Abstract
Background/Aims
Alzheimer’s disease (AD) clinical trials enroll two participants: a patient and a study partner. The primary caregiver most often fills the role of study partner and most trial study partners are spousal caregivers.
Methods
AD trial inclusion criteria were applied to baseline data from 5674 probable AD dementia research participants in the National Alzheimer’s Coordinating Center Uniform Data Set. Eligibility was compared among patients with spousal, adult child, and other types of study partners.
Results
Patients with spousal study partners were more frequently eligible than patients with adult child study partners. Compared to patients with spousal study partners, patients with adult child study partners were more frequently ineligible because of age, residence in skilled nursing facility, excluded low scores on the MMSE, excluded high score on Hachinski ischemia scale, and failure to fulfill a minimum number of weekly visits with the study partner.
Conclusions
In this sample, patients with adult child study partners were less likely to qualify for AD clinical trials than were patients with spousal study partners. This may contribute to the lower representation of patients with adult child caregivers in these studies.
Keywords: Alzheimer’s disease, dementia, clinical trials, caregiver, study partner, recruitment
Background/Aims
The clinical trial is essential for developing better therapies for Alzheimer’s disease (AD), but these trials often experience slow recruitment [1]. Among the challenges to timely recruitment is that, at each visit, a single informant, commonly referred to as the participant’s “study partner,” must accompany the patient. The study partner is responsible for the patient’s adherence to study protocol and study medication and provides critical information pertaining to adverse events and changes in the patient’s cognitive and functional abilities.
Caregivers typically fulfill the role of AD trial study partner. At least half of primary AD caregivers are adult children or children-in-law. The Alzheimer’s Association Facts and Figures reported that 48% of all adult caregivers are under age 50 [2] and one report from 2004 suggests that as much as 68% of caregivers provide care for a parent, parent-in-law, or grandparent [3]. Most AD clinical trials, however, enroll predominantly spousal caregiver-patient dyads. In six recent AD Cooperative Study (ADCS) trials (nonsteroidal antiinflammatory drugs rofecoxib and naproxen [4], folate and B vitamin supplementation [5], docosahexaenoic acid (DHA)[6], simvastatin [7], valproate [8], and huperzine [9]), for example, 67% of study partners were spouses while only 26% were daughters, sons, daughters-in-law, or sons-in-law [Grill et al, in preparation].
The discrepancy between the proportion of adult children who are caregivers and the smaller proportion who are study partners suggests that patients with adult child caregivers may be less likely to be eligible for an AD clinical trial. To examine this hypothesis, we developed AD trial inclusion and exclusion criteria adapted from a completed AD trial [6] and then, using data from the National Institute on Aging (NIA)-funded natural history study of AD, compared the proportions of AD patients participating with adult child and spousal study partners who were trial eligible.
Methods
Data sources
The National Alzheimer’s Coordinating Center Uniform Data Set (NACC UDS) is a repository for longitudinal data collected from approximately 30 current or previously NIA-funded AD Centers nationwide (www.alz.washington.edu). The UDS was initiated in 2005. In the current analyses, baseline data collected on or before March 1, 2011 were examined.
Study inclusion criteria
Though the NACC UDS includes individuals with dementia caused by a variety of underlying pathologies, individuals with mild cognitive impairment (MCI), and individuals with no demonstrable cognitive impairment, the current analyses focus on persons with a diagnosis of probable AD at baseline. Patients were categorized as having a spouse, adult child (including a son, daughter, son-in-law, or a daughter-in-law), or person of another relationship as a study partner. Within each study partner category, we calculated the proportion that was eligible for clinical trial criteria. Finally, we examined the criteria that most often resulted in ineligibility.
To examine eligibility for AD clinical trials, we developed a set of trial inclusion criteria, adapted from ADCS DHA trial [6]: ages 50 to 85; diagnosed as having dementia by NINCDS-ADRDA criteria and enrolled in the NACC database as probable AD; score between 14 and 26 on the mini mental status examination (MMSE; [10]); residing in the community or in assisted living (but not in a skilled nursing facility); and having a study partner who had contact with the patient at least three times per week.
