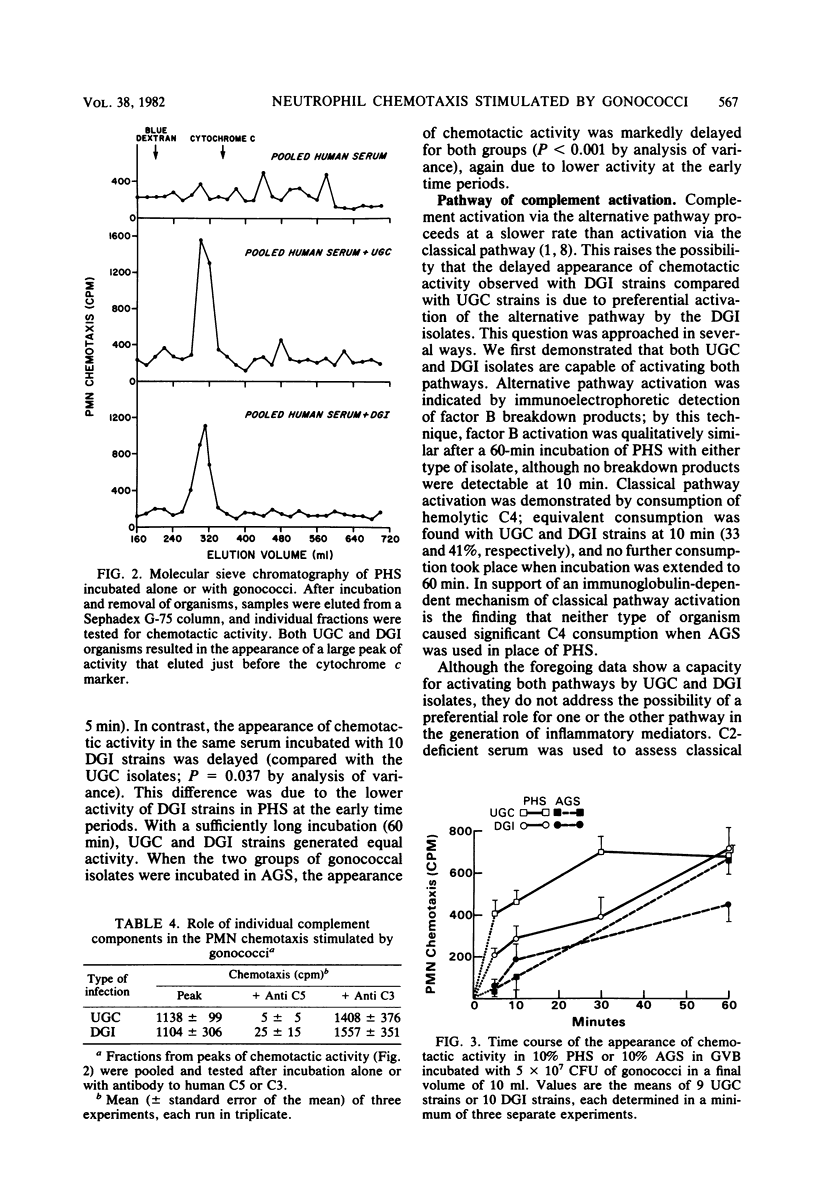
Gonococci isolated from patients with uncomplicated gonorrhea or disseminated infection were examined for their ability to stimulate neutrophil chemotaxis in vitro. A neutrophil chemotactic response was not observed when as many as 10(9) colony-forming units of gonococci were incubated in buffer alone. However, a striking response was observed when 4 x 10(7) colony-forming units were incubated in 10% pooled normal human serum. Activation of complement was required for chemotaxis as demonstrated by complement consumption and failure of chemotactic activity generation in serum treated with heat or EDTA. Chromatography of activated serum demonstrated a single peak of chemotactic activity with an apparent molecular weight of 15,000 and was shown to be due to C5a. Examination of the kinetics of chemotactic factor generation demonstrated that local isolates stimulated a rapid response (about 60% maximal in 5 min), whereas the response to disseminated isolates was delayed (50% maximal in 20 to 30 min). Chemotactic activity generated by both types of isolates was suppressed at early time periods in agammaglobulinemic serum, indicating that immunoglobulins contribute to the generation of activity. Both pathways of complement activation were utilized by the two types of gonococci, but there was preferential dependence on the alternative pathway for disseminated strains and on the classical pathway for local isolates. We suggest that delayed stimulation of complement-dependent neutrophil migration may account in part for the infrequency of genital symptoms and may contribute to the mechanism of dissemination in patients with systemic gonococcal infection.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark R. A., Frank M. M., Kimball H. R. Generation of chemotactic factors in guinea pig serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1973 Apr;1(3):414–426. doi: 10.1016/0090-1229(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. Natural and immune human antibodies reactive with antigens of virulent Neisseria gonorrhoeae: immunoglobulins G, M, And A. J Bacteriol. 1967 Jul;94(1):141–148. doi: 10.1128/jb.94.1.141-148.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C., Knapp J. S., Hale J., Holmes K. K. Asymptomatic gonorrhea in men: caused by gonococci with unique nutritional requirements. Science. 1977 Jun 17;196(4296):1352–1353. doi: 10.1126/science.405742. [DOI] [PubMed] [Google Scholar]
- Draper D. L., James J. F., Brooks G. F., Sweet R. L. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect Immun. 1980 Mar;27(3):882–888. doi: 10.1128/iai.27.3.882-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Lee T. J., Sparling P. F. Penicillin sensitivity and serum resistance are independent attributes of strains of Neisseria gonorrhoeae causing disseminated gonococcal infection. Infect Immun. 1977 Mar;15(3):834–841. doi: 10.1128/iai.15.3.834-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handsfield H. H., Lipman T. O., Harnisch J. P., Tronca E., Holmes K. K. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med. 1974 Jan 17;290(3):117–123. doi: 10.1056/NEJM197401172900301. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Counts G. W., Beaty H. N. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- Holmes K. K., Weisner P. J., Pedersen A. H. The gonococcal arthritis-dermatitis syndrome. Ann Intern Med. 1971 Sep;75(3):470–471. doi: 10.7326/0003-4819-75-3-470. [DOI] [PubMed] [Google Scholar]
