Introduction
Physical signs of corpus callosum (CC) dysfunction in some ALS patients have been reported in the form of mirror movements, which reflect a disruption in communication between motor hemispheres while an individual performs motor tasks (1). These studies suggest that sub-clinical levels of this damage may occur as an early manifestation of the disease, warranting further in vivo study of the CC in these patients. This proposition, as well as knowledge of its involvement in landmark histologic studies (2,3), has prompted the study of the CC with diffusion tensor imaging. While diffusion tensor imaging (DTI) results show that there are consistent abnormal microstructural changes manifesting in the CC in ALS (4–6), few studies have examined macro-structural differences of this region. One identified reduced gross size of the CC compared to controls (7), while another found evidence for increased white matter density (8). Given these discrepant results, the aim of this study was to examine size differences in geometrically-defined sub-regions of the CC in the native space of brain magnetic resonance images of patients with ALS compared to healthy controls. A difference in CC size observed in either direction would add another dimension to the current understanding of the micro-structural changes observed in studies cited above. For instance, a confirmed increase in CC area in ALS patients might indicate an underlying proliferative process such as reactive astrocytosis in response to neuronal death, whereas a smaller CC area in patients could instead imply isolated atrophy of neurons. A confirmation of CC macro-structural change could warrant additional study of the CC of individuals at various stages of the disease in order to illuminate the role of this structure in the natural history of motor neuron degeneration.
Materials and methods
Study participants
Patients diagnosed with ALS (El Escorial criteria) by a neuromuscular physician were recruited from the University of Michigan Motor Neuron Disease Clinic. Twenty-five ALS patients (17 male, 19 limb onset, mean age 59 years, mean ALSFRS-R 38, mean disease duration 20 ± 15 months, range disease duration 1–61 months) were included. To maintain a functionally uniform group, patients were selected under conditions previously described (9), and without any other confounding mental disorder or neurological disease. They were mean age-matched to 22 healthy volunteers (11 male, mean age 57 years). Healthy volunteers were recruited from the general community. This study was approved by the Institutional Review Board at the University of Michigan and all participants gave written informed consent for participation.
Image acquisition
Magnetic resonance imaging procedures took place at the University of Michigan using a GE 3T Excite 2 magnet (General Electric, Milwaukee, WI). High-resolution axially oriented 3D anatomical volumes were acquired over a 4.7-min duration (spoiled-gradient-recall, TE = 1.85 ms, TR = 9.06 ms, flip-angle = 15 °, 2562 matrix, 256 mm FOV, 1.2-mm slice thickness, 124 slices).
Corpus callosum size analysis
All procedures were conducted using methods similar to Fling et al. (10,11). Briefly, each participant's corpus callosum (CC) was outlined manually in the same mid-sagittal slice of the high-resolution T1-weighted structural images (appropriately oriented to true mid-sagittal slice to remove variance of participant head placement in MR coil). Each CC was then segmented geometrically into seven sub-regions, as defined by Witelson (12). To reduce variance from boundary instability between the first two anterior regions, they were combined, yielding six CC regions for analysis (see Figure 1 in Fling et al. (11)). The interpretations of the anatomical correlates of these sub-regions are identical to those used in Fling et al. Notably for the study of ALS, area 5 is the region shown to contain fibers passing between primary motor cortices (11).
Figure 1.
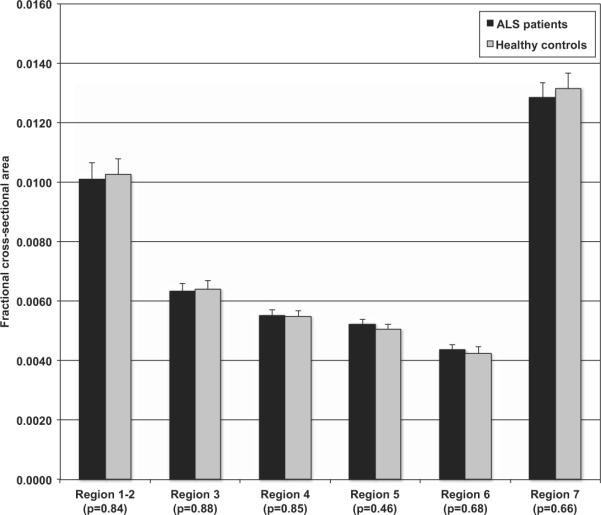
Mean size and standard error are displayed for each group in each of the resulting six regions (1–2, 3, 4, 5, 6, 7) of the CC. No significant differences were observed. HC: healthy controls; ALS: patients.