Exclusion criteria were recent or active cardiovascular disease (e.g. heart attack, atrial fibrillation); presence of a pacemaker (representing a discrepancy from the DHA trial, since most current AD trials include neuroimaging with magnetic resonance imaging); medical conditions that might otherwise cause or contribute to cognitive impairment, including vitamin B12 deficiency, thyroid disease, alcohol or other substance abuse, Parkinson’s disease, seizures, or traumatic brain injury; history of stroke; Hachinski ischemia scale score greater than 4; and geriatric depression scale (GDS) score greater than 6. For the medical conditions, patients were not excluded if the condition was characterized as remote or inactive. For vitamin B12 and thyroid deficiency, this was assumed to separate patients with a current active condition from those with a previous diagnosis adequately treated. The use of the following concomitant medications was exclusionary: lithium, anti-Parkinsonian medications, MAO-B Inhibitors, tricyclic antidepressants and other anticholinergic drugs (including diphenhydramine), stimulants (i.e. modafinil and methylphenidate), narcotic analgesics, first generation antipsychotics, atypical antipsychotics, and anticonvulsants. Finally, patients were excluded if the local site investigator questioned the reliability of the study partner.
Data analyses
Descriptive statistics (mean, standard deviation, and percentages) were calculated for both the total study population of probable AD patients and their study partners, and the subset of probable AD patients and study partners who were trial eligible. The frequency of each reason for trial ineligibility was also calculated. Groups were compared by Chi square test (X2), Fisher’s exact test (FE), ANOVA, and Kruskal Wallis (KW) test, as appropriate. Pairwise comparisons (X2, FE, Wilcoxon Rank Sum (WRS), and t-tests) were reported for tests where an overall effect was found to be significant. The proportion of patients eligible by trial inclusion criteria were calculated and compared across demographic categories by X2 and FE tests.
Next, we used multivariable logistic regression to examine the relationship between the following independent variables and the odds of being eligible for the clinical trial: study partner type, age, age2, Hachinksi score, GDS, race, ethnicity, and baseline MMSE. Age was treated as a quadratic variable, to account for the use of young and old exclusion criteria. Because of the potential association with age for other variables (e.g. Hachinski ischemia scale), age was also included as a linear variable. Independent variables were evaluated for significance using a Wald test at the 0.05 alpha level.
To account for the large number of comparisons performed, a significance level of 0.001 was applied to all tests of statistical significance.
Human subjects protection
Each patient or an authorized informant provided written informed consent, approved by the local Institutional Review Boards at each participating AD Center.
Results
NACC AD patients
