Abstract
We have previously shown that the main factor responsible for the faster [Ca2+]i decline rate with β-adrenergic (β-AR) stimulation is the phosphorylation of phospholamban (PLB) rather than the increase in systolic Ca2+ levels. The purpose of this study was to correlate the extent of augmentation of PLB Serine16 phosphorylation to the rate of [Ca2+]i decline. Thus, ventricular myocytes were isolated from neuronal nitric oxide synthase knockout (NOS1−/−) mice, which we observed had lower basal PLB Serine16 phosphorylation levels, but equal levels during β-AR stimulation. Ca2+ transients (Fluo-4) were measured in myocytes superfused with 3mM extracellular Ca2+ ([Ca2+]o) and a non-specific β-AR agonist isoproterenol (ISO, 1μM) with 1mM [Ca2+]o. This allowed us to get matched Ca2+ transient amplitudes in the same myocyte. Similar to our previous work, Ca2+ transient decline was significantly faster with ISO compared to 3mM [Ca2+]o, even with matched Ca2+ transient amplitudes. Interestingly, when we compared the effects of ISO on Ca2+ transient decline between NOS1−/− and WT myocytes, ISO had a larger effect in NOS1−/− myocytes, which resulted in a greater percent decrease in the Ca2+ transient RT50. We believe this is due to a greater augmentation of PLB Serine16 phosphorylation in these myocytes. Thus, our results suggest that not only the amount but the extent of augmentation of PLB Serine16 phosphorylation are the major determinants for the Ca2+ decline rate. Furthermore, our data suggest that the molecular mechanisms of Ca2+ transient decline is normal in NOS1−/− myocytes and that the slow basal Ca2+ transient decline is predominantly due to decreased PLB phosphorylation.
Keywords: Phospholamban, NOS1, calcium, β-adrenergic, myocyte
INTRODUCTION
An enhanced lusitropic response is a hallmark of β-adrenergic (β-AR) stimulation 1. This is, in part, due to the faster [Ca2+]i uptake into the sarcoplasmic reticulum (SR) by the SR Ca2+ ATPase (SERCA). SERCA’s function is inhibited by its regulatory protein, phospholamban (PLB) 2. The PLB-mediated inhibition of SERCA is relieved by its dissociation, which is caused by an increase in systolic Ca2+ levels and/or phosphorylation of PLB 3. During β-AR stimulation, not only is PLB phosphorylated at Serine16 by the cAMP-dependent protein kinase (PKA) but there are higher systolic Ca2+ levels as well. In our previous study 4, we set out to determine the major factor in the faster [Ca2+]i decline during β-AR stimulation (i.e., the high systolic levels of [Ca2+]i or PLB phosphorylation). We demonstrated that the faster [Ca2+]i decline rate with β-AR stimulation was due to the phosphorylation of PLB, specifically at Serine16, instead of the increase in systolic Ca2+ levels.
Cardiac relaxation is altered in many cardiomyopathies such as heart failure 5–7. The slowed relaxation can be attributed to changes in myofilament kinetics and/or slowed [Ca2+]i decline. Fully understanding each aspect of muscle relaxation will be critical for successful treatment of these diseases. Thus, the purpose of this study was to correlate the extent of augmentation of PLB Serine16 phosphorylation to the rate of [Ca2+]i decline. Previous studies have shown that neuronal nitric oxide synthase (NOS1) signaling is able to modulate basal PLB phosphorylation 8, 9. Namely, basal PLB phosphorylation at Serine16 is decreased in NOS1 knockout (NOS1−/−) compared to wildtype (WT) myocytes 8, 9. However, during β-AR stimulation, PLB Serine16 phosphorylation levels are similar in NOS1−/− and WT myocytes 8, 9. Since NOS1−/− myocytes have lower basal PLB Serine16 phosphorylation but equal phosphorylation levels with β-AR stimulation, the extent of the increase of PLB Serine16 phosphorylation is greater in NOS1−/− compared to WT myocytes. We hypothesize that not only the amount but the extent of augmentation of PLB Serine16 phosphorylation are the key determinants of the Ca2+ transient decline rate. We expect a greater effect of ISO that should result in a more prominent effect on Ca2+ transient decline in NOS1−/− vs WT myocytes.
METHODS AND MATERIALS
Cardiomyocyte isolation
Ventricular myocytes were isolated from NOS1−/− and C57BL/6 (WT) (Jackson Laboratories, Bar Harbor, Maine) as previously described 8. Briefly, the heart was cannulated and hung on a Langendorff apparatus. It was then perfused with Ca2+ free tyrode solution for 4 min. The solution was then switched to tyrode solution containing Liberase Blendzyme II (0.077 mg/ml) (Roche Applied Science, Indianapolis, IN). After 3–5 min, the heart was taken down, the ventricles minced, and myocytes were dissociated by trituration. Subsequently the myocytes were filtered through mesh, centrifuged, and resuspended in Tyrode solution containing 200 μmol/L Ca2+. Myocytes were used within 6 hours of isolation. All the animal protocols and procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
Measurement of Myocyte Ca2+ transients
Ca2+ transient measurements were performed as previously described 10. Briefly, myocytes were loaded at room temperature with Fluo-4 AM (10 μmol/L, Molecular Probes, Eugene, OR) for 30 min, and then another 30 min were allowed for intracellular de-esterification. The solution for de-esterification was Tyrode solution containing 200 μmol/L Ca2+. The instrumentation used for cell fluorescence measurements was a Cairn Research Limited (Faversham, UK) epifluorescence system. [Ca2+]i was measured by Fluo-4 epifluorescence with excitation at 480±20 nm and emission at 535±25 nm. The illumination field was restricted to collect the emission of a single cell. Data were expressed as ΔF/F0, where F is the fluorescence intensity and F0 is the intensity at rest. Myocytes were stimulated at 1 Hz via platinum electrodes connected to a Grass Telefactor S48 stimulator (West Warwick, RI).
Western blot for PLB Serine16 phosphorylation
Whole hearts were perfused with the various experimental solutions (3mM [Ca2+]o or ISO) for 3 min using a Langendorff apparatus, homogenized, and analyzed via western blot as previously described 11. Membranes were probed using a custom antibody to PLB (Zymed, San Francisco, CA), Serine16 phosphospecific antibody (Badrilla, Leeds, UK) and normalized to calsequestrin (ABR, Golden, CO). Data expressed as Serine16 phosphorylation normalized to total PLB.
Solutions and drugs
Normal Tyrode (NT) solution consisted of (in mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, 5 HEPES, pH 7.4 adjusted with NaOH or HCl. Isoproterenol (ISO, 1 μmol/L, a non-selective β-AR agonist) was prepared fresh each day. All chemicals were from Sigma (St. Louis, MO).
Experimental protocol
Our experimental protocol consisted of the myocyte first being superfused with control solution (NT with 1 mM [Ca2+]) until steady-state was reached, the myocyte was then superfused with 3 mM [Ca2+] NT solution, which resulted in increased systolic Ca2+ levels. After reaching steady-state, the solution was then switched back to control solution (1mM [Ca2+] NT) resulting in the washout of 3 mM [Ca2+]o and the Ca2+ transient amplitude returning back to basal levels (~ 3 minutes). The myocyte was then superfused with 1μM ISO (with 1mM [Ca2+]o) which increased myocyte systolic Ca2+ levels. Experiments were performed at room temperature.
Statistics
Data were presented as mean±SEM. Differences between groups were evaluated for statistical significance (P < 0.05) by ANOVA for multiple groups or paired/unpaired Student’s t tests for two groups.
RESULTS
Ca2+ transient kinetics experimental protocol
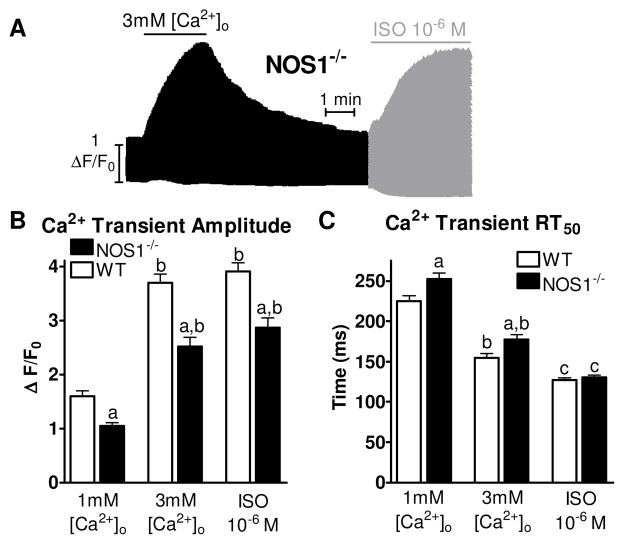
Shown in Figure 1A is a representative experiment showing Ca2+ transients over time recorded in a NOS1−/− myocyte. The maximal Ca2+ transient amplitude for each treatment for NOS1−/− myocytes and adapted data for WT myocytes 4 is shown in Figure 1B. 3 mM [Ca2+]o and ISO caused a significant increase in maximal Ca2+ transient amplitude compared to 1 mM [Ca2+]o in WT and NOS1−/− myocytes. However, NOS1−/− myocytes had significantly lower maximal Ca2+ transient amplitudes at 1 mM [Ca2+]o, 3 mM [Ca2+]o, and ISO compared to WT myocytes. Shown in Figure 1C is the Ca2+ transient decline rate measured as time to 50% relaxation (RT50) with 1 mM [Ca2+]o, 3 mM [Ca2+]o, and ISO. 3 mM [Ca2+]o and ISO resulted in a faster RT50 compared to 1 mM [Ca2+]o in WT and NOS1−/− myocytes. However, NOS1−/− myocytes had significantly slower RT50 at 1 mM and 3 mM [Ca2+]o. Interestingly, RT50 with ISO is similar between WT and NOS1−/− myocytes. Thus, Ca2+ transient decline was slower in NOS1−/− myocytes, but normalized with β-AR stimulation.
Figure 1.
Experimental Protocol. A) Representative time plot of the experimental protocol. B) Summary data (mean±sem) of maximum Ca2+ transient amplitude with 1 mM [Ca2+]o, 3mM [Ca2+]o, or ISO from WT (clear bar) and NOS1−/− (black bar) myocytes. C) Summary data (mean±sem) of Ca2+ transient decline measured as the RT50. a P<0.05 vs corresponding WT, b P<0.05 vs 1 mM [Ca2+]o, c P<0.05 vs 1mM and 3mM [Ca2+]o.
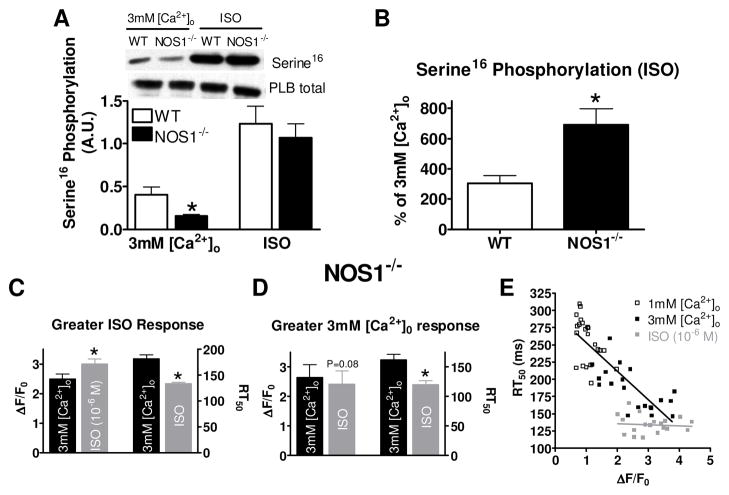
Since we observed differences in Ca2+ transient decline in NOS1−/− myocytes with 3mM [Ca2+]o and ISO treatments, we further analyzed Ca2+ transient decline in NOS1−/− myocytes. We first examined PLB phosphorylation. We measured PLB Serine16 phosphorylation under similar experimental conditions (i.e., perfusion with 3 mM [Ca2+]o or ISO for 3 min, which is a similar time point in which we matched the Ca2+ transient amplitudes). Shown in Figure 2A, WT and NOS1−/− myocytes perfused with ISO had significantly increased Serine16 phosphorylation compared with myocytes perfused with 3 mM [Ca2+]o. However, NOS1−/− myocytes perfused with 3 mM [Ca2+]o, had decreased Serine16 phosphorylation levels but similar phosphorylation levels with ISO when compared to WT myocytes. Further analysis reveals that ISO produces a much larger increase in PLB Serine16 phosphorylation in NOS1−/− myocytes compared to WT myocytes (Figure 2B). Thus, the extent of the increase of PLB Serine16 phosphorylation with ISO was greater in NOS1−/− vs WT myocytes.
Figure 2.
Effects of ISO and 3mM [Ca2+]o on WT and NOS1−/− PLB Serine16 phosphorylation and Ca2+ transient decline in NOS1−/− myocytes. A) Summary data (mean±sem) of PLB Serine16 phosphorylation with ISO or 3mM [Ca2+]o (A.U.- arbitrary units). B) Summary data (mean±sem) of the ISO mediated increase in PLB Serine16 phosphorylation expressed as the % of 3mM [Ca2+]o. Summary data (mean±sem) of Ca2+ transient amplitude (left) and decline (right) in NOS1−/− myocytes which had a higher maximal Ca2+ transient amplitude to ISO (C) or 3 mM [Ca2+]o (D). *P<0.05) vs corresponding WT or 3 mM [Ca2+]o. E) Individual values of peak [Ca2+]i versus RT50 with Ca2+ (black, r2=0.70) and ISO (gray, r2=0.007).
Although the maximum Ca2+ transient amplitudes with 3mM [Ca2+]o and ISO were not statistically different, the faster Ca2+ transient decline rates with ISO may have resulted from the slightly higher peak systolic Ca2+ levels. Therefore, we further investigated the Ca2+ transient decline by grouping the NOS1−/− myocytes that had a higher maximal response to ISO together (n=18) and the myocytes that had a higher maximal response to 3mM [Ca2+]o together (n=5). When grouping the data, the myocytes that had a higher maximum peak systolic Ca2+ with ISO also had a faster RT50 (Figure 2C). In this case, it is unknown if the faster Ca2+ transient decline with ISO is due to higher systolic Ca2+ levels or PLB Serine16 phosphorylation. However, when comparing the group that had a higher maximum peak systolic Ca2+ with 3mM [Ca2+]o (Figure 2D), ISO still resulted in a faster Ca2+ transient decline RT50. We observed the same phenomenon in WT myocytes 4. We further analyzed this relationship between maximum peak systolic Ca2+ levels and RT50. We plotted the maximum peak systolic Ca2+ levels with 1mM [Ca2+]o, 3mM [Ca2+]o, and ISO against their respective RT50 (Figure 2E). The higher peak systolic Ca2+ in the 1mM and 3mM [Ca2+]o group correlated with a faster RT50 (slope of −41.3±4.3). However, in the ISO group, there was no correlation between peak systolic Ca2+ and RT50 (slope of −1.5±4.3). We observed the same phenomenon in WT myocytes 4. These data suggest that with ISO at 1 mM [Ca2+]o, unlike in the absence of ISO, the rate of Ca2+ transient decline is not dependent on peak Ca2+ transient amplitude. These data suggest that during β-AR stimulation PLB Serine16 phosphorylation is the major factor responsible for the faster Ca2+ transient decline rate. Furthermore, these data suggest that the molecular mechanisms for Ca2+ transient decline are normal in NOS1−/− myocytes.
Ca2+ Transient kinetics with matched amplitudes
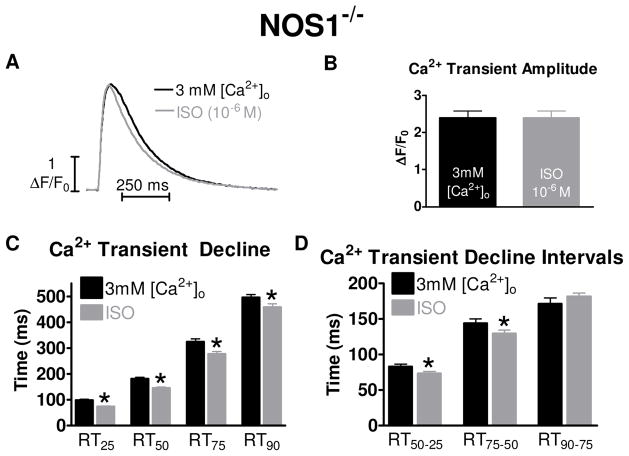
For accurate comparisons of Ca2+ transient decline rates, it has been shown that one must match the Ca2+ transient amplitudes 12. By using our experimental protocol, we were able to match Ca2+ transient amplitude levels between the 3mM [Ca2+]o and ISO groups and reduce the variability of Ca2+ handling between cells exposed to 3mM [Ca2+]o and ISO by superfusing each myocyte with both solutions. Representative matched individual Ca2+ transient traces are shown in Figure 3A and the matched peak values of 3mM [Ca2+]o and ISO are shown in Figure 3B.
Figure 3.
Effects of 3 mM [Ca2+]i and ISO on Ca2+ transient decline with matched Ca2+ transient amplitudes in NOS1−/− myocytes. A) Representative trace of matched [Ca2+]i amplitudes with 3 mM [Ca2+]o (black) or 10−6 M ISO (gray). B) Summary data (mean±sem) of matched Ca2+ transient amplitude with 3mM [Ca2+]o (black) or ISO (gray). C) Summary data (mean±sem) of Ca2+ transient decline with 3mM [Ca2+]o (black) or ISO (gray). (D) Summary data (mean±sem) of Ca2+ transient decline time intervals with 3mM [Ca2+]o or ISO. * P<0.05 vs corresponding 3 mM [Ca2+]o.
Using the matched Ca2+ transient amplitude data, we examined the effects of 3mM [Ca2+]o and ISO on Ca2+ transient decline by analyzing the time it takes the Ca2+ transient to decline by 25% (RT25), 50% (RT50), 75% (RT75) and 90% (RT90) from its peak amplitude. The Ca2+ decline with ISO at each time point was significantly faster compared to 3mM [Ca2+]o (Figure 3C). Our previous study 4 showed that the faster effect of ISO occurred only in the first 50% of the decline in WT myocytes. This was determined by dividing the declining Ca2+ transient into intervals: RT50-25, RT75-50, and RT90-75. For example, we subtracted the RT25 from the RT50 to get the RT50-25 interval, reflecting the time of the Ca2+ transient to decline from 25% from the peak amplitude to the 50% point. Data are shown in Figure 3D. The RT50-25 and RT75-50 intervals were significantly different between 3mM [Ca2+]o and ISO. Thus, these data suggest that faster Ca2+ transient decline with ISO compared to 3mM [Ca2+]o occurs in the first 75% in NOS1−/− myocytes.
Different effect of ISO on Ca2+ transient decline in NOS1−/− vs WT myocytes
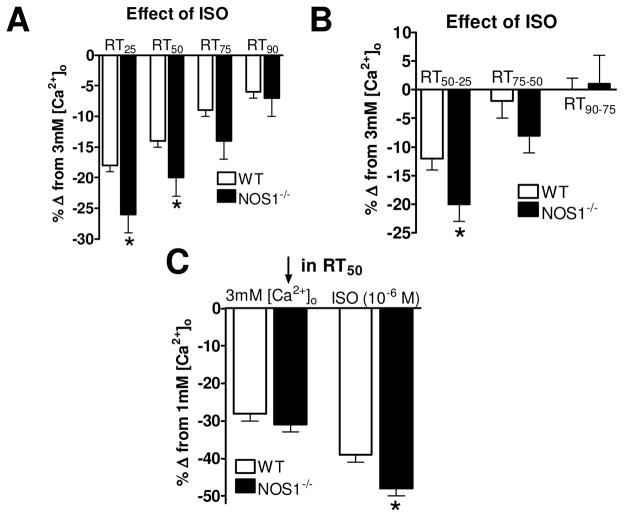
The interval data observed in the NOS1−/− myocytes are somewhat different than what was observed in WT myocytes (i.e., first 75% vs 50% of the decline) 4. Hence, we wanted to further investigate if there were any more differences in the effects of ISO on Ca2+ transient decline between WT and NOS1−/− myocytes. This was done by examining the effects of ISO on the decline rate (i.e., RT25, RT50, RT75, and RT90) of matched Ca2+ transients as a percent change of the decline with 3mM [Ca2+]o. For example, at the RT25, ISO in WT myocytes resulted in 18±1% faster decline compared to 3mM [Ca2+]o, but in NOS1−/ − myocytes, ISO had a 26±3% faster decline (P<0.05 vs WT). ISO also had a faster decline at the RT50 in NOS1−/− myocytes, but not at the RT75 or the RT90 (Figure 4A). We performed the same analysis investigating the effects of ISO in WT and NOS1−/− myocytes on our relaxation time intervals. Shown in Figure 4B, ISO had a significantly greater effect at the RT50-25 interval in NOS1−/− vs WT myocytes. This pronounced effect of ISO on Ca2+ transient decline also resulted in a greater effect in reducing the Ca2+ transient RT50 when inspecting the maximal responses to ISO (Figure 4C). That is, ISO resulted in a 48±2% faster Ca2+ transient RT50 (compared to the RT50 with 1mM [Ca2+]o) in NOS1−/ − myocytes, but only a 39±2% in WT myocytes (P<0.05 vs NOS1−/−). There was no difference in the effects of 3mM [Ca2+]o on Ca2+ decline between NOS1−/− and WT myocytes. Thus, these data suggest that ISO has a more prominent effect to augment Ca2+ transient decline in NOS1−/− myocytes.
Figure 4.
Effect of ISO on Ca2+ transient decline in NOS1−/− vs WT myocytes. A) Summary data (mean±sem) of the effects of ISO on Ca2+ transient decline normalized to 3mM [Ca2+]o in WT (clear bars) and NOS1−/− (black bars) myocytes. B) Summary data (mean±sem) of the effects of ISO on Ca2+ transient decline time intervals normalized to 3mM [Ca2+]o in WT (clear bars) and NOS1−/− (black bars) myocytes. C) Summary data (mean±sem) of the effects of maximum 3mM [Ca2+]o (left) and ISO (right) on Ca2+ transient RT50 normalized to 1mM [Ca2+]o in WT (clear bars) and NOS1−/− (black bars) myocytes. * P<0.05 vs corresponding WT.
DISCUSSION
The lowering of [Ca2+]i is the initiating event that permits relaxation. The majority of the decline of [Ca2+]i is due to Ca2+ resequestation into the SR via the SERCA/PLB complex and extrusion from the cell by the Na+/Ca2+ exchanger (NCX). In murine myocytes, the bulk (>95%) of the [Ca2+]i is resequestered back into the SR via SERCA 13, which is reversibly inhibited by PLB 3. Ca2+ binding to SERCA results in the dissociation of PLB from SERCA to relieve this inhibition. PLB is also a key phosphoprotein in the heart, which can be phosphorylated on Serine16 through PKA or on Threonine17 through Ca2+/calmodulin-dependent protein kinase. Phosphorylation at either site also results in the dissociation of PLB from SERCA. Thus, increasing [Ca2+]i or PLB phosphorylation results in greater Ca2+ resequestation into the SR and faster Ca2+ decline.
Ca2+ decline during β-AR stimulation
Stimulation of the β-AR receptor results in increased myocyte contraction and faster relaxation 1. In terms of Ca2+ handling, this will lead to an increase in peak systolic Ca2+ levels and faster decline. The higher systolic Ca2+ levels during β-AR stimulation should result in PLB dissociation and greater SR Ca2+ uptake. Furthermore, β-AR stimulation results in the phosphorylation of PLB at Serine16,14, which will also dissociate PLB from SERCA. We have previously shown that in WT myocytes the major factor for the faster Ca2+ decline during β-AR stimulation is PLB Serine16 phosphorylation.
In addition to the greater Ca2+ uptake into the SR with β-AR stimulation, greater Ca2+ extrusion from the cell could also enhance the Ca2+ transient decline rate 15. Thus, increased NCX, which removes Ca2+ from the cytosol throughout the action potential (besides phase 0), could result in faster Ca2+ transient decline. However, we believe that NCX does not play a role in the effects of β-AR stimulation on Ca2+ transient decline. Studies have found that β-AR stimulation does not affect NCX function 16. In addition, NCX plays a minor role in the Ca2+ decline rates in mouse myocytes (<5%), so an effect, if any, would be very minor at best. Further, using the NCX knockout mouse, there was no difference in Ca2+ transient decline rates with β-AR stimulation in WT compared to knockout myocytes 17.
Besides greater Ca2+ uptake or extrusion, β-AR stimulation also decreases myofilament Ca2+ sensitivity via TnI phosphorylation. It is widely accepted that TnI phosphorylation during β-AR stimulation accelerates relaxation 18, 19. A previous study 20 has shown that in unloaded myocyte experiments, there is little effect of TnI phosphorylation on myocyte relengthening. However, under loaded conditions (e.g. trabeculae), TnI phosphorylation does play a significant role in relaxation. Thus, under our experimental conditions, (i.e. unloaded myocyte) we believe that TnI does not play a role. Hence, with our previous work 4 showing that the major mechanism for the faster Ca2+ transient decline is PLB Serine16 phosphorylation, we wanted to extend this observation and correlate the extent of augmentation of PLB Serine16 phosphorylation to the rate of [Ca2+]i decline.
NOS1 signaling in myocytes
Nitric oxide produced via NOS1 is an important cardiac signaling molecule 21. Many studies have shown that NOS1 is a key modulator of cardiac myocyte function. By using cardiac myocyte NOS1 overexpressing mice 22, NOS1−/− mice 8, 23–26, and specific NOS1 inhibitors 8, 26, studies found that NOS1 signaling results in enhanced inotropy, lusitropy and augments the functional response to β-AR stimulation. In terms of Ca2+ handling, NOS1 signaling will accelerate Ca2+ transient decline under basal conditions and increase basal and β-AR stimulated systolic Ca2+ levels. Hence, NOS1−/− myocytes have slowed basal Ca2+ transient decline and depressed basal systolic Ca2+ levels. A molecular component of NOS1 regulation of myocyte contraction occurs via modulation of basal PLB Serine16 phosphorylation levels. We8 and others9, 27 have shown that WT myocytes with acute NOS1 inhibition or NOS1−/− myocytes have reduced basal levels of PLB Serine16 phosphorylation, while myocyte-specific NOS1 overexpression results in increased basal PLB Serine16 phosphorylation. Interestingly, studies 8, 9 have shown that NOS1−/− myocytes have similar PLB Serine16 phosphorylation levels and Ca2+ transient decline rates during β-AR stimulation. Since NOS1−/− myocytes have a lower basal PLB Serine16 phosphorylation but equal phosphorylation levels with ISO, the extent of the increase of PLB Serine16 phosphorylation is greater in NOS1−/− compared to WT myocytes. Our current results examining PLB Serine16 phosphorylation (Fig 2A and 2B) are consistent with these previous studies, in which we show that ISO resulted in a 692±107% increase in PLB Serine16 phosphorylation in NOS1−/− myocytes but only a 306±51% increase in WT myocytes. Thus, NOS1−/− myocytes represent a straightforward model system to investigate the role of PLB Serine16 phosphorylation augmentation in modulating the Ca2+ transient decline.
Ca2+ transient decline in NOS1−/− and WT myocytes with high extracellular Ca2+ vs ISO
We investigated Ca2+ transient decline kinetics with ISO and 3mM [Ca2+]o in NOS1−/− myocytes and compared them to WT data 4. As shown in Figure 1C, WT had significantly faster Ca2+ transient RT50 during 1mM [Ca2+]o and 3mM [Ca2+]o compared to NOS1−/− myocytes. The slowed Ca2+ decline during 1mM [Ca2+]o and 3mM [Ca2+]o is consistent with lower basal PLB Serine16 phosphorylation levels and agrees with previous work 8, 9. Moreover, the Ca2+ transient RT50 with ISO is similar between WT and NOS1−/− myocytes, which suggest that β-AR stimulation normalizes the Ca2+ transient RT50 in NOS1−/− myocytes via similar PLB Serine16 phosphorylation levels between WT and NOS1−/− myocytes and agrees with previous work 4. Nevertheless, the faster RT50 with ISO (compared to 3 mM [Ca2+]o) in the NOS1−/− myocytes may be due to the higher (although not significant) systolic Ca2+ levels. However, if we group the NOS1−/− myocytes that had a larger Ca2+ transient amplitude with 3mM [Ca2+]o (Figure 2D), the Ca2+ transient decline with ISO was still faster than 3mM [Ca2+]o. Thus, this highlights the importance of PLB Serine16 phosphorylation in modulating the rate of Ca2+ decline.
In addition to PLB Serine16 phosphorylation, an increase in diastolic SR Ca2+ leak via increased ryanodine receptor (RyR2) activity could contribute to slowed basal Ca2+ transient decline. Indeed, Gonzalez et al 28 previously demonstrated that NOS1−/− myocytes have increased basal diastolic SR Ca2+ leak via increased RyR2 activity and slowed basal [Ca2+]i decline. Thus, there is a balance shift towards increased SR Ca2+ release that slows the Ca2+ decline. However, we observed decreased RyR2 activity (and diastolic SR Ca2+ leak) 29 and decreased basal PLB Serine16 phosphorylation in NOS1−/− myocytes (8 and Figure 2A). Thus, our data suggests, that the balance is shifted towards a decrease in SR Ca2+ uptake that is responsible for the slowed basal [Ca2+]i decline. During β-AR stimulation, RyR2 activity is increased 30, 31. However, there is no difference in RT50 between WT and NOS1−/− myocytes (in which PLB Serine16 phosphorylation is the same). Furthermore, our previous work 4 showed that increased RyR activity with β-AR stimulation contributed to a faster rate of [Ca2+]i rise, but had no effect on [Ca2+]i decline. Therefore, during β-AR stimulation, the balance is shifted to greater SR Ca2+ uptake via PLB Serine16 phosphorylation that accelerates Ca2+ decline. Thus, if RyR2 activity was the major reason for the slowed Ca2+ transient decline, then we should still have observed slowed Ca2+ transient decline with ISO in the NOS1−/− myocytes vs WT.
We also examined the relationship between maximum systolic Ca2+ levels and the Ca2+ transient RT50 (Figure 2E). These data show that in the absence of ISO as systolic Ca2+ levels increased there was a direct relationship for a faster Ca2+ transient decline. However, this is not the case during β-AR stimulation. That is, the ISO group (at 1 mM [Ca2+]o) was not dependent upon systolic Ca2+ levels. We did not measure this relationship with ISO at 3 mM [Ca2+]o and cannot establish that the same phenomenon will occur. The ISO at 1 mM [Ca2+]o data are similar to what we observed in WT myocytes 4. Thus, we suggest that the molecular mechanisms for the Ca2+ transient decline are not different between NOS1−/− and WT myocytes but just slower under basal conditions due to decreased PLB Serine16 phosphorylation.
As suggested in previous studies 4, 12, to properly analyze Ca2+ transient decline between groups, one should use matched Ca2+ transient amplitudes. Thus, we investigated the RT25, RT50, RT75, RT90 and the time intervals (RT50-25 and RT75-50) of the Ca2+ transient decline with matched Ca2+ transient amplitudes (Figure 3). ISO resulted in a faster decline at all time points and a faster decline at the RT50-25 and RT75-50 intervals. This suggests that ISO has its greatest effect in increasing Ca2+ decline during the initial 75%. The effects of PLB Serine16 phosphorylation are observed in the initial 75% of the decline in NOS1−/− myocytes because, we believe that the increase in systolic Ca2+ with 3 mM [Ca2+]o (or ISO) will not dissociate all the PLB from SERCA. However, phosphorylation of PLB Serine16 results in the dissociation of more PLB from SERCA (compared to 3 mM [Ca2+]o) resulting in a faster decline during the initial 50% in WT and 75% in NOS1−/− Ca2+ transient decline.
Ca2+ transient decline in NOS1−/− vs WT myocytes
Our data suggest that ISO has a more pronounced effect on Ca2+ transient decline in NOS1−/− myocytes compared to WT myocytes. This effect of ISO also resulted in a greater decline in RT50 in NOS1−/− vs WT myocytes (Figure 4C). We believe this greater effect of ISO in NOS1−/− myocytes is due to the lower basal PLB Serine16 phosphorylation. Thus, there is a greater quantitative increase in PLB Serine16 phosphorylation with ISO that results in a greater effect on Ca2+ transient decline. Furthermore, although not significant, there was a trend that 3mM [Ca2+]o had a greater effect on RT50 in NOS1−/− myocytes (Figure 4C). NOS1−/− myocytes also had a steeper slope in the peak systolic Ca2+ levels with 1mM and 3mM [Ca2+]o and their respective RT50 (Figure 2) compared to WT myocytes 4. We believe that this may be due to the increased SERCA/PLB ratio in the NOS1−/− myocytes 24, 32. These data highlight the major role of PLB phosphorylation in modulating Ca2+ transient decline. Such that there is a compensatory change in SERCA/PLB ratio in NOS1−/− myocytes, but basal Ca2+ transient decline is still slower (compared to WT myocytes), which we believe is due to decreased basal PLB Serine16 phosphorylation.
In conclusion, our data suggest that the molecular mechanisms for the decline of [Ca2+]i is similar between NOS1−/− and WT myocytes and emphasizes that not only the amount but the extent of augmentation of PLB Serine16 phosphorylation are key determinants for the Ca2+ transient decline rate.
Molecular mechanisms of [Ca2+]i decline are similar in NOS1−/− and WT myocytes
β-AR stimulation resulted in greater effect on [Ca2+]i decline in NOS1−/− myocytes
The amount of PLB phosphorylation is major determinant for the [Ca2+]i decline rate
Extent of augmentation of PLB phosphorylation also is a major determinant
Acknowledgments
GRANTS
This research was supported by the American Heart Association (Established Investigator Award 0740040N, PML Janssen) and the National Institutes of Health, R01JL091986, JP Davis; K02HL094692, R01HL079283, MT Ziolo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res. 2001;89(5):373–375. [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4(7):566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 4.Roof SR, Shannon TR, Janssen PM, Ziolo MT. Effects of increased systolic Ca(2) and phospholamban phosphorylation during beta-adrenergic stimulation on Ca(2) transient kinetics in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301(4):H1570–1578. doi: 10.1152/ajpheart.00402.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varian KD, Kijtawornrat A, Gupta SC, Torres CA, Monasky MM, Hiranandani N, Delfin DA, Rafael-Fortney JA, Periasamy M, Hamlin RL, Janssen PM. Impairment of diastolic function by lack of frequency-dependent myofilament desensitization rabbit right ventricular hypertrophy. Circ Heart Fail. 2009 Sep;2(5):472–481. doi: 10.1161/CIRCHEARTFAILURE.109.853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43(5):523–531. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JP, Tikunova SB. Ca(2+) exchange with troponin C and cardiac muscle dynamics. Cardiovasc Res. 2008 Mar 1;77(4):619–626. doi: 10.1093/cvr/cvm098. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294(6):C1566–1575. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102(2):242–249. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 10.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Biphasic effect of SIN-1 is reliant upon cardiomyocyte contractile state. Free Radic Biol Med. 2008;45(1):73–80. doi: 10.1016/j.freeradbiomed.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77(2):353–361. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol. 1995;268(1 Pt 1):C271–277. doi: 10.1152/ajpcell.1995.268.1.C271. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 14.Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta -agonists. J Biol Chem. 2000 Dec 8;275(49):38938–38943. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 15.Terracciano CM, Souza AI, Philipson KD, MacLeod KT. Na+-Ca2+ exchange and sarcoplasmic reticular Ca2+ regulation in ventricular myocytes from transgenic mice overexpressing the Na+-Ca2+ exchanger. J Physiol. 1998 Nov 1;512 (Pt 3):651–667. doi: 10.1111/j.1469-7793.1998.651bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsburg KS, Bers DM. Isoproterenol does not enhance Ca-dependent Na/Ca exchange current in intact rabbit ventricular myocytes. J Mol Cell Cardiol. 2005 Dec;39(6):972–981. doi: 10.1016/j.yjmcc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res. 2004 Sep 17;95(6):604–611. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- 18.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005 Apr 1;66(1):12–21. 21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995 Jun;76(6):1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278(3):H769–779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- 21.Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and myocardial function. J Mol Cell Cardiol. 2008;45(5):625–632. doi: 10.1016/j.yjmcc.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loyer X, Gomez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, Charue D, Vaudin E, Zhang W, Sainte-Marie Y, Robidel E, Marty I, Mayer B, Jaisser F, Mercadier JJ, Richard S, Shah AM, Benitah JP, Samuel JL, Heymes C. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008;117(25):3187–3198. doi: 10.1161/CIRCULATIONAHA.107.741702. [DOI] [PubMed] [Google Scholar]
- 23.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416(6878):337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fradley M, Shoukas AA, Berkowitz DE, Hare JM. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92(12):1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 25.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Viatchenko-Karpinski S, Sun J, Gyorke I, Benkusky NA, Kohr MJ, Valdivia HH, Murphy E, Gyorke S, Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588(Pt 15):2905–2917. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loyer X, Gomez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, Charue D, Vaudin E, Zhang W, Sainte-Marie Y, Robidel E, Marty I, Mayer B, Jaisser F, Mercadier JJ, Richard S, Shah AM, Benitah JP, Samuel JL, Heymes C. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008 Jun 24;117(25):3187–3198. doi: 10.1161/CIRCULATIONAHA.107.741702. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104(51):20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Viatchenko-Karpinski S, Sun J, Gyorke I, Benkusky NA, Kohr MJ, Valdivia HH, Murphy E, Gyorke S, Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588(Pt 15):2905–2917. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. Dec;120(12):4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007 Feb 16;100(3):391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 32.Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92(5):e52–59. 22. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]