Abstract
Humans and wildlife are exposed to environmental pollutants that have been shown to interfere with the thyroid hormone system and thus may affect brain development. Our goal was to expose pregnant rats to propylthiouracil (PTU) to measure the effects of a goitrogen on white matter development in offspring using magnetic resonance imaging (MRI) and volumetric analysis. We exposed pregnant Sprague Dawley (SD) rats to 3 or 10 ppm PTU from gestation day 7 (GD7) until postnatal day 25 (P25) to determine the effects on white matter (WM), gray matter (GM), and hippocampus volumes in offspring. We sacrificed offspring at P25 but continued the life of some offspring to P90 to measure persistent effects in adult animals. P25 offspring exposed to 10 ppm PTU displayed lowered levels of triiodothyronine (T3) and thyroxine (T4); cerebral WM, GM, and total brain volumes were significantly lower than the volumes in control animals. P90 adults exposed to 10 ppm PTU displayed normal T3 levels but lowered T4 levels; WM, GM, total brain, and hippocampal volumes were significantly lower than the volumes in control adults. Both P25 and P90 rats exposed to 10 ppm PTU displayed significant reductions in percent WM as well as heterotopias in the corpus callosum. Exposure to 3 ppm PTU did not produce any significant effects. These results suggest that MRI coupled with volumetric analysis is a powerful tool in assessing the effects of thyroid hormone disruption on white matter development and brain structure. This approach holds great promise in assessing neurotoxicity of xenobiotics in humans and wildlife.
Keywords: Myelination, White matter, Magnetic Resonance Imaging, Brain, Thyroid Hormone
1. INTRODUCTION
Thyroid hormones (TH) are critical in neurodevelopment (Gilbert and Zoeller 2010; Zoeller, 2002). They influence multiple processes including granule cell proliferation, neuronal migration, synaptogenesis, gliogenesis, and myelination (Howdeshell, 2002). Developmental hypothyroidism has been shown to selectively reduce the number of myelinated axons in the rat brain without affecting the total number of axons (Berbel et al., 1994; Guadano-Ferraz et al., 1994). Reductions in myelination caused by developmental TH deficiencies have been associated with various neurological symptoms including cognitive deficits, sensory loss, and motor impairments (Gilbert and Zoeller 2010; Zoeller, 2002).
Magnetic resonance imaging (MRI) is an excellent tool to study myelination. MRI offers a non-invasive (i.e., can image live specimens) and non-destructive method of acquiring a permanent archive of external and internal brain structure data (Montie et al., 2008, 2009). This technique allows thin virtual sections of the entire brain to be acquired where histological processing is not possible or practical. MRI provides excellent contrast between white matter (WM) and gray matter (GM), and when coupled with image analysis techniques, can be used to accurately determine brain volumes (Montie et al., 2008, 2009). MRI has been used to measure the volume of the hippocampus in rats and humans that were hypothyroid during development (Hasegawa et al., 2010; Wheeler et al., 2011). However, MRI and volumetric techniques have not been used to assess the effects of thyroid hormone disruption on WM volume. Given the known effects of developmental hypothyroidism on myelination, MRI and volumetric analysis has the potential to provide new insight on the effects of thyroid hormone disrupting chemicals (e.g., polychlorinated biphenyls (PCBs), brominated flame retardants (PBDEs), Triclosan) on myelination.
The overall goal of this study was to use MRI and volumetric analysis to investigate the effects of thyroid hormone disruption on WM development. Our specific objectives were to: (a) induce hypothyroidism during gestation and lactation in rat dams using propylthiouracil (PTU) as a model goitrogen to produce hypothyroid offspring; (b) sacrifice offspring at weaning (P25) and image brains to determine WM, GM, and hippocampus volumes (i.e., ex vivo imaging); (c) terminate PTU exposure at P25 and allow remaining offspring to recover thyroid function and mature to adulthood (P90); sacrifice these adults and image brains ex vivo to determine brain volumes from adults; (d) image some adults live (i.e., in vivo imaging) to determine WM, GM, and hippocampus volumes. Evaluation of brain structure in adult offspring following cessation of dosing at weaning allowed us to determine if changes in brain structure were persistent. This approach can serve as the basis for future studies using non-invasive, in vivo MR imaging to study the persistent effects of environmental pollutants on brain development in humans and wildlife.
2. MATERIALS AND METHODS
2.1 Animals and Experimental Treatment
Animal experiments were conducted at the University of South Florida (USF; St. Petersburg FL) with approval by the USF Institutional Animal Care and Use Committee (IACUC), permit #R3486. Timed pregnant Sprague Dawley (SD) rat dams (Harlan Laboratories, Indianapolis, IN) arrived at our facilities at gestation day 5 (GD5), defined as 5 days after insemination. Rats were housed in individual plastic cages with woodchip bedding, 12 hour light/dark cycles, and food/water provided ad libitum. Rat dams were randomly assigned to three treatment groups: control (n = 8), 3 ppm 6-propyl-2-thiouracil (PTU; Sigma Aldrich Corp., St. Louis, MO) (n = 8), or 10 ppm PTU (n = 8) in drinking water. Dosing began at GD7 and continued until weaning of offspring on P25. At P5, pups were culled to ten for each litter (approximately 5 males and 5 females). Each week, dams were weighed individually, while male and female pups were grouped and weighed separately by litter. On P25, offspring were weaned, and dams were weighed and anesthetized under 3–5% isoflurane gas followed by intraperitoneal injections of 50 mg/kg ketamine and 5 mg/kg xylazine; blood was collected by heart stick to measure serum T3 and T4 concentrations. At the same time, 46 P25 male offspring (14 control offspring from 7 litters; 16 3 ppm offspring from 8 litters; 16 10 ppm offspring from 8 litters; a maximum of two offspring per litter for each treatment) were weighed individually, anesthetized, and had blood collected in a similar fashion. Offspring were then perfused with 1× phosphate-buffered saline (PBS) followed by 10% buffered formalin phosphate (Fisher Scientific Inc., Hampton, NH). Brains of these P25 offspring were removed from the skull and stored in formalin for ex vivo imaging. Two male offspring from each litter were housed in pairs and placed on control drinking water until sacrifice on P90; food and water were provided ad libitum. Rats were weighed individually at weekly intervals. At P90, 42 of the 46 animals were anesthetized and had blood collected by heart stick to measure T3 and T4 levels. These rats were then perfused and had brains removed as previously described. Four P90 adults (2 control, 2 10 ppm PTU) were allowed to live to P93 for in vivo imaging. After the completion of in vivo MRI, blood was collected for TH measurements and brains were perfused and removed for ex vivo MR imaging.