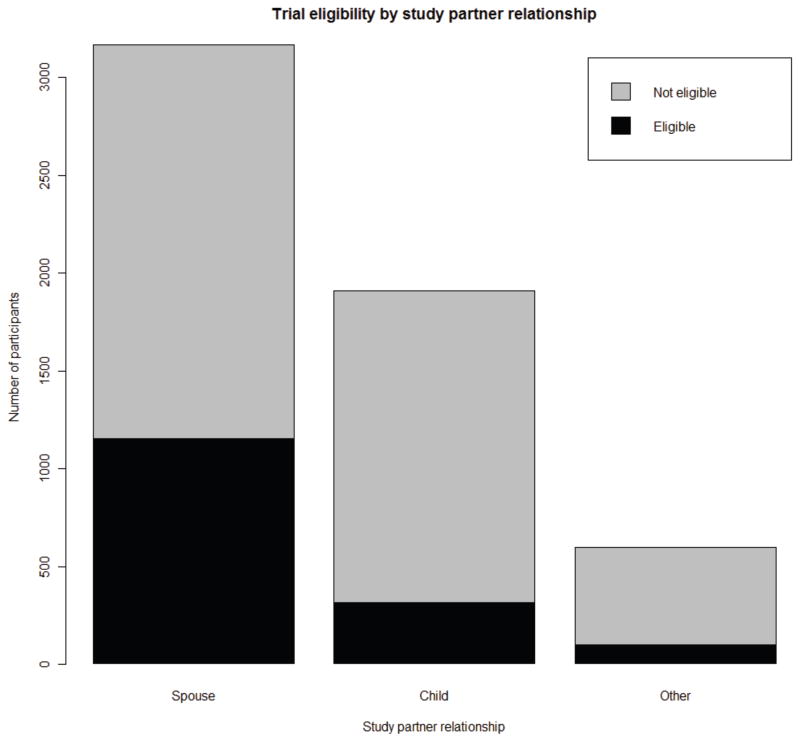
Data from 5,674 AD patients with probable AD enrolled in the NACC natural history study were examined. Demographic characterizations of the population are described in Table 1. A significantly greater proportion of patients enrolled in NACC with spousal study partners than adult child or other types of study partners (Figure, X2, p<0.0001). Compared to patients with adult child study partners, patients with spousal study partners were younger, more educated, more often male and White, and more likely to take AD medications (see Table 1). Patients with adult child study partners were more often female and Black or Hispanic.
Table 1.
Demographic characterizations of the NACC AD dementia subjects.
| Characteristic | Spouse Caregiver | Adult Child Caregiver | Other Caregiver | Overall P value, (statistic) | Spouse vs Adult Child P value, (statistic) | Spouse vs Other P value, (statistic) | Adult Child vs Other P value, (statistic) |
|---|---|---|---|---|---|---|---|
| N (%) | 3167 (56%) | 1909 (34%) | 598(11%) | ||||
| Age, mean (SD) | 74.0(9.3) | 80.2 (8.0) | 76.5 (11.6) | P<0.0001 (ANOVA) | P<0.0001 (t-test) | P<0.0001 (t-test) | P<0.0001 (t-test) |
| Female, n (%) | 1247 (39%) | 1596 (84%) | 458 (77%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) |
| White, n (%) | 2786 (88%) | 1350 (71%) | 391 (65%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0135 (X2) |
| Black, n (%) | 242 (8%) | 384 (20%) | 143 (24%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0467 (X2) |
| American Indian/Alaska Native, n (%) | 18 (.6%) | 21 (1%) | 9 (2%) | P=0.0238 (X2) | - | - | - |
| Native Hawaiian/Pacific Islander, n (%) | 1 (.03%) | 2 (.1%) | 0 (0%) | P=0.6855(FE) | - | - | - |
| Asian, n (%) | 49 (2%) | 28 (1%) | 8 (1%) | P=0.9191 (X2) | - | - | - |
| Hispanic, n (%) | 162 (5%) | 287 (15%) | 83 (14%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.4873 (X2) |
| Education, mean years (SD) | 14.9 (3.4) | 12.3 (3.9) | 13.1 (4.3) | P<0.0001 (KW) | P<0.0001 (WRS) | P<0.0001 (WRS) | P<0.0001 (WRS) |
| Donepezil, n (%) | 1717 (54%) | 881 (46%) | 238 (40%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0064 (X2) |
| Galantamine, n (%) | 333 (11%) | 137 (7%) | 36 (6%) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0007 (X2) | P=0.3302 (X2) |
| Rivastigmine, n (%) | 235 (7%) | 94 (5%) | 26 (4%) | P=0.0002 (X2) | P=0.0005 (X2) | P=0.0067 (X2) | P=0.5646 (X2) |
| Memantine, n (%) | 1414 (45%) | 620 (32%) | 168 (28%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0439 (X2) |
| Taking any approved AD medication, n (%) | 2439 (77%) | 1239 (65%) | 338 (57%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0002 (X2) |
Figure.
The proportions of total and trial eligible NACC UDS probable AD patients by study partner type. A significantly greater proportion (P<0.0001) of patients had spousal study partners than either those with adult child or another study partner type. A greater proportion of patients with spousal study partners were eligible, P<0.0001 compared to those with adult child or another type of study partner.
