Abstract
The present study investigates sleep, mood, and the proposed bidirectional relationship between the two in psychiatric disorders. Participants with interepisode bipolar disorder (n = 49), insomnia (n = 34), and no psychiatric history (n = 52) completed seven consecutive days of sleep diaries and mood measures. The interepisode bipolar and insomnia participants exhibited greater sleep disturbance than the healthy control individuals. Negative mood was equally heightened in both interepisode bipolar disorder and insomnia, and there were no differences between the three groups in positive mood. Total wake time was associated with next morning negative mood in bipolar disorder, whereas evening negative mood was associated with subsequent total wake time in both bipolar disorder and insomnia. Additionally, positive mood was associated with subsequent total wake time for the insomnia group. Results support the theory that disruptions in nighttime sleep and daytime mood may be mutually maintaining and suggest the potential importance of transdiagnostic or universal processes.
Keywords: sleep, bipolar disorder, insomnia, Profile of Mood States, transdiagnostic
Sleep disturbance and mood disturbance are features of, and highly comorbid across, psychiatric disorders (e.g., Benca, Obermeyer, Thisted, & Gillin, 1992; Benca et al., 1997; Harvey, 2008b). Moreover, a bidirectional relationship has been proposed whereby disruptions in nighttime sleep and daytime mood may be mutually reinforcing (Harvey, 2008a; Wehr, Sack, & Rosenthal, 1987). This bidirectional model, termed the sleep–mood cycle, may be applicable across disorders. Given the lack of empirical evaluation of this theory, the broad aim of the present study is to examine two disorders in which the cycle is likely to be most germane: bipolar disorder and insomnia.
Bipolar disorder is ranked in the top 10 leading causes of disability worldwide (World Health Organization, 2001), affecting as many as 1 in 25 individuals (Kessler et al., 2005). Bipolar disorder is characterized by mood disturbance, typically comprising manic episodes and depressive episodes (American Psychiatric Association, 2000). Importantly, bipolar disorder is also associated with significant sleep disturbance. During mania there is a reduced need for sleep. During depression individuals typically suffer from insomnia or hypersomnia (American Psychiatric Association, 2000; Harvey, 2008b).
Surprisingly, accruing evidence suggests that sleep disturbance continues even when individuals are interepisode (Harvey, Schmidt, Scarna, Semler, & Goodwin, 2005; Millar, Espie, & Scott, 2004). The interepisode period is also important because individuals suffer from other significant symptomatology and impairment (e.g., Harrow, Goldberg, Grossman, & Meltzer, 1990; MacQueen et al., 2003), including mood dysregulation. For example, there is preliminary evidence that interepisode bipolar patients exhibit affective lability and increased reactivity (Fukuda, Etoh, Iwadate, & Ishii, 1983; Tsuang, Woolson, & Fleming, 1979), particularly with regard to positive affect (for review see Gruber, in press; Johnson, 2005). The evidence is mixed regarding interepisode patients' negative effect. A number of studies indicate no increase in reactivity relative to healthy individuals (e.g., Ruggero & Johnson, 2006; Sutton & Johnson, 2002), whereas others demonstrate an increase in neural response to negative stimuli relative to healthy controls (Chang et al., 2004; Yurgelun-Todd et al., 2000). The present study sought to clarify the presence of negative and positive mood in the interepisode period.
In terms of the sleep–mood cycle, in interepisode bipolar disorder there is already some evidence to support the theory that sleep disturbance influences daytime mood. Sleep disturbance is associated with manic episode onset in a majority of individuals with bipolar disorder (e.g., Jackson, Cavanagh, & Scott, 2003; Wehr et al., 1987), and a more recent study observed that sleep disturbance predicted depressive symptoms (Perlman, Johnson, & Mellman, 2006). Bauer et al. (2006) used the cross correlation function to examine the particular time lag that maximized the sleep–mood relationship, observing that mood shifts toward depression or mania most commonly occurred the morning immediately after a sleep change. The present study will further examine the association between sleep disturbance and next-morning positive and negative mood in the interepisode period.