2.2 T3 and T4 assay
Blood collected by heart stick from dams, offspring (P25), and adults (P90 and P93) was allowed to clot on ice for a minimum of 30 min. Serum was separated via centrifugation of clotted samples and stored at −80°C for analyses by radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA). All samples for total T4 and total T3 measurements were run in duplicate and the intra- and inter- assay variations were below 10%. The lowest calibrator run for T3 was 10 ng/dL and for T4 5 ng/mL. Based on greater than 95% specific binding, the sensitivity of the radioimmunoassay for total T4 was 5 ng/ml. Results below this limit of quantification were recorded at 5 ng/ml for statistical purposes.
2.3 Magnetic Resonance Imaging Acquisition
MR imaging of excised P25 and P90 brains (i.e., ex vivo imaging) and live anesthetized P93 rats (i.e., in vivo imaging) was performed at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility of the McKnight Brain Institute at the University of Florida (Gainesville, FL). MRI measurements were completed on ex vivo brains (N = 41: n = 7, control P25 offspring; n = 5, 3 ppm P25 offspring; n = 8, 10 ppm P25 offspring; n = 8, control P90 adults; n = 6, 3 ppm P90 adults; n = 7, 10 ppm P90 adults) that exhibited minimal excision and perfusion damage. Excised brain MR images were acquired at 750 MHz on a Bruker 17.6 Tesla 89 mm vertical bore imaging spectrometer (Bruker Corp, Billerica, MA) with a 20 mm birdcage coil (M2M Imaging Corp., Cleveland, OH). Before imaging, excised brains were placed in PBS for approximately 24–48 hours to remove free fixative. Then, brains were placed in a 20 mm NMR tube (model 20PP, Wilmad-LabGlass, Vineland, NJ) in Fluorinert (3M Corp, St. Paul, MN). P25 and P90 ex vivo MR images were acquired using a three-dimensional gradient echo sequence with the following parameters: recovery time of 150 ms, echo time of 15 ms, 2 averages, and acquisition matrix 300 × 150 × 200 with isotropic resolution of 80 μm. Each three-dimensional image acquisition required 140 minutes.
For in vivo imaging of P93 adults (n = 4; 2 control and 2 10 ppm), rats were anesthetized with 4 % Isoflurane in O2 at 2.0 L/min followed by subcutaneous injections of 0.8 mg Xylazine, dissolved in 2 mL of lactated Ringers solution (Hospira, Lake Forrest, IL). The Ringer’s solution was provided to maintain the physical condition of the rats during the extended MRI scans. Each rat was placed in a supine position, in a custom made MRI compatible stereotaxic frame and cradle, to allow repeatable positioning and to prevent motion artifacts. Anesthesia was maintained during imaging with 1.5 – 2.0 % Isoflurane in O2 at 1 L/min. Respiration was monitored and maintained at less than 50 breaths/min by adjusting the Isoflurane. Physiological temperature was monitored using a rectal probe and maintained using an air flow heater (SA Instruments, Stony Brook, NY). In vivo MR images of these P93 adults were acquired with spin echo sequences at 470 MHz using a 11.1 Tesla 40 cm horizontal bore imaging spectrometer (Bruker Corp, Billerica, MA) using a custom linear transmit-receive surface coil. All images were acquired with a field-of-view of 27 mm × 21 mm in a matrix of 180 × 140 and 59 slices of 500 μm slice thickness with 4 signal averages. Spin density images were acquired using a recovery time of 7000 ms and echo time of 10 ms in a total acquisition time of 65.3 mins. T2-weighted images were acquired with a recovery time of 7000 ms and echo time of 45 ms in a total acquisition time of 65.3 mins. After completion of in vivo imaging, brains of these rats were then excised and imaged ex vivo using the methods previously described. Following MRI, select brains were sectioned coronally at 50 μm with a vibratome, transferred to slides, and stained with cresyl violet.