NACC study partners
Relative to adult children or other types of study partners, spousal study partners were older and more often male and White (see Table 2). Adult children and other types of study partners were younger, more often female, and more often Black and Hispanic (see Table 2). Adult child study partners had higher levels of education than either spousal or other types of study partners. Other types of study partners were more frequently discrepant to the patient’s race than either spousal or adult child study partners and were more frequently discrepant to the patient’s ethnicity than adult child study partners.
Table 2.
Demographic characterizations of the NACC AD study partners.
| Characteristic | Spouse Caregiver | Adult Child Caregiver | Other Caregiver | Overall P value (statistic) | Spouse vs Adult Child P value (statistic) | Spouse vs Other P value (statistic) | Adult Child vs Other P value (statistic) |
|---|---|---|---|---|---|---|---|
| Age, mean | 71.3 (10.0) | 51.8 (9.0) | 59.2 (15.1) | P<0.0001 (ANOVA) | P<0.0001 (t-test) | P<0.0001 (t-test) | P<0.0001 (t-test) |
| Female, n (%) | 1913 (60%) | 1418 (74%) | 485 (81%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0007 (X2) |
| White, n (%) | 2798 (88%) | 1350 (71%) | 382 (64%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0016 (X2) |
| Black, n (%) | 230 (7%) | 384 (20%) | 145 (24%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.0307 (X2) |
| American Indian/Alaska Native, n (%) | 3 (.1%) | 19 (1%) | 8 (1%) | P<0.0001 (FE) | P<0.0001 (FE) | P<0.0001 (FE) | P=0.4966 (FE2) |
| Native Hawaiian/Pacific Islander, n (%) | 3 (.1%) | 3 (.2%) | 0 (0%) | P=0.8352 (FE) | - | - | - |
| Asian, n (%) | 55 (2%) | 27 (1%) | 12 (2%) | P=0.5320 (X2) | - | - | - |
| Race discrepant from study partner*, n (%) | 54 (2%) | 16 (1%) | 26 (5%) | P<0.0001 (X2) | P=0.0167 (X2) | P<0.0001 (X2) | P<0.0001 (X2) |
| Hispanic, n (%) | 170 (5%) | 288 (15%) | 81 (14%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.3804 (X2) |
| Ethnicity discrepant from study partner, n (%) | 74 (2%) | 15 (1%) | 20 (3%) | P<0.001 (X2) | P<0.0001 (X2) | P=0.1405 (X2) | P<0.0001 (X2) |
| Education, mean years (SD) | 15.0 (3.0) | 15.4 (2.7) | 14.7 (3.1) | P<0.0001 (KW) | P<0.0001 (WRS) | P<0.1576 (WRS) | P<0.0001 (WRS) |
Patients eligible for clinical trial criteria
Twenty seven percent of AD patients were eligible by hypothetical clinical trial criteria (Figure). A greater proportion of patients with spousal study partners were trial eligible (36%), compared to patients with an adult child (16%) (X2, p<0.0001) or another type of study partner (16%) (X2, p<0.0001). Within each study partner category, trial eligible patients were younger than their ineligible counterparts (data not shown). There were no significant differences between eligible and ineligible patients within study partner categories in the proportion of eligible patients by gender, race or Hispanic ethnicity, level of education, or record of treatment with AD medications.