There has been sparse research on the other direction of the sleep–mood cycle, that mood influences subsequent sleep. To the best of our knowledge, only one study has examined this direction of the model. This study demonstrated that bipolar individuals did not benefit from a positive presleep mood, unlike nonbipolar individuals who fell asleep more quickly after a positive mood induction. Interestingly, a negative mood induction compared to a neutral mood induction decreased the time to fall asleep in individuals with interepisode bipolar disorder (Talbot, Hairston, Eidelman, Gruber, & Harvey, 2009). The latter result is contrary to the sleep–mood cycle, but it likely relates to low arousal that was inherent to the study's in-laboratory methodology. The current study is designed to provide clarification in individuals' natural environment.
The second disorder examined in the present study is insomnia. Insomnia is one of the most prevalent psychological health problems, affecting at least 10% of the United States population (e.g., Simon & VonKorff, 1997). Insomnia is defined by sleep disturbance, including difficulty initiating sleep, maintaining sleep, or waking in the morning not feeling restored. These symptoms must occur for a period of one month or more, and the disturbance must not be attributable to another mental disorder (American Psychiatric Association, 2000; Edinger et al., 2004). While the role of mood in insomnia has not been extensively examined (Harvey, McGlinchey, & Gruber, 2010), several studies suggest that insomnia is associated with increased mood disturbance compared to normal sleepers (Bonnet & Arand, 1997; Monroe, 1967; Roth, Roehrs, & Pies, 2007; Vgontzas & Kales, 1999). However, an important review of this topic indicated that some studies have observed no significant mood differences between insomnia and healthy control groups (Riedel & Lichstein, 2000), with no clear methodological differences (e.g., type of measure, participant screening criteria) clearly accounting for the disparate findings. Hence, the current study aims to clarify the mixed findings relating to mood symptoms in insomnia.
There is initial evidence of an association between sleep and mood in insomnia, suggesting the sleep–mood cycle may be relevant. For example, Buysse et al. (2007) observed that individuals with insomnia endorsed less positive and more negative moods associated with sleep disturbance compared to good sleepers. Considering the other direction of the cycle—mood affecting sleep—insomnia patients' use of emotion-oriented coping strategies has been found to negatively impact sleep via increasing presleep stress and cognitive arousal (Morin, Rodrigue, & Ivers, 2003). Given the lack of other studies addressing these relationships, further research is needed to evaluate the sleep–mood cycle in insomnia.
In sum, the present study sought to extend and clarify the current literature on sleep and mood in bipolar disorder and insomnia. Participants in three groups—interepisode bipolar disorder, insomnia, and healthy control—were studied in a naturalistic setting using an experience sampling methodology. The study had three aims. First, we sought to replicate differences in nighttime sleep. The hypothesis was that the bipolar and insomnia groups would exhibit greater sleep disturbance than the control group (Edinger et al., 2004; Harvey et al., 2005). Second, we examined daytime mood. We expected that the bipolar and insomnia groups would exhibit greater negative mood than the control group. Moreover, we expected the bipolar group would exhibit greater negative mood and positive mood than the insomnia and control groups. The rationale is that negative mood appears to be characteristic of both bipolar disorder and insomnia, but positive and negative mood disturbances are central deficits of bipolar disorder (American Psychiatric Association, 2000). Third, we examined the relationship between sleep and mood. The hypothesis tested was that sleep disturbance would be associated with greater subsequent morning negative mood in the bipolar and insomnia groups, compared with the control group, and that sleep disturbance would be associated with greater subsequent morning positive mood in the bipolar group compared with the insomnia and control groups (Buysse et al., 2007; Perlman et al., 2006). We also predicted that evening negative mood disturbance would be associated with greater subsequent sleep disturbance in the insomnia group, compared with the bipolar and control groups (Morin et al., 2003; Talbot et al., 2009), and that evening positive mood would be associated with greater subsequent sleep disturbance in the bipolar group, compared with the insomnia and control groups (Talbot et al., 2009).