To control for total brain size variance, the CC area was normalized to intracranial area (ICA). The ICA mask was drawn for each individual using the same mid-sagittal slice used for the CC outlining. Three independent raters (blinded to group) created both CC and ICA masks, and all masks were drawn using an in-house MATLAB program (MATLAB, MathWorks Inc. R2006b).
From the independently drawn CC masks, the two raters' masks with the highest fractional area overlap were chosen for analysis. The final CC mask was taken as the spatial union of these two masks; any disagreement on sub-region boundary was split. Final ICA masks were generated similarly. Area (mm2) for both measures was the product of the number of voxels in each mask and the cross-sectional area of each voxel. Normalized areas were recorded for each individual from these values for statistical analysis. A repeated measures ANOVA was conducted to examine CC size differences between groups, including gender as a fixed effect to account for known gender CC size differences (13). To test specific a priori hypotheses of regional CC size differences, independent sample t-tests were performed between groups. All analyses were performed in SPSS v.19 (SPSS Inc., an IBM Company, 1989, 2010).
Results
The ANOVA showed no differences between the ALS and control groups, F(1, 45) = .456, p = .503, η2 = .010; and no interaction between group and CC area, F(5, 225) = .651, p = .661, η2 = .015. There was a significant effect of gender on area, F(1,45) = 6.058, p = .018, η2 = .123. However, there was no significant interaction between group and gender, F(1,45) = .616, p = .437, η2 = .014. Box's test of equality showed significant variance differences when gender was included as a fixed effect, p = .047. However, when gender was removed from the model the variance in CC size between groups was no longer significant, p = .097. This suggests that found variance differences were driven by gender and were not specific to ALS. All t-test comparisons showed no significant differences between any of the CC sub-regions at a significance level of 0.05 (see Figure 1). We also observed that these results did not change after removal of the bulbar-onset patients.
Discussion
We designed our study and analysis to explicitly examine the question: “Are there corpus callosum size differences to be found in patients with ALS?” Our data indicate there are no differences. Gender does have an effect on CC size, but this is consistent with previous findings in healthy individuals (13); moreover, this effect does not appear to interact with group membership in the present results. A previous study's (7) method was constrained by taking video images of MRI scanner renderings of structural images, and it is unclear whether these were appropriately oriented to provide a true mid-sagittal slice. The second study examining the CC in ALS (8) relied on voxel based morphometry, which warps the participant's structural image towards a template. Our methodology used measurements of the CC in the native space of the participant from a true mid-sagittal structural image slice. Given that our data represent a sample of ALS patients from a single clinic, and the conflicting results from different imaging techniques, future studies might benefit from multicenter pooling of data, as previously called for by Turner et al. (14). This would facilitate higher statistical power for the interpretation of neuroimaging studies for ALS.
The current results suggest that any pathophysiological processes occurring in the CC in these patients as demonstrated in the DTI literature (4–6) are perhaps too subtle, or do not manifest in a way to be observed with the resolution of structural MR imaging, as in commentary by Unrath et al. (15). An alternative explanation may be that any fiber atrophy occurring in the CC due to neuronal degeneration is offset by a consequent reactive gliosis, a characteristic proliferative response of local astrocytes to various brain insults (16). Notably, the presence of reactive astrocytic proliferation has been described in various areas of the brain in post mortem studies of ALS patients (2,3). Whatever drives the current findings, it is clear from the diffusion tensor imaging literature as well as post mortem studies that CC impairment is part of the pathogenesis of the disease, and the results of this study imply a secondary role for the use of CC macro-structural analysis in the disease course.
Acknowledgements
We would like to thank Pooja Modi, Donald McNair, and Rebecca Hovatter for their contributions to their work in drawing CC masks. We are also sincerely grateful for the time volunteered by all participants.
Funding for this study was provided by NIH RO1-NS052514 (RCW) and Basic Radiological Sciences Award (RCW).
Footnotes
Declaration of interest: The authors have no disclosures of interest to make regarding this work.
The authors alone are responsible for the content and writing of the paper.
References
- 1.Wittstock M, Wolters A, Benecke R. Transcallosal inhibition in amyotrophic lateral sclerosis. Clin Neurophysiol. 2007;118:301–7. doi: 10.1016/j.clinph.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Lawyer T, Netsky M. Amyotrophic lateral sclerosis. AMA Arch Neurol Psychiatry. 1953;69:171–92. doi: 10.1001/archneurpsyc.1953.02320260029002. [DOI] [PubMed] [Google Scholar]
- 3.Smith MC. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 1960;23:269–82. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res. 2010;1348:156–64. doi: 10.1016/j.brainres.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 5.Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology. 2010;75:1645–52. doi: 10.1212/WNL.0b013e3181fb84d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douaud G, Filippini N, Knight S, Talbot K, Turner MR. Integration of Structural and Functional Magnetic Resonance Imaging in Amyotrophic Lateral Sclerosis. Brain. 2011;134:3470–9. doi: 10.1093/brain/awr279. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi H, Fukuyama H, Ouchi Y, Nagahama Y, Kimura J, Asato R, et al. Corpus callosum atrophy in amyotrophic lateral sclerosis. J Neurol Sci [Internet] 1995;134:189–96. doi: 10.1016/0022-510x(95)00220-6. Available from: http://ac.els-cdn.com.proxy.lib.umich.edu/0022510X95 002206/1-s2.0-0022510X95002206-main.pdf?_tid = 4684c8 d2f6b63a73921768228e4cb0ee&acdnat = 1333109631_687f bb68cd5bbeab3dc80dc84cfc7ba4. [DOI] [PubMed] [Google Scholar]
- 8.Kassubek J, Unrath A, Huppertz HJ, Lul é D, Ethofer T, Sperfeld AD, et al. Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel based morphometry of 3-D MRI. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:213–20. doi: 10.1080/14660820510038538. [DOI] [PubMed] [Google Scholar]
- 9.Jelsone-Swain LM, Fling BW, Seidler RD, Hovatter R, Gruis K, Welsh RC. Reduced Interhemispheric Functional Connectivity in the Motor Cortex during Rest in Limb-Onset Amyotrophic Lateral Sclerosis. Front Syst Neurosci. 2010;4:158. doi: 10.3389/fnsys.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential callosal contributions to bimanual control in young and older adults. J Cogn Neurosci [Internet] 2011;23:2171–85. doi: 10.1162/jocn.2010.21600. Available from: http://www.mitpressjournals.org/doi/pdf/10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fling BW, Peltier SJ, Bo J, Welsh RC, Seidler RD. Age differences in interhemispheric interactions: callosal structure, physiological function, and behavior. Front Neurosci. 2011;5:38. doi: 10.3389/fnins.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witelson SF. Hand and gender differences in the isthmus and genu of the human corpus callosum. A post mortem morphological study. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 13.Luders E, Thompson PM, Toga AW. The Development of the Corpus Callosum in the Healthy Human Brain. Journal of Neuroscience. 2010;30:10985–90. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner MR, Grosskreutz J, Kassubek J, Abrahams S, Agosta F, Benatar M, et al. Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. The Lancet Neurology. 2011;10:400–3. doi: 10.1016/S1474-4422(11)70049-7. [DOI] [PubMed] [Google Scholar]
- 15.Unrath A, Ludolph AC, Kassubek J. Alterations of the Corpus Callosum as an MR Imaging-Based Hallmark of Motor Neuron Diseases. American Journal of Neuroradiology. 2011;32:E90. doi: 10.3174/ajnr.A2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karam C, Scelsa SN. Can vitamin D delay the progression of ALS? Medical Hypotheses. 2011;76:643–5. doi: 10.1016/j.mehy.2011.01.021. [DOI] [PubMed] [Google Scholar]