Trial eligible patients with spousal study partners were younger (mean age = 72.6) than trial eligible patients with adult child study partners (mean age = 76.6, WSR test, p<0.0001) but not those with another type of study partner (mean age = 72.5, WSR test, p=0.97). In the logistic regression model, the relationship between study partner type and trial eligibility remained significant (OR=0.44, 95% CI: 0.37 – 0.52, p<0.0001 for adult child vs. spousal study partner; OR=0.41, 95% CI: 0.31 – 0.53, p<.0001 for other vs. spousal study partner) when controlling for age, baseline MMSE, race, ethnicity, GDS and Hachinski scale sores. Similarly, when examining only age-eligible participants, differences in the odds of eligibility were still significant when comparing the study partner groups and controlling for all other factors (OR=0.42, 95% CI: 0.35–0.50, p<.0001 for adult child vs. spousal study partner; OR=0.41, 95% CI: 0.31 – 0.54, p<.0001 for other vs. spousal study partner). Among only the age-eligible NACC participants, 40.5% of participants with a spousal study partner, 22.4% of those with an adult child study partner, and 23.0% of those with another study partner met remaining eligibility criteria.
Eligible patients with spousal study partners were more often male, more often White, had higher education, and were more likely to take AD medications than were the trial eligible patients with an adult child or another type of study partner (data not shown). Trial eligible patients with an adult child study partner were significantly more likely than eligible patients with another study partner type to take AD medications (data not shown).
Reasons for trial ineligibility
Table 3 lists the reasons for trial ineligibility. Common reasons for ineligibility were age (18% of the overall sample); the presence of another possible cause of memory impairment (18% of the overall sample); taking exclusionary medications (16% of the overall sample); inadequate visit frequency with the informant (15% of the overall sample); cardiac exclusion criteria (12% of the overall sample); and exclusionary score on the GDS (11% of the overall sample). The most common reason for trial exclusion was failure to meet MMSE criteria (30% of all participants). Though there were no differences in the total proportions with exclusionary scores on the MMSE, the nature of exclusion differed significantly among the groups. Eleven percent of patients with a spousal study partner were excluded for too high MMSE and 15% for too low MMSE. In contrast, more (20%, X2, p<0.0001) patients with an adult child study partner were excluded for too low MMSE while fewer (7%, X2, p<0.0001) were excluded for too high scores. Similarly fewer patients (9%, X2, p=0.13) with other study partners were excluded for high MMSE scores and more patients (17%, X2, p=0.20) with other study partners were excluded for high MMSE scores, relative to those with spousal study partners, though neither difference reached statistical significance.
Table 3.
Reasons for trial exclusion.
| Criteria | Spouse Caregiver | Adult Child Caregiver | Other Caregiver | Overall P value | Spouse vs Adult Child P value | Spouse vs Other P value | Adult Child vs Other P value |
|---|---|---|---|---|---|---|---|
| Age, n (%) | 332 (10%) | 528 (28%) | 168 (28%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.8357 (X2) |
| Age <50 | 31 (1%) | 4 (.2%) | 15 (3%) | P<0.0001 (X2) | P=.0007 (FE) | P=0.0018(X2) | P<0.000(FE) |
| Age >85 | 301 (10%) | 524 (27%) | 153 (26%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.3704(X2) |
| MMSE, n (%) | 828 (27%) | 514 (28%) | 156 (27%) | P=0.5641 (X2) | - | - | - |
| MMSE <14 | 465 (15%) | 373 (20%) | 100 (17%) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.2002 (X2) | P=0.1245 (X2) |
| MMSE >26 | 363 (11%) | 141 (7%) | 56 (9%) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.1347 (X2) | P=0.1166 (X2) |
| Residence criteria, n (%) | 90 (3%) | 124 (6%) | 42 (7%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.6549 (X2) |
| Informant visit frequency, n (%) | 21 (.7%) | 642 (34%) | 189 (32%) | P<0.0001 (X2) | P<0.0001 (X2) | P<0.0001 (X2) | P=0.3587 (X2) |
| Informant reliability, n (%) | 78 (2%) | 41 (2%) | 30 (5%) | P=0.0005 (X2) | P=0.4722 (X2) | P=0.0006 (X2) | P=0.0002 (X2) |
| Cardiac medical exclusion, n (%) | 279 (9%) | 196 (11%) | 53 (9%) | P=0.2067 (X2) | - | - | - |
| Neurologic medical exclusion, n (%) | 96 (3%) | 54 (3%) | 24 (4%) | P=0.3362 (X2) | - | - | - |
| Other memory cause, n (%) | 446 (14%) | 302 (16%) | 99 (16%) | P=0.1210 (X2) | - | - | - |
| Hachinksi > 4, n (%) | 68 (2%) | 70 (4%) | 18 (3%) | P=0.0054 (X2) | P=0.0013 (X2) | P=0.1952 (X2) | P=0.4463 (X2) |