2.4 Volumetric Image Analysis
Volumetric analysis of MRI data sets was performed using AMIRA 5.3.3 (Mercury Computer Systems, San Diego CA) and followed the same procedures as described previously (Montie et al., 2008, 2009). For WM and GM volumes, brains (N = 45; 41 ex vivo, 4 in vivo) were segmented (i.e., the process of assigning pixels to a material that represents a particular brain structure) visually based on the contrast between white and gray matter. Segmentations were performed in the coronal plane beginning at the forceps minor corpus callosum and ending at the posterior end of the hippocampus. The total brain was not analyzed because of excision damage to the olfactory bulbs and cerebellum in some animals. The ventricles were collapsed in most ex vivo brains and were not segmented. Heterotopias (i.e., a structural deformity where abnormal clusters of neurons appear within the corpus callosum) were segmented as GM and not WM. These deformities were also segmented individually. Percent white matter of the cerebral hemispheres was calculated for each specimen by dividing the volume of cerebral WM by the total segmented volume. Percent hippocampus was calculated by dividing the volume of the hippocampus by the total segmented volume. Percentages of total volumes were used to describe changes in WM and hippocampus that were caused by selective reductions of these structures, as opposed to a reduction in total brain volume. Brains were segmented by six independent analysts who were randomly assigned brains to process and who remained blind to the treatment condition. High inter-rater reliability was demonstrated by having the six analysts segment the same brain (see Supplemental Material, Table 1).
2.5 Statistical Analysis
Statistical calculations were completed using SYSTAT version 11.00.01 (SYSTAT Software, Richmond, CA). Repeated measures ANOVAs (α = 0.05) were performed to examine the effect of PTU exposure on body weight of the dams, P25 offspring, and P90 adults. One-way ANOVAs (α = 0.05) were performed to determine the effect of PTU exposure on T3 and T4 levels, brain volumes (total, WM, GM, hippocampus), and percentages of WM and hippocampal tissue. ANOVA assumptions of homogeneity of variances were examined and fulfilled these assumptions. If a significant effect was discovered, pair-wise comparisons were conducted using the Tukey-Kramer post-hoc test.
3. RESULTS
3.1 Effects of PTU Exposure on Body Weight
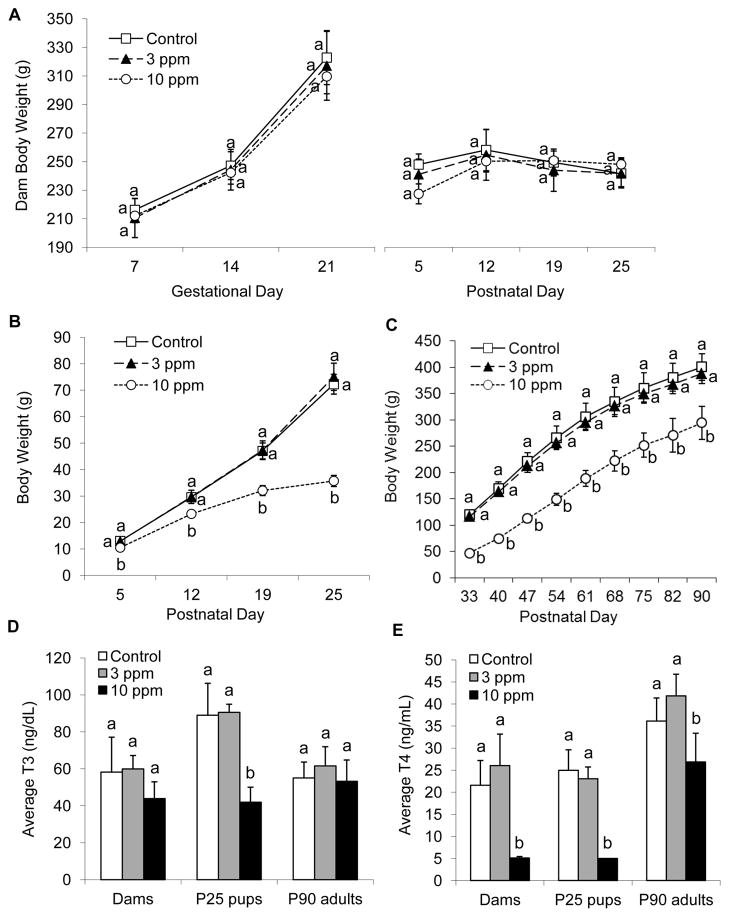
PTU exposure had no significant effect on weights of treated dams during gestation or lactation (Fig. 1A). Body weights of offspring exposed to 10 ppm PTU were significantly lower than the weights of control and 3 ppm dosed offspring (P < 0.05; Fig. 1B). Weights of rats from P33 to P90 that were exposed to 10 ppm PTU from GD7 to P25 were significantly lower than weights of control animals and adults previously exposed to 3 ppm PTU (P < 0.05; Fig. 1C).
Figure 1.
Weight and thyroid hormones of imaged rats exposed to propylthiouracil (PTU) from GD7 to P25. (A) Weights of dams from the first day of PTU exposure at gestation day 7 (GD7) until postnatal day 25 (P25). (B) Weights of P25 offspring and (C) weights of P90 adults. Note significant reductions in weight of offspring and adults exposed to 10 ppm PTU. (D) Average T3 concentrations of dams, P25 offspring, and P90 adults exposed to PTU. (E) Average T4 concentrations of dams, P25 offspring, and P90 adults exposed to PTU. Values that share the same letter are not significantly different from each other (P > 0.05); error bars show standard deviation. Note reduced T3 and T4 levels in offspring exposed to 10 ppm PTU and a recovery in T3 and near recovery in T4 levels in P90 adults previously exposed to 10 ppm PTU. Seven of eight dams and five of five P25 offspring exposed to 10 ppm PTU had T4 values below the minimum detection level.