Method
Participants
Participants included 49 adults (ages 18–65) with bipolar I (n = 43) or bipolar II (n = 6) disorder who were currently interepisode, 34 adults with insomnia, and 52 healthy adults with no history of psychiatric or sleep disorders. Participants were recruited through Internet advertisements and flyers distributed to psychiatric clinics in the San Francisco Bay Area community. A telephone interview was completed to initially screen for eligibility. Individuals were excluded from participation on the basis of history of severe head trauma, stroke, neurological disease, severe medical illness, current mood episode, or current alcohol or substance abuse or dependence. Individuals who were considered likely to be eligible based on the initial telephone screen were invited to the laboratory for an extensive diagnostic interview session.
Individuals in the bipolar group were eligible to participate if they (a) met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000) criteria for a diagnosis of bipolar I or bipolar II disorder and (b) did not meet criteria for narcolepsy, sleep apnea, or periodic limb movement disorder based on the Duke Structured Interview for Sleep Disorders (DSISD; Edinger et al., 2004). In addition, bipolar participants were included only if they met criteria for interepisode symptom cutoffs based on prior research (Chengappa et al., 2003; Thompson et al., 2005): a score of less than 8 on the Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, 1978) and a score of less than 12 on the Inventory of Depressive Symptomatology, Clinician Rating (IDS-C; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). Given that bipolar disorder is typically associated with the presence of comorbid psychiatric diagnoses (Kessler et al., 2005), participants were not excluded on the basis of comorbid diagnoses other than current alcohol or substance abuse or dependence. Thirty-four of 49 bipolar participants (69%) had at least one current comorbid Axis I disorder. The average number of comorbidities for bipolar participants was 0.76 (SD = 0.97). Current comorbidities included specific phobia (n = 11), generalized anxiety disorder (n = 7), social phobia (n = 7), agoraphobia (n = 3), obsessive-compulsive disorder (n = 2), panic disorder (n = 2), posttraumatic stress disorder (n = 2), anorexia nervosa (n = 1), binge eating disorder (n = 1), and hypochondriasis (n = 1). All bipolar participants were required to be in the care of a psychiatrist. All but three of the 49 bipolar participants were taking psychotropic medication. The mean number of psychotropic medications taken was 2.3 (SD = 1.50). Seventy-six percent were taking mood stabilizers, 71% antidepressants, 71% antipsychotics, 57% anxiolytics, and 2% prescription sleep aids.
Participants in the insomnia group were eligible if they (a) met diagnostic criteria for a diagnosis of primary insomnia as assessed by the DSISD; (b) did not meet criteria for current sleep disorders, including narcolepsy, sleep apnea and periodic limb movement disorder; and (c) had scores of less than 8 on the YMRS and less than 12 on the IDS-C. Given that insomnia is commonly comorbid with one or more Axis I disorders (e.g., Ohayon, 1997), we did not exclude insomnia participants on the basis of comorbidities, with the exception of current alcohol or substance abuse or dependence and current depression. We ensured that insomnia was the primary diagnosis, defined as the disorder currently most distressing and disabling (Di Nardo et al., 1993). Fifteen of the 34 insomnia participants (44%) had at least one current comorbid Axis I disorder. The average number of comorbidities for insomnia participants was 0.56 (SD = 0.79). Current comorbidities included generalized anxiety disorder (n = 5), specific phobia (n = 5), binge eating disorder (n = 4), dysthymia (n = 3), social phobia (n = 2), panic disorder (n = 1), and posttraumatic stress disorder (n = 1). One participant was taking an antidepressant and one participant was taking a prescription sleep aid.
Participants in the control group were eligible if they (a) did not meet DSM–IV–TR criteria for any past or current Axis I disorder based on the SCID; (b) did not meet criteria for any past or current sleep disorder based on the DSISD; and (c) had scores of less than 8 on the YMRS and less than 12 on the IDS-C. No control participants were taking psychotropic medications.
Measures
Structured Clinical Interview for DSM–IV
The Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1995) is a semistructured interview designed to assess DSM–IV–TR diagnostic criteria for Axis I disorders. The SCID has been shown to have good reliability (Skre, Onstad, Torgersen, & Kringlen, 1991; Williams et al., 1992). Trained psychology doctoral students and postdoctoral fellows administered the SCID to all participants to assess current and lifetime Axis I disorders. Fifteen randomly selected audiotapes of SCID interviews were rated by a set of independent reviewers in order to check diagnostic reliability. These were randomly selected equally for the bipolar, control, and insomnia participants. Ratings matched 100% (κ = 1.00) of the primary diagnoses made by the original interviewer. This indicates strong interrater reliability, though the use of a “skip-out” strategy (implemented if initial required criteria for a particular disorder were not met) may have reduced the number of potential disagreements with the original interviewer.