- Ingwer I., Petersen B. H., Brooks G. Serum bactericidal action and activation of the classic and alternate complement pathways by Neisseria gonorrhoeae. J Lab Clin Med. 1978 Aug;92(2):211–220. [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar R. L., Drutz D. J. Perihepatitis and hepatitis as complications of experimental endocarditis due to Neisseria gonorrhoeae in the rabbit. J Infect Dis. 1977 Jul;136(1):37–42. doi: 10.1093/infdis/136.1.37. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- LEVINE L., COWAN K. M., OSLER A. G., MAYER M. M. Studies on the role of Ca++ and Mg++ in complement fixation and immune hemolysis. I. Uptake of complement nitrogen by specific precipitates and its inhibition by ethylenediamine tetraacetate. J Immunol. 1953 Nov;71(5):359–366. [PubMed] [Google Scholar]
- Petersen B. H., Lammel C. J., Stites D. P., Brooks G. F. Human seminal plasma inhibition of complement. J Lab Clin Med. 1980 Oct;96(4):582–591. [PubMed] [Google Scholar]
- Polhill R. B., Jr, Newman S. L., Pruitt K. M., Johnston R. B., Jr Kinetic assessment of alternative complement pathway activity in a hemolytic system. II. Influence of antibody on alternative pathway activation. J Immunol. 1978 Jul;121(1):371–376. [PubMed] [Google Scholar]
- Price R. J., Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979 Jul;32(1):61–66. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Goldenberg D. L. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981 Aug;95(2):175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- SCHUMACHER G. F., STRAUSS E. K., WIED G. L. SERUM PROTEINS IN CERVICAL MUCUS. Am J Obstet Gynecol. 1965 Apr 15;91:1035–1049. doi: 10.1016/0002-9378(65)90700-3. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Gonococcal color and opacity variants: virulence for chicken embryos. Infect Immun. 1978 Nov;22(2):359–364. doi: 10.1128/iai.22.2.359-364.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. L., Osler A. G. Dual pathways of complement interaction with guinea pig immunoglobulins. J Immunol. 1971 Nov;107(5):1268–1273. [PubMed] [Google Scholar]
- Scherer R., Braun-Falco O. Alternative pathway complement activation:a possible mechanism inducing skin lesions in benign gonococcal spesis. Br J Dermatol. 1976 Sep;95(3):303–309. doi: 10.1111/j.1365-2133.1976.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Ochs H. D., Buchanan T. M. Immunoglobulin class responsible for gonococcal bactericidal activity of normal human sera. J Immunol. 1979 May;122(5):1771–1779. [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Phillips J. K., Gewurz H., Mergenhagen S. E. A neutrophil chemotatic factor derived from C'5 upon interaction of guinea pig serum with endotoxin. J Immunol. 1969 Sep;103(3):413–422. [PubMed] [Google Scholar]
- Spink W. W., Keefer C. S. STUDIES OF GONOCOCCAL INFECTION. II. THE BACTERIOLYTIC POWER OF THE WHOLE DEFIBRINATED BLOOD OF PATIENTS WITH GONOCOCCAL ARTHRITIS. J Clin Invest. 1937 Mar;16(2):177–183. doi: 10.1172/JCI100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. E., Reynolds G., Short H. B., Thornsberry C., Biddle J. W., Jacobs N. F., Rein M. F., Zaidi A., Young F. E., Shulman J. A. Auxotypes and antibiotic susceptibility patterns of Neisseria gonorrhoeae from disseminated and local infections. Sex Transm Dis. 1978 Oct-Dec;5(4):127–131. doi: 10.1097/00007435-197810000-00001. [DOI] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]