3.2 Effects of PTU Exposure on Serum TH Concentrations
PTU exposure did not have any significant effect on serum T3 concentrations in dams (Fig. 1D). Exposure did lower serum T4 concentrations significantly in dams exposed to 10 ppm PTU (P < 0.05); 3 ppm exposure had no significant effect (Fig. 1E). The concentrations of T3 and T4 in P25 offspring exposed to 10 ppm PTU were significantly lower than the levels of control offspring (P < 0.05; Fig. 1D, E); 3 ppm exposure had no significant effect. Earlier exposure to PTU did not affect the concentrations of T3 in P90 adults (Fig. 1D), but in the rats that were imaged ex vivo, exposure to 10 ppm PTU did alter the concentration of T4 (P < 0.05; Fig. 1E). However, this result is likely due to a sampling error because when TH values of all exposed rats were included in statistical analysis, including animals that were not imaged, circulating T4 levels were not different from the levels in control animals (P < 0.05) (see Supplemental Material, Figure 1).
3.3 Effects of PTU exposure on brains of offspring
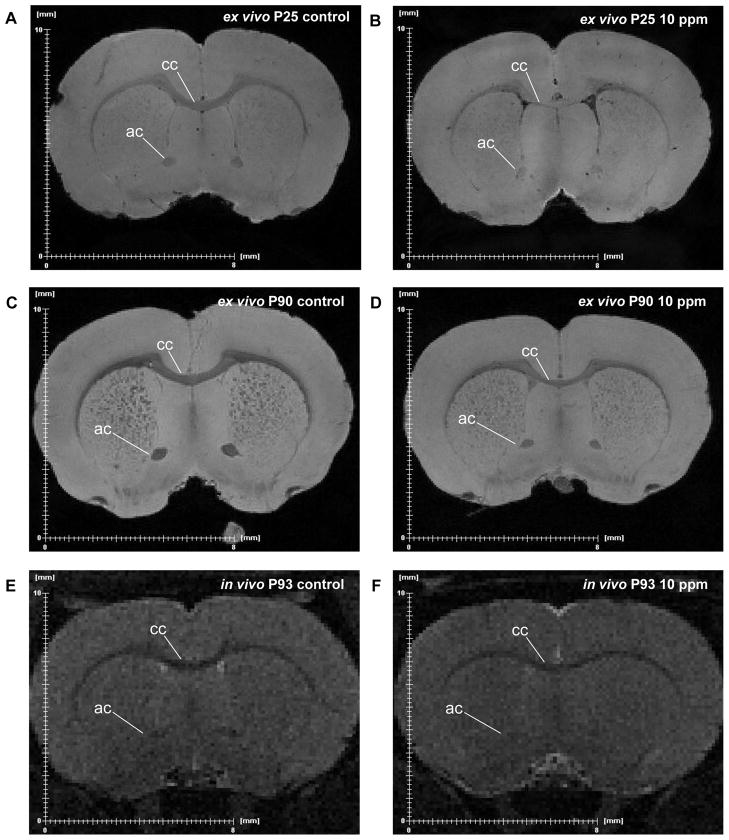
Ex vivo MR images of P25 offspring exposed to 10 ppm PTU revealed a decrease in size and an increase in signal intensity of major WM bodies, such as the corpus callosum and anterior commissure, when compared to control offspring (Fig. 2A, B). This difference was maintained to adulthood (i.e., the P90 adults) despite termination of treatment at P25 (Fig. 2C, D). These effects were also seen in T2-weighted in vivo images of P93 adults previously exposed to 10 ppm PTU (Fig. 2E, F).
Figure 2.
Representative examples of ex vivo and in vivo magnetic resonance images of rats exposed to propylthiouracil (PTU) from GD7 to P25. (A) Ex vivo MR images of a control P25 pup and (B) a P25 pup exposed to 10 ppm PTU. (C) Ex vivo MR images of a control P90 adult and (D) a P90 adult exposed to 10 ppm PTU from GD7 to P25. (E) In vivo MR images of a live control P93 adult and (F) a live P93 adult exposed to 10 ppm PTU from GD7 to P25. The locations of the sections are approximately equivalent. Note the increased signal intensity (i.e. brighter WM tracts) and decreased size of the corpus callosum (cc) and anterior commissure (ac) in the rats exposed to 10 ppm PTU. While in vivo images have reduced image quality, a reduction in size of the cc and ac are still evident in the 10 ppm exposed rats.
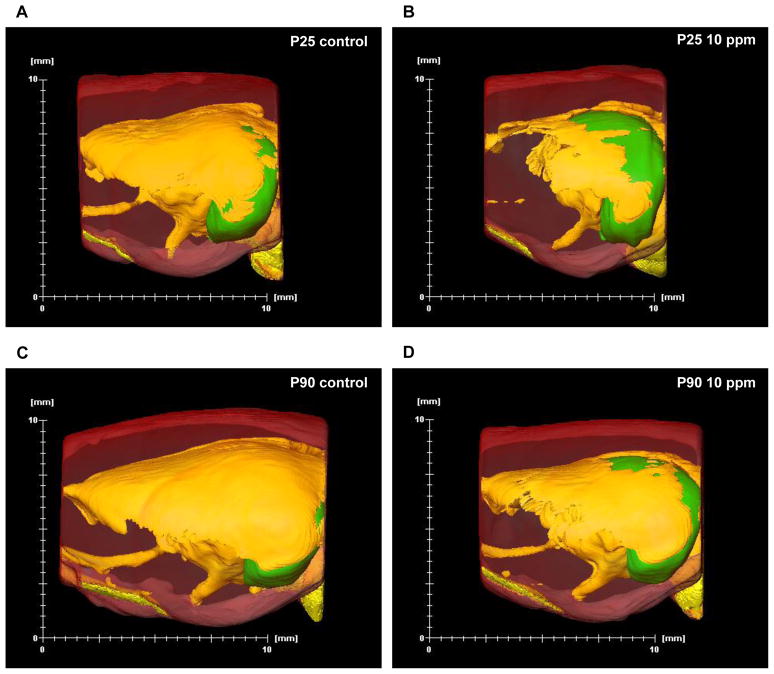
Three-dimensional (3D) reconstructions of P25 pup brains exposed to 10 ppm PTU showed a reduction in total brain volume and a global loss of WM when compared to control animals (Fig. 3A vs. Fig. 3B). 3D reconstructions of P90 adult rat brains previously exposed to 10 ppm PTU also showed a reduction of total brain volume and a global loss of WM when compared to control animals (Fig. 3C vs. Fig. 3D). However, 3D reconstructions did illustrate some brain recovery, as indicated by the gain of WM (Fig. 3D vs. 3B).