| GDS >6, n (%) | 281 (9%) | 217 (11%) | 87 (15%) | P<0.0001 (X2) | P=0.0038 (X2) | P<0.0001 (X2) | P=0.0376 (X2) |
| Excluded medication, n (%) | 432 (14%) | 298 (16%) | 96 (16%) | P=0.0856 (X2) | - | - | - |
Other differences in the rate of exclusion among the study partner categories were apparent (Table 3). A greater proportion of patients with adult child (34%) and other types of study partners (32%) than spousal study partners (0.7%, X2, p<0.0001) were excluded for informant visit frequency. Similarly, a greater proportion of patients with adult child (28%) and other types of study partners (28%) than with spousal study partners (10%) were excluded for age (X2, p<0.0001). Patients with spousal study partners also were less likely to be excluded for residence criteria (relative to both the adult children and other study partner groups, X2, p<0.0001), score on the Hachinski ischemia scale (relative to the adult child group, X2, p<0.0013), and score on the GDS (relative to the other study partner group, X2, p<0.0001). The relationship between GDS and eligibility was modified by age (Wald test, p<0.0001), but the relationship between Hachinski ischemia scale and eligibility was not (Wald test, p=0.92). Other types of study partner were more frequently (5%) deemed unreliable by local investigators than were adult children (2%, X2, p=0.0002) or spousal study partners (2%, X2, p=0.0006).
Discussion
Few AD patients are eligible to participate in clinical trials [11] and fewer still actually enroll. In this study, using inclusion criteria meant to broadly represent the current state of mild-to-moderate AD dementia trials, 27% of NACC research participants with probable AD were deemed trial eligible. Common reasons for trial exclusion were disease severity (as measured by the MMSE), age, and medical contraindications.
In this study, patients with a spousal study partner were more likely to meet eligibility criteria than were patients with adult children or other types of study partners. Among the spousal caregiver group, a greater proportion was excluded for too high MMSE, suggesting that many of the ineligible participants in this group may eventually become eligible as a result of the natural progression of AD. Together, these results parallel the low prevalence of nonspousal study partner-AD patient dyad participation in mild-to-moderate trials and suggest that such discrepancies may, in part, be due to differential exclusion by trial entry criteria.
Patients with adult child study partners were more likely than those with a spousal study partner to be excluded for reasons that are not corrigible. These include age, low MMSE, living situation (living in a skilled nursing facility), inadequate frequency of visits with the informant, and exclusionary scores on the Hachinski ischemia scale. In a model that controlled for age and MMSE, the difference among the study partner groups remained significant, suggesting that unmeasured qualities of the patient or caregiver independently impacts trial eligibility. It remains unclear, however, if the increased exclusion of patients with non-spousal study partners observed here is sufficient to explain the discrepancy between the high overall prevalence of AD patients with nonspousal caregivers and the low numbers of such patients in AD trials.
The current results suggest that AD patient-adult child caregiver dyads’ low rates of participation in research are not limited to clinical trials of new medications. In the NACC UDS, a natural history study with few exclusion criteria that requires annual follow-up with a clinician and neuropsychological testing, there was a significantly greater representation of AD patients with spousal study partners than with adult children or other types of informants. This suggests that adult child caregiver-patient dyads’ low rate of participation in trials does not simply reflect that the patients cared for by adult children are more likely to be excluded because of co-morbidities and related medical factors. Instead, their low rate may reflect logistical challenges, low awareness of research opportunities, or more negative attitudes toward participation. Adult child caregivers, who may still hold full time employment or have young children for whom they also provide care, likely do face increased logistical challenges to research participation. They are also, however, more likely to see their burden as overwhelming [12] and to view patient quality of life as poor [13,14], which may negatively impact the likelihood of research participation. Spousal caregivers, alternatively, may hold closer relationships to the patient [15] and may be more motivated to seek out research participation.