Duke Structured Interview for Sleep Disorder
The Duke Structured Interview for Sleep Disorder (DSISD; Edinger et al., 2004) is a semistructured interview that assesses research diagnostic criteria for sleep disorders. The DSISD has been shown to have good reliability and validity (Edinger et al., 2009). Again, 15 randomly selected audiotapes of DSISD interviews were rated by a set of independent reviewers for diagnostic reliability. Ratings matched 100% (κ = 1.00) of the primary diagnoses made by the original interviewer.
Young Mania Rating Scale
The Young Mania Rating Scale (YMRS; Young et al., 1978) is an 11-item measure used to assess the severity of manic symptoms, with each item rated on a five-point scale. It has been shown to have good reliability and validity (Young et al., 1978).
Inventory of Depressive Symptomatology, Clinician Rating
The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C; Rush et al., 1996) is a widely used 30-item instrument assessing depressive symptoms, with each item rated on a four-point scale. The measure has demonstrated good reliability and validity (Rush et al., 1996).
Sleep diary
Following the standard recommendations for sleep research (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006), the sleep diary in the present study included questions to assess time to fall asleep (i.e., sleep onset latency), total length of time awake during the night (i.e., wake after sleep onset), amount of time awake between the final awakening and the time of getting out of bed (i.e., terminal wakefulness), number of awakenings during the night, time spent in bed, total sleep time, bed time, final wake time, and final arising time. In addition, total wake time was calculated as sleep onset latency + wake after sleep onset + terminal wakefulness (e.g., Morin, Kowatch, & Wade, 1989). Sleep efficiency was calculated by dividing total sleep time by time in bed and multiplying this value by 100. The sleep diary has been shown to be a reliable estimate (Morin & Espie, 2003) and is considered the gold standard subjective measure of sleep (Buysse et al., 2006).
Profile of Mood States—Short Form
The Profile of Mood States—Short Form (POMS-SF; Shacham, 1983) is a widely used 37-item scale derived from the original 65-item Profile of Mood States (McNair, Lorr, & Druppleman, 1971). The POMS-SF indices mood via six broad scales (tension-anxiety, depression-dejection, anger-hostility, fatigue, confusion, and vigor). From this scale an overall negative mood index was derived, comprising tension-anxiety, depression-dejection, anger-hostility, and confusion. Fatigue was excluded from negative mood to more clearly separate mood from tiredness in the context of sleep disturbance. The vigor scale comprised the positive affect measure, on the basis of previous research indicating it as strong measure of positive affect (Watson & Clark, 1994). The POMS-SF has established reliability and validity (McNair et al., 1971; Shacham, 1983).
Procedure
After completing the initial telephone screen described above, participants who appeared likely to be eligible completed seven days of sleep diaries and mood measures at home. Specifically, each morning on waking participants completed the sleep diary and POMS-SF. Each evening between 4:00 p.m. and bedtime participants again completed the POMS-SF. Participants called a voicemail box to record their answers each morning and evening. There were no differences between groups in percentage of entries that participants time-stamped by telephone (bipolar M = 73% entries, SD = 31%; insomnia M = 64% entries, SD = 35%; control M = 64% entries, SD = 32%).
After the one-week sleep and mood diary, participants visited the laboratory. Postdoctoral and doctoral student interviewers assessed the diagnostic status and symptom severity of participants by administering the SCID, the DSISD, the YMRS, and the IDS-C in the laboratory. Diaries of ineligible participants were destroyed (n = 76).
Analysis Plan
To examine hypotheses, we conducted three sets of analyses. In the first, we averaged each of the sleep and mood variables across the seven days. A multivariate analysis of variance (MANOVA) was conducted on the sleep variables and then on the mood variables. When significant multivariate effects were observed on dependent measures, post hoc t tests were conducted on those measures.