Figure 3.
Representative examples of three-dimensional reconstructions of brains of P25 offspring and P90 adults exposed to propylthiouracil (PTU) from GD7 to P25. (A) Sagittal views of a control P25 pup and (B) a P25 pup exposed to 10 ppm PTU. (C) Sagittal views of a control P90 adult and a (D) P90 adult exposed to PTU from GD7 to P25. Yellow = white matter; translucent red = gray matter; green = hippocampus. Note the global loss of white matter as well as a reduction in total brain size in both the P25 pup and P90 adult exposed to 10 ppm PTU.
Cerebral WM, cerebral GM, and total cerebral volumes derived from ex vivo MR images of P25 offspring exposed to 10 ppm were significantly lower than the volumes of control and 3 ppm exposed offspring (P < 0.01; Table 1). PTU exposure did not affect the hippocampus volumes of P25 offspring (Table 1). Percent volume of WM of offspring exposed to 10 ppm PTU was significantly lower than the percentages of the control and 3 ppm exposed offspring (P < 0.001; Table 1). Percent volume of hippocampus was not significantly affected by PTU exposure (Table 1). Cerebral WM, cerebral GM, total cerebral, and hippocampal volumes of P90 adults previously exposed to 10 ppm PTU were significantly lower than the volumes of control animals and rats previously exposed to 3 ppm PTU (P < 0.01; Table 1). Percent WM of P90 adults previously exposed to 10 ppm PTU was significantly lower than percent WM of control adults (P < 0.001; Table 1). Percent hippocampus in P90 adults was not significantly impacted by prior PTU exposure (Table 1). Volumes derived from in vivo images of the P93 adults treated with 10 ppm PTU were similar to the volumes derived from ex vivo images of the same animals. In both cases, WM volume and percent WM were reduced (See Supplemental Material, Table 2). However, the image quality in vivo was poor compared to the quality of the ex vivo images. This difference resulted from reduced magnet strength (11.1 vs. 17.6 T).
Table 1.
Average Brain Volumes (mm3) from Ex Vivo MRI of Rats Exposed to Propylthiouracil (PTU)
| P25 | Control | 3 ppm PTU | 10 ppm PTU |
|---|---|---|---|
| WM | 89.25 ± 11.11a | 101.66 ± 10.23a | 54.18 ± 9.63b |
| GM | 798.94 ± 38.83a | 831.61 ± 21.00a | 740.3 ± 34.80b |
| Total | 888.19 ± 46.06a | 933.28 ± 21.39a | 794.48 ± 36.44b |
| Hippocampus | 66.85 ± 5.20ab | 70.99 ± 2.18a | 61.85 ± 4.65b |
| %WM | 10.03 ± 0.98a | 10.89 ± 1.06a | 6.82 ± 1.16b |
| %Hippocampus | 7.52 ± 0.31a | 7.61 ± 0.08a | 7.78 ± 0.44a |
| P90 | Control | 3 ppm PTU | 10 ppm PTU |
|---|---|---|---|
| WM | 183.37 ± 14.61a | 178.87 ± 9.50a | 139.23 ± 10.79b |
| GM | 978.35 ± 22.98a | 1018.1 ± 17.07a | 868.29 ± 41.73b |
| Total | 1161.72 ± 32.83a | 1196.97 ± 12.28a | 1007.52 ± 46.58b |
| Hippocampus | 90.86 ± 4.13a | 93.2 ± 2.83a | 79.84 ± 5.18b |
| %WM | 15.77 ± 0.95a | 14.95 ± 0.85ab | 13.82 ± 0.90b |
| %Hippocampus | 7.82 ± 0.19a | 7.79 ± 0.23a | 7.92 ± 0.35a |
WM = White Matter; GM = Gray Matter; Total = Total Segmented Volume; %WM = Percent White Matter; % Hippocampus = Percent Hippocampus. Values that share the same letter are not significantly different from each other (i.e., P > 0.05)
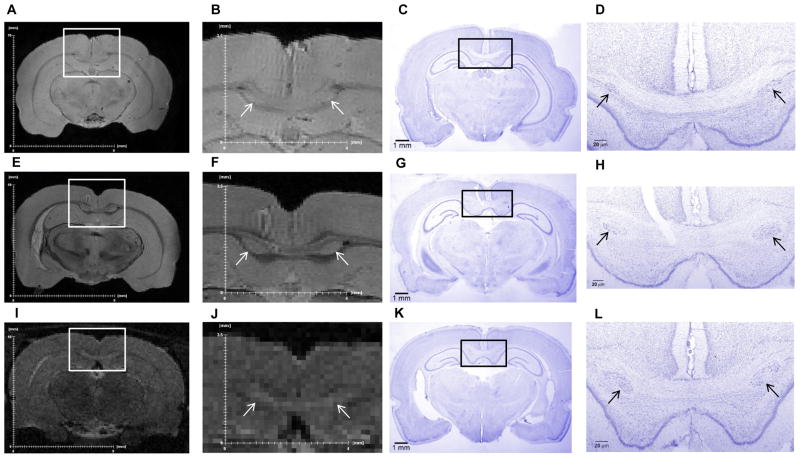
Heterotopias in the corpus callosum were identified in five of the eight P25 offspring exposed to 10 ppm PTU but in none of the control or 3 ppm exposed offspring (Table 2; Fig. 4A–D). Heterotopias were also identified in six of the seven P90 adults exposed to 10 ppm from GD7 to P25 but in none of the control rats or in P90 adults previously exposed to 3 ppm PTU (Table 2; Fig. 4E–H). Heterotopias were also identified in the in vivo MR images of the P93 adults previously exposed to 10 ppm but not in the control P93 adults (Fig. 4I–L). Verification of the presence of the heterotopia in imaged brains was ascertained by histology and cresyl violet staining (Fig. 4).