Minority patients are more likely receive care from nonspousal family members [16,17]; have less access to medical care and lower rates of AD medication use [18,19]; and have lower participation rates in AD clinical trials [20]. In NACC, a larger proportion of minority patients exists among the nonspousal caregiver-patient dyads and these patients less commonly took AD medications. Similarly, the nonspousal caregiver groups were more frequently excluded for low scores on the MMSE and minorities are often more severe in their disease at diagnosis [21], potentially inhibiting their participation in trials enrolling mild-to-moderate disease. We found no differences, however, in race between eligible and ineligible patients within study partner categories in the current study. The potential interaction between caregiver type and race and ethnicity will require further study, but increasing enrollment of AD patients cared for by nonspouses may represent a mechanism for improving minority representation in trials.
This study has some weaknesses. The NACC is not a population study and these results should not be extrapolated to the broader disease population. This limitation, however, may be minimized by the facts that the NACC has lenient inclusion criteria, varying slightly among Centers but excluding few [22]. Furthermore, clinical trial enrollment is often viewed as a more involved form of research participation (relative to natural history studies) and clinical trials in AD often recruit from natural history studies such as the NACC UDS.
Besides race and ethnicity, other factors not measured in the NACC may impact or interact with the effect of caregiver type on trial enrollment. These factors may include socioeconomic and other cultural characteristics. Additionally, the data included in the current study were over a period of six years, which could introduce a cohort effect.
Though it is clear that patients cared for by adult children or other nonspouse relationships are underrepresented in AD trials, these data do not inform whether AD patients cared for by a nonspouse are a reasonable target for trial recruitment or how trial designs or recruitment strategies would need to be adjusted to increase their representation. We do not make the argument that the differences observed here account for the low numbers of nonspousal dyads in trials. Even if this were the case, removing age limits or widening the MMSE criteria for a trial would be associated with significant risk for scientific error or unsafe trials. Instead, studies are needed to examine if adult child and other nonspouse caregivers are adequate informants in the setting of a trial and methods to increase their participation must be developed if they are.
In summary, among patients in a large national multisite natural history study of AD, those with spousal study partners are more often eligible for standard clinical trial enrollment criteria. These results may begin to explain why many AD clinical trials have low levels of patient enrollees with nonspousal study partners, such as adult child caregivers. Further work will be necessary to better understand and develop interventions to overcome the barriers to research participation by AD patients with nonspousal caregivers.
Acknowledgments
Funding: Dr. Grill is supported by NIA AG016570 and by the Sidell-Kagan Foundation. Dr. Karlawish is supported by the Marian S. Ware Alzheimer Program and NIA P30-AG01024. The NACC database is supported by UO1 AG016976.
References
- 1.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in alzheimer’s disease clinical trials. Alzheimers Res Ther. 2010;2:34. doi: 10.1186/alzrt58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association: 2008 alzheimer’s disease facts and figures. Alzheimers Dement. 2008;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association. Families care: Alzheimer’s disease caregiving in the united states, 2004. wwwalzorg; 2004. Alzheimer’s association and national alliance for caregiving. [Google Scholar]
- 4.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on alzheimer disease progression: A randomized controlled trial. Jama. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 5.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, Thal LJ. High-dose b vitamin supplementation and cognitive decline in alzheimer disease: A randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, Shinto L, Aisen PS. Docosahexaenoic acid supplementation and cognitive decline in alzheimer disease: A randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, Aisen PS. A randomized, double-blind, placebo-controlled trial of simvastatin to treat alzheimer disease. Neurology. 2011;77:556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tariot PNAP, Cummings J, Jakimovich L, Schneider L, Thomas R, Becerra L, Loy R. The adcs valproate neuroprotection trial: Primary efficacy and safety results. ICAD. 2009 01-04-04. [Google Scholar]
- 9.Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, Jin S, Thomas R, Aisen PS. A phase ii trial of huperzine a in mild to moderate alzheimer disease. Neurology. 2011;76:1389–1394. doi: 10.1212/WNL.0b013e318216eb7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Schneider LS, Olin JT, Lyness SA, Chui HC. Eligibility of alzheimer’s disease clinic patients for clinical trials. J Am Geriatr Soc. 1997;45:923–928. doi: 10.1111/j.1532-5415.1997.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 12.Conde-Sala JL, Garre-Olmo J, Turro-Garriga O, Vilalta-Franch J, Lopez-Pousa S. Differential features of burden between spouse and adult-child caregivers of patients with alzheimer’s disease: An exploratory comparative design. Int J Nurs Stud. 2010;47:1262–1273. doi: 10.1016/j.ijnurstu.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Conde-Sala JL, Garre-Olmo J, Turro-Garriga O, Lopez-Pousa S, Vilalta-Franch J. Factors related to perceived quality of life in patients with alzheimer’s disease: The patient’s perception compared with that of caregivers. Int J Geriatr Psychiatry. 2008 doi: 10.1002/gps.2161. [DOI] [PubMed] [Google Scholar]
- 14.Conde-Sala JL, Garre-Olmo J, Turro-Garriga O, Vilalta-Franch J, Lopez-Pousa S. Quality of life of patients with alzheimer’s disease: Differential perceptions between spouse and adult child caregivers. Dement Geriatr Cogn Disord. 2010;29:97–108. doi: 10.1159/000272423. [DOI] [PubMed] [Google Scholar]
- 15.Norton MC, Piercy KW, Rabins PV, Green RC, Breitner JC, Ostbye T, Corcoran C, Welsh-Bohmer KA, Lyketsos CG, Tschanz JT. Caregiver-recipient closeness and symptom progression in alzheimer disease. The cache county dementia progression study. J Gerontol B Psychol Sci Soc Sci. 2009;64:560–568. doi: 10.1093/geronb/gbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janevic MR, Connell CM. Racial, ethnic, and cultural differences in the dementia caregiving experience: Recent findings. Gerontologist. 2001;41:334–347. doi: 10.1093/geront/41.3.334. [DOI] [PubMed] [Google Scholar]
- 17.Association As. 2010 alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Mehta KM, Yin M, Resendez C, Yaffe K. Ethnic differences in acetylcholinesterase inhibitor use for alzheimer disease. Neurology. 2005;65:159–162. doi: 10.1212/01.wnl.0000167545.38161.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner AJ, McClendon MJ, Sami SA, Ogrocki PK, Adams KB, Smyth KA. Factors affecting usage patterns of memantine in alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:137–143. doi: 10.1097/WAD.0b013e31815ccd68. [DOI] [PubMed] [Google Scholar]
- 20.Faison WE, Schultz SK, Aerssens J, Alvidrez J, Anand R, Farrer LA, Jarvik L, Manly J, McRae T, Murphy GM, Jr, Olin JT, Regier D, Sano M, Mintzer JE. Potential ethnic modifiers in the assessment and treatment of alzheimer’s disease: Challenges for the future. Int Psychogeriatr. 2007;19:539–558. doi: 10.1017/S104161020700511X. [DOI] [PubMed] [Google Scholar]
- 21.Livney MG, Clark CM, Karlawish JH, Cartmell S, Negron M, Nunez J, Xie SX, Entenza-Cabrera F, Vega IE, Arnold SE. Ethnoracial differences in the clinical characteristics of alzheimer’s disease at initial presentation at an urban alzheimer’s disease center. Am J Geriatr Psychiatry. 2011;19:430–439. doi: 10.1097/JGP.0b013e3181f7d881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The uniform data set (uds): Clinical and cognitive variables and descriptive data from alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]