In the second set of analyses, we examined the potential effect of time on sleep and mood. To investigate patterns of sleep and mood over the course of the week, we used HLM 6.06 to perform multilevel analyses. Sleep and mood measures were nested within individuals. For each of the sleep and mood outcome measures, we first modeled overall effects of time predictors using dummy coded day variables and then added group status as a predictor (dummy coded as control = 0, bipolar = 1, insomnia = 0 or control = 0, bipolar = 0, insomnia = 1) to examine group by time interactions. The effects were interpreted off the omitted group. In these analyses the independent variables were centered, and both intercepts and slopes were allowed to vary as individual variability was predicted to be high.
The latter analyses were not significant (described in Results), indicating time did not have an effect on sleep and mood over the course of the week and consequently that a single-level framework could be adopted. Hence, in the third set of analyses, we abandoned multilevel analyses. Hierarchical multiple regression models were used to examine the sleep and mood relationships. We examined morning mood as the dependent variable immediately after nighttime sleep based on the timing of this relationship established by previous research (Bauer et al., 2006). We examined nighttime sleep immediately after evening mood as the dependent variable given that it is most proximal to evening mood. We entered the sleep or mood predictor in the first model, the main effects of bipolar and insomnia group status in the second model, and the interactions between group status and the sleep or mood predictor in the third model. The continuous variables were centered in the interactions. The same database structure as for the HLM analyses was used. Hence, all daily data points were used in the regression analyses rather than only seven-day aggregates, allowing transient fluctuations in individuals' responses to be captured.
Data Reduction
For the time trend and sleep–mood relationship analyses, total wake time was used as the sleep outcome measure on the basis of previous research (Eidelman, Talbot, Gruber, & Harvey, 2010; McCrae et al., 2008; Morin et al., 1989). As described above, participants were asked to record their nightly sleep and daily morning and evening moods for seven consecutive days. Four bipolar participants did not record their morning or evening moods across the seven days. Two control participants did not record their nightly sleep across the seven days. In addition, some participants missed recordings during the seven days. We examined the mean number of days of data across groups for the main outcome variables: total wake time, morning positive mood, morning negative mood, evening positive mood, and evening negative mood. There were no differences between groups in the mean number of days of recording (total wake time: bipolar M = 6.59 nights, SD = 0.86; insomnia M = 6.82 nights, SD = 0.52; control M = 6.66 nights, SD = 0.75; morning positive mood, morning negative mood: bipolar M = 5.98 days, SD = 1.25; insomnia M = 6.32 days, SD = 1.22; control M = 6.12 days, SD = 1.34; evening positive mood, evening negative mood: bipolar M = 5.84 days, SD = 1.31; insomnia M = 6.26 days, SD = 1.38; control M = 6.10 days, SD = 1.26).
Results
Participant Characteristics
There were no significant differences between groups in any of the demographic variables except age, with the insomnia participants being significantly younger than the bipolar and control participants (see Table 1). The groups differed on the IDS-C and YMRS. The bipolar and insomnia groups exhibited more depressive symptoms than the control group. The bipolar group exhibited more manic symptoms than the insomnia and control groups. All symptoms were well below established clinical cutoffs (Rush et al., 1996; Young et al., 1978).
Table 1.