Table 2.
Volumes of Heterotopias from Ex Vivo MRI of Rats Exposed to Propylthiouracil (PTU)
| Animal ID | Age | PTU dose | Heterotopia volume (mm3) | Maternal T3 (ng/dL) | % of Control Dam T3 | Maternal T4 (ng/mL) |
|---|---|---|---|---|---|---|
| 17 | P25 | 10 ppm | 0.027 | 49.6 | 85.3 | < LOD |
| 19 | P25 | 10 ppm | 0.053 | 49.3 | 84.7 | < LOD |
| 17b | P25 | 10 ppm | 0.052 | 49.6 | 85.3 | < LOD |
| 21b | P25 | 10 ppm | 0.013 | 25.4 | 43.7 | < LOD |
| 22b | P25 | 10 ppm | 0.020 | 45.9 | 78.8 | < LOD |
|
| ||||||
| Avg vol. | 0.033 | |||||
| ±St.Dev | 0.018 | |||||
|
| ||||||
| 18 | P90 | 10 ppm | 0.013 | 46.6 | 80.0 | < LOD |
| 20 | P90 | 10 ppm | 0.033 | 43.4 | 74.6 | < LOD |
| 22 | P90 | 10 ppm | 0.308 | 45.9 | 78.8 | < LOD |
| 23 | P90 | 10 ppm | 0.020 | 45.9 | 78.8 | < LOD |
| 22b | P90 | 10 ppm | 0.122 | 45.9 | 78.8 | < LOD |
| 24b | P90 | 10 ppm | 0.013 | 36.5 | 62.7 | < LOD |
|
| ||||||
| Avg vol. | 0.085 | |||||
| ±St.Dev | 0.117 | |||||
PTU = propylthiouracil; T3 = triiodothyronine; T4 = thyroxine; LOD = limit of detection.
Percent of control dam T3 shows the % reduction in T3 levels of the dams exposed to 10 ppm PTU. This value was calculated by dividing the T3 level of the dam exposed to 10 ppm PTU by the average T3 value for control dams.
Figure 4.
Heterotopias observed in ex vivo MR images, in vivo MR images, and histological sections of rats exposed to 10 ppm propylthiouracil (PTU) from GD7 to P25. (A, B) Ex vivo MR images and (C, D) histological sections of the same P25 rat exposed to 10 ppm PTU. (E, F) Ex vivo MR images and (G, H) histological sections of the same P90 rat exposed to 10 ppm PTU. (I, J) In vivo MR images of a live P93 rat exposed to 10 ppm PTU and (K, L) and corresponding histological sections of the same animal. Slides were stained with cresyl violet. White and black boxes show magnified areas; heterotopias are indicated by white and black arrows. The locations of the MRI slices and histological sections are approximately equivalent.
4. DISCUSSION
4.1 Developmental PTU exposure
The main finding of the present study was that TH disruption affected WM volume and brain structure, which could be measured using MRI and volumetric analysis. Dams exposed to 10 ppm PTU displayed lowered T4 levels compared to the levels of control animals, a result consistent with previous studies (Gilbert and Sui, 2006; Goodman and Gilbert, 2007). In dams, the reduction in TH synthesis decreased TH availability during gestation, a time during development when the fetus is dependent on maternal T4 (Gilbert and Zoeller, 2010; Morreale de Escobar et al., 2004; Zoeller and Rovet, 2004). Exposure to PTU during nursing lowered T3 and T4 levels in P25 offspring exposed to 10 ppm PTU. P25 offspring exposed to 10 ppm PTU also had lowered body weights and developmental delays (i.e., craniofacial defects and impaired motor movements), symptoms consistent with developmental TH deficiency (as reviewed in Gilbert and Zoeller, 2010). P25 offspring exposed to 3 ppm PTU displayed normal TH levels, a result which was not observed in previous studies of thyroid hormone disruption with PTU (e.g., Gilbert and Sui, 2006; Goodman and Gilbert, 2007; Sharlin et al., 2008; Shibutani et al., 2009). These differences may be due to the strain of rats used in these experiments (Harlan Sprague Dawley vs. Long Evans vs. Charles River Sprague Dawley).
Significant reductions in WM, GM, and total cerebral volumes, as well as a reduction in the percent WM, was observed in P25 offspring exposed to 10 ppm PTU. Previous histological studies have shown that TH reductions can specifically decrease myelination without affecting the number of axons in the rat brain (Berbel et al., 1994; Guadano-Ferraz et al., 1994). In our study, volumetric reductions in WM in offspring exposed to 10 ppm PTU likely reflect a decrease in myelin and an increase in signal intensity, which was most likely due to an increase in the water content in those areas expected to be occupied by myelin. P25 offspring exposed to 3 ppm PTU did not exhibit reductions in serum hormones nor did they have lower WM or GM volumes. PTU exposure did not significantly affect hippocampal volume or percent hippocampus in P25 offspring at either dose level, a result supported by a previous volumetric study with methimazole, another goitrogen (Hasegawa et al., 2010).
Gray matter heterotopias (i.e., a structural deformity where abnormal clusters of neurons appear within the corpus callosum) were observed in P25 offspring exposed to 10 ppm PTU. Heterotopias have been identified histologically in rats developmentally exposed to PTU and methimazole (Goodman and Gilbert, 2007; Shibutani et al., 2009). The formation of heterotopias in TH deficient rats may arise from neuronal migration errors, as TH deficiency alters radial glial cell development (Auso et al., 2004; Goodman and Gilbert, 2007). While heterotopias were observed in only five of the eight 10 ppm PTU dosed P25 offspring in our study, it is possible that small heterotopias were not detectable using MRI. Heterotopias were not observed in any of the 3 ppm PTU exposed animals, a finding that may be related to the potentially small size or absence of heterotopias, as this dose of PTU, in our hands, did not lower serum hormones in dams or offspring. Goodman and Gilbert (2007) have detected heterotopias histologically in Long Evans rats exposed to 2, 3 and 10 ppm PTU, dose levels that were accompanied by significant reductions in serum T4. Shibutani et al. (2009) did not detect any heterotopias in rats exposed to 3 ppm and 12 ppm PTU despite significant reductions in T4. However, both studies used different exposure periods (Goodman and Gilbert, GD6 to P30; Shibutani et al., GD10 to P20) and different rat strains than our study, which may account for discrepancies in findings. To the best of our knowledge this is the first study to identify PTU exposure induced heterotopias in live animals.
4.2 Long-term effects of PTU exposure
To determine if structural changes observed in offspring exposed to PTU during gestation and lactation were permanent, dosing was halted at P25 to give the thyroid hormone system an opportunity to return to a euthyroid state. General appearance of P90 adult offspring previously exposed to 10 ppm PTU was normal despite significant deficits in body weight (30%). T3 and T4 levels in adults returned to control levels (See Fig. 1D, E and Supplemental Material, Figure 1).
A reduction in WM, GM, and total cerebral volumes, as well as a reduction in the percent WM, was observed in adults previously exposed to 10 ppm PTU. However, some recovery of WM volume between P25 and P90 occurred in the 10 ppm exposed offspring, as measured by the increase in the percentage of WM. In the control animals, the percent WM increased from 10.03% at P25 to 15.77% at P90, while in the 10 ppm rats, the percent WM increased from 6.82% at P25 to 13.82% at P90. An interesting observation was that the increase in percent WM of rats previously exposed to 10 ppm PTU was greater than the increase in control animals (7% vs. 5.7%). It may be possible that myelination occurred at an accelerated rate after the return to euthyroid conditions, as a result of recovery from developmental delays originally induced by PTU exposure.
Hippocampus volume was significantly lower in adults previously exposed to 10 ppm PTU as compared to volumes in adults that were never exposed to PTU. This observation is most likely a consequence of lower total brain volumes in exposed rats, since the percent hippocampus was comparable between control and PTU-exposed rats. The presence of heterotopia seen in vivo and ex vivo in P90 brains exposed to 10 ppm PTU indicates there is no resolution of these malformations associated with the return to euthyroid conditions, an observation consistent with previous reports (Goodman and Gilbert, 2007).
4.3 Future Implications
The detection of reductions in WM volume and the presence of heterotopias in adult rats previously exposed to PTU demonstrate the ability of MRI and volumetric analysis to reveal irreversible alterations in brain structure caused by developmental TH disruption. In vivo imaging is particularly useful in that live imaging allows for noninvasive and repeated measures of the same animal over time. This approach could be readily applied to examine the effects of a TH disrupter across critical developmental windows and in particular brain regions where TH is specifically required. While the resolution of the in vivo 11.1 T imaging used in this study was not as clear as the resolution in the ex vivo 17.6 T imaging, advancements in imaging technology will soon allow for the acquisition of higher resolution in vivo images. For example, since the completion of this study, the development of a headcoil for imaging live rats at 17.6 T was initiated at University of Florida Gainesville.
In the present study, we have shown that MRI and volumetric analysis are excellent tools for examining the effects of thyroid hormone disruption on brain structure with specific regard to myelination. Incorporation of MRI approaches will aid in our understanding of the neurotoxicity of several environmental pollutants that affect the thyroid system and thus possibly brain development in humans and wildlife populations (e.g., PCBs, PBDEs, and Triclosan) (Costa and Giordano, 2007; Kuriyama et al., 2007; Paul et al., 2010; Sharlin et al., 2006; Zoeller et al., 2002). The ability to detect WM reductions and brain structure abnormalities in rats that were caused by developmental TH disruption, but as adults show normal thyroid hormone levels, may be particularly relevant to epidemiological studies. It is possible that humans and wildlife that have been exposed to TH disruptors during development may exhibit normal TH levels at adulthood but harbor long-lasting changes in brain structure. The application of MRI and volumetric analysis, along with other MRI technologies such as diffusion weighted imaging for fiber tract analysis, may be extremely helpful in detecting these abnormalities in live animals and humans.
Supplementary Material
Highlights.
Postnatal Day 25 (P25) rats exposed to 10 ppm propylthiouracil (PTU) had lowered levels of T3 and T4 thyroid hormones.
P90 adult rats previously exposed to 10 ppm PTU displayed normal T3 levels but lowered T4 levels.
Both P25 and P90 rats exposed to 10 ppm PTU displayed reductions in white matter volume (WM) and percent WM.
Heterotopias of the corpus callosum were identified in magnetic resonance images of P25 and P90 rats exposed to 10 ppm PTU.
Acknowledgments
The authors thank the staff of the animal facility at the All Children’s Hospital in St. Petersburg, Florida at the University of South Florida for their help in animal care, blood collection, brain fixation and removal, and Garrett Astary and William Triplett of the University of Florida for their assistance with MR imaging and data processing. We would also like to thank the following students from the University of South Carolina Beaufort for their help in segmentation of brain structures: Ilton Cubero, Justin LaFrance, Jessica Perrulli, and Kathleen Armstrong. We thank Drs. Karl Jensen and Shonagh O’Leary-Moore for their comments on an earlier version of this manuscript. MRI data were supported through the National High Magnetic Field Laboratory and obtained at the Advanced Magnetic Resonance Imaging and Spectroscopy Facility in the McKnight Brain Institute of the University of Florida. This study was supported under a Subaward with the University Corporation of Atmospheric Research (UCAR) under Grant No. NA06OAR4310119 (Training Tomorrow’s Ecosystem and Public Health Leaders Using Marine Mammals as Sentinels of Oceanic Change) with the National Oceanic and Atmospheric Administration (NOAA), US Department of Commerce. We also acknowledge the partners of this training grant: University of California Davis Wildlife Health Center, The Marine Mammal Center, and Northwest Fisheries Science Center (NWFSC). Additional funding was provided by the National Institute of General Medical Sciences (NIGMS) grant no. P20GM103499 of the National Institute of Health (NIH), and the National Institute of Neurological Diseases and Stroke (NINDS) grant no. R01NS063360 of the NIH. This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
Competing Financial Interests Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auso E, Lavado-Autric R, Cuevas E, Escobar del Rey F, Morreale de Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–47. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- Berbel P, Guadano-Ferraz A, Angulo A, Ramon Cerezo J. Role of thyroid hormones in the maturation of interhemispheric connections in rats. Behav Brain Res. 1994;64:9–14. doi: 10.1016/0166-4328(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Sui L. Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res. 2006;1069:10–22. doi: 10.1016/j.brainres.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Zoeller RT. Neurotoxciology. 3. New York, NY: Informa Healthcare USA; 2010. Thyroid hormones-impact on the developing brain: possible mechanisms of neurotoxicity; pp. 81–111. [Google Scholar]
- Goodman JH, Gilbert ME. Modest thyroid hormone insufficiency during development induces a cellular malformation in the corpus callosum: a model of cortical dysplasia. Endocrinology. 2007;148:2593–7. doi: 10.1210/en.2006-1276. [DOI] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Escobar del Rey F, Morreale de Escobar G, Innocenti G, Berbel P. The development of the anterior commissure in normal and hypothyroid rats. Brain Res Dev Brain Res. 1994;81:293–308. doi: 10.1016/0165-3806(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kida I, Wada H. A volumetric analysis of the brain and hippocampus of rats rendered perinatal hypothyroid. Neurosci Lett. 2010;479:240–4. doi: 10.1016/j.neulet.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110:337–48. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama SN, Wanner A, Fidalgo-Neto AA, Talsness CE, Koerner W, Chahoud I. Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology. 2007;242:80–90. doi: 10.1016/j.tox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Montie EW, Pussini N, Schneider GE, Battey TWK, Dennison S, Barakos J, et al. Neuroanatomy and volumes of brain structures of a live california sea lion (zalophus californianus) from magnetic resonance images. Anat Rec. 2009;292:1523–47. doi: 10.1002/ar.20937. [DOI] [PubMed] [Google Scholar]
- Montie EW, Schneider G, Ketten DR, Marino L, Touhey KE, Hahn ME. Volumetric neuroimaging of the atlantic white-sided dolphin (lagenorhynchus acutus) brain from in situ magnetic resonance images. Anat Rec. 2008;291:263–82. doi: 10.1002/ar.20654. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151:U25–U37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. Developmental triclosan exposure decreases maternal and neonatal thyroxine in rats. Environ Toxicol Chem. 2010;29:2840–4. doi: 10.1002/etc.339. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Bansal R, Zoeller RT. Polychlorinated biphenyls exert selective effects on cellular composition of white matter in a manner inconsistent with thyroid hormone insufficiency. Endocrinology. 2006;147:846–58. doi: 10.1210/en.2005-0778. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology. 2008;149:2527–36. doi: 10.1210/en.2007-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M, Woo G-H, Fujimoto H, Saegusa Y, Takahashi M, Inoue K, et al. Assessment of developmental effects of hypothyroidism in rats from in utero and lactation exposure to antithyroid agents. Reprod Toxicol. 2009;28:297–307. doi: 10.1016/j.reprotox.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Wheeler SM, Willoughby KA, McAndrews MP, Rovet JF. Hippocampal size and memory functioning in children and adolescents with congenital hypothyroidism. J Clin Endocrinol Metab. 2011;9:E1427–E34. doi: 10.1210/jc.2011-0119. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Dowling ALS, Herzig CTA, Iannacone EA, Gauger KJ, Bansal R. Thyroid hormone, brain development, and the environment. Environ Health Perspect. 2002;110:355–61. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.