Participant Characteristics
| Group |
||||
|---|---|---|---|---|
| Variable | Bipolar (n = 49) | Insomnia (n = 34) | Control (n = 52) | Test statistic |
| Gender | χ2 = .649 | |||
| Male | 15 | 8 | 17 | |
| Female | 34 | 26 | 35 | |
| Race/ethnicity | χ2 = .117 | |||
| African American | 5 | 3 | 4 | |
| Asian American | 2 | 10 | 7 | |
| Caucasian | 34 | 16 | 34 | |
| Hispanic | 3 | 0 | 3 | |
| Native American | 1 | 0 | 0 | |
| Other | 4 | 4 | 4 | |
| Employment status | χ2 = .185 | |||
| Full-time/part-time/student | 34 | 29 | 42 | |
| Unemployed/retired | 15 | 5 | 10 | |
| Marital status | χ2 = .136 | |||
| Single | 25 | 27 | 31 | |
| Married/partnered | 15 | 4 | 13 | |
| Divorced/separated/widowed | 9 | 3 | 8 | |
| Mean age (SD) | 36.78 (11.75) | 29.79 (9.92) | 36.85 (11.64) | F(2, 133) = 4.93** |
| Mean years of education (SD) | 15.37 (2.16) | 14.74 (1.90) | 15.21 (2.01) | F(2, 133) = 1.01 |
| Mean IDS-C (SD) | 8.00 (4.06) | 9.00 (5.17) | 2.93 (2.77) | F(2, 109) = 26.36*** |
| Mean YMRS (SD) | 2.80 (2.34) | 1.40 (1.50) | 1.02 (1.41) | F(2, 109) = 11.15*** |
Note. Mean values presented. IDS-C = Inventory of Depressive Symptomatology, Clinician Rating; YMRS = Young Mania Rating Scale.
p < .01.
p < .001.
Sleep and Mood Parameters
Sleep parameters
A multivariate analysis of variance (MANOVA) was conducted on the sleep variables with age as a covariate. A significant multivariate effect was observed on the following dependent measures: sleep onset latency, F(2, 102) = 23.28, p < .001; number of awakenings, F(2, 102) = 8.97, p < .001; wake after sleep onset, F(2, 102) = 15.77, p < .001; total wake time, F(2, 102) = 23.05, p < .001; and sleep efficiency, F(2, 102) = 28.31, p < .001. There were no significant differences in terminal wakefulness, total sleep time, bedtime, final wake time, final arising time, or time in bed.
Post hoc t tests were conducted on sleep onset latency, number of awakenings, wake after sleep onset, total wake time, and sleep efficiency. Both diagnostic groups differed from controls on all five variables. The diagnostic groups differed from one another on only two variables: the insomnia group had longer sleep onset latency and more wake after sleep onset than the bipolar group. Overall, then, the insomnia group exhibited worse sleep than both the healthy controls and patients with bipolar disorder. Mean values for these variables for each group are presented in Table 2.
Table 2.
Sleep Parameters Across Groups
| Group |
|||
|---|---|---|---|
| Variable | Bipolar | Insomnia | Control |
| Sleep onset latency | 25.32 (18.18)a | 33.75 (19.67)b | 8.22 (5.04)c |
| Wake after sleep onset | 19.31 (17.22)a | 36.02 (35.56)b | 10.21 (17.51)c |
| Terminal wakefulness | 30.27 (38.42)a | 27.24 (19.53)a | 16.53 (18.39)a |
| Number of awakenings | 1.55 (0.90)a | 1.82 (0.96)a | 1.02 (0.93)b |
| Total wake time | 74.89 (48.02)a | 97.01 (49.62)a | 34.97 (30.67)b |
| Time in bed | 505.66 (67.31)a | 512.86 (52.15)a | 483.67 (50.65)a |
| Total sleep time | 450.19 (68.98)a | 429.67 (48.20)a | 456.32 (50.12)a |
| Sleep efficiency | 85.45% (7.65%)a | 80.97% (9.25%)a | 93.09% (5.35%)b |
| Bedtime | 11:57p.m. (84.24)a | 12:21a.m. (95.51)a | 12:10a.m. (80.40)a |
| Waketime | 7:49 a.m (82.26)a | 8:24a.m. (72.70)a | 7:55a.m. (68.80)a |
| Final arising time | 8:22a.m. (82.05)a | 8:51a.m. (69.06)a | 8:12a.m. (74.83)a |
Note. Mean values are presented. Standard deviations are in parentheses. Means having the same subscript are not significantly different at p < .05. All values are in minutes unless otherwise noted.
Mood parameters
A between-groups MANOVA was conducted on the four mood outcomes (morning positive mood, morning negative mood, evening positive mood, and evening negative mood) with age as a covariate, indicating a significant multivariate effect for both morning negative mood, F(2, 130) = 12.91, p < .001, and evening negative mood, F(2, 130) = 10.46, p < .001. Post hoc t tests indicated that the control group had less morning negative mood than the bipolar group (p < .001) and the insomnia group (p < .001). The control group also had less evening negative mood than the bipolar group (p < .001) and the insomnia group (p < .001). There were no differences between the bipolar and insomnia groups on morning negative mood or evening negative mood. Results of the MANOVA indicated no significant effect for morning positive mood or evening positive mood. Mean values for these variables for each group are presented in Table 3.
Table 3.
Mood Parameters From the Profile of Mood States Across Groups
| Group |
|||
|---|---|---|---|
| Variable | Bipolar | Insomnia | Control |
| Morning | |||
| Positive mood | 4.20 (4.38)a | 4.86 (4.08)a | 6.17 (4.07)a |
| Negative mood | 13.78 (10.96)a | 12.58 (9.52)a | 5.13 (5.83)b |
| Evening | |||
| Positive mood | 4.05 (3.79)a | 3.90 (3.41)a | 4.60 (3.68)a |
| Negative mood | 13.53 (12.57)a | 11.47 (9.08)a | 4.76 (6.96)b |
Note. Mean values are presented. Standard deviations are in parentheses. Means having the same subscript are not significantly different at p < .05.
Time Trends
Multilevel analyses for the five outcome variables (total wake time, morning positive mood, morning negative mood, evening positive mood, evening negative mood) including group status yielded significant intercepts but no significant slopes. The significant intercepts were expected and paralleled the results exhibited by the MANOVAs described above. The lack of significant slopes indicated that time did not differentially affect sleep or mood across the groups. These analyses confirm that the previously conducted MANOVAs did not obscure group differences in time pattern over the course of the study.
Sleep–Mood Relationships
Sleep–subsequent morning mood relationship
Hierarchical multiple regression models were used to examine the sleep and mood relationships. When morning positive mood was regressed on previous night's total wake time for the full sample, including time variables (Days 1–7) as predictors, more total wake time was associated with lower morning positive mood, p < .01. When bipolar and insomnia diagnostic group status were added as predictors, both bipolar (p < .001) and insomnia (p < .05) group status predicted lower morning positive mood. Hence, both total wake time and group status were significant predictors of morning positive mood.
To examine whether the total wake time-morning positive mood relationship differed by diagnostic group, two interaction terms were added to the regression analysis (bipolar × total wake time and insomnia × total wake time). Neither interaction term was significant, indicating that diagnostic status did not moderate the total wake time-morning positive mood relationship.
Next, morning negative mood was regressed on previous night's total wake time for the full sample, including time variables (Days 1–7) as predictors. More total wake time was associated with higher morning negative mood, p < .001. When bipolar and insomnia diagnostic group status were added as predictors, both bipolar (p < .001) and insomnia (p < .001) group status predicted higher morning negative mood. Hence, both total wake time and group status were significant predictors of morning negative mood.
To examine whether the total wake time–morning negative mood relationship differed by diagnostic group, two interaction terms were added to the regression analysis (bipolar × total wake time and insomnia × total wake time). The bipolar interaction term was significant (p < .05), indicating that bipolar disorder diagnostic status moderated the total wake time–morning negative mood relationship. The insomnia interaction term was not significant. See Table 4 and Figure 1.
Table 4.
Models: Morning Positive and Negative Mood Predicted by Previous Night's Total Wake Time
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | SE | B | β | SE | B | β | SE | |
| Morning positive mood predicted by total wake time | |||||||||
| Total wake time (previous night) | −0.01** | −0.10 | 0.01 | −0.01 | 0.003 | 0.01 | −0.01 | −0.14 | 0.01 |
| Bipolar | −1.66*** | −0.15 | 0.44 | −1.48*** | −0.13 | 0.46 | |||
| Insomnia | −1.10* | −0.09 | 0.48 | −1.19* | −0.10 | 0.51 | |||
| Bipolar × total wake time (previous night) | 0.003 | 0.02 | 0.01 | ||||||
| Insomnia × total wake time (previous night) | 0.01 | 0.10 | 0.01 | ||||||
| R 2 | 0.02 | 0.03 | 0.04 | ||||||
| Morning negative mood predicted by total wake time | |||||||||
| Total wake time (previous night) | 0.03*** | 0.19 | 0.01 | 0.02** | 0.10 | 0.01 | 0.00 | 0.00 | 0.01 |
| Bipolar | 7.88*** | 0.31 | 0.96 | 8.32*** | 0.33 | 0.10 | |||
| Insomnia | 6.20*** | 0.23 | 1.06 | 6.78*** | 0.25 | 1.11 | |||
| Bipolar × total wake time (previous night) | 0.03* | 0.11 | 0.02 | ||||||
| Insomnia × total wake time (previous night) | 0.02 | 0.06 | 0.02 | ||||||
| R 2 | 0.06 | 0.14 | 0.14 | ||||||
p < .05.
p < .01.
p < .001.
Figure 1.
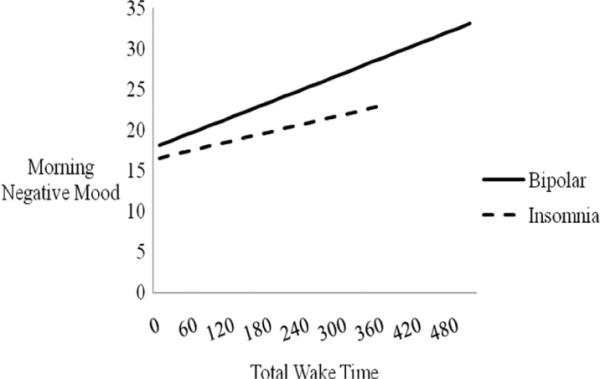
Total wake time (in minutes) predicting morning negative mood, moderated by group status. The estimated sleep–mood relationships based on the regression equations for each group are presented. Lines are depicted only to the maximum value for the independent variable in each group. The bipolar × total wake time interaction is significant (p < .05). Note that the control group slope = 0.00 and hence no line is depicted.
Evening mood–subsequent sleep relationship
Next, regression analyses were conducted in the second direction to examine the degree to which evening mood affects subsequent total wake time. When total wake time was regressed on evening positive mood for the full sample, evening positive mood was not associated with subsequent total wake time. When bipolar and insomnia diagnostic group status were added as predictors, both bipolar (p < .001) and insomnia (p < .001) group status predicted total wake time. Hence, group status, but not evening positive mood, was a significant predictor of total wake time.
To examine whether the evening positive mood–total wake time relationship differed by diagnostic group, two interaction terms were added to the regression analysis (bipolar × evening positive mood and insomnia × evening positive mood). The insomnia interaction term was significant (p < .05), indicating that insomnia diagnostic status moderated the evening positive mood-total wake time relationship. The bipolar interaction term was not significant. See Table 5 and Figure 2.
Table 5.
Models: Total Wake Time Predicted by Previous Evening's Positive and Negative Mood
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | SE | B | β | SE | B | β | SE | |
| Total wake time predicted by evening positive mood | |||||||||
| Evening positive mood | −0.33 | −0.02 | 0.50 | 0.07 | 0.01 | 0.47 | −0.42 | −0.03 | 0.77 |
| Bipolar | 33.94*** | 0.24 | 5.27 | 33.55*** | 0.23 | 5.25 | |||
| Insomnia | 53.67*** | 0.35 | 5.66 | 54.67*** | 0.36 | 5.65 | |||
| Bipolar × evening positive mood (previous night) | −0.60 | −0.03 | 1.07 | ||||||
| Insomnia × evening positive mood (previous night) | 3.17* | 0.11 | 1.24 | ||||||
| R2 | 0.02 | 0.12 | 0.13 | ||||||
| Total wake time predicted by evening negative mood | |||||||||
| Evening negative mood | 0.66*** | 0.13 | 0.18 | 0.26 | 0.05 | 0.18 | −0.82 | −0.146 | 0.43 |
| Bipolar | 32.58*** | 0.23 | 5.57 | 36.40*** | 0.25 | 5.79 | |||
| Insomnia | 50.58*** | 0.33 | 5.82 | 55.62*** | 0.36 | 6.05 | |||
| Bipolar × evening negative mood (previous night) | 1.44** | 0.19 | .50 | ||||||
| Insomnia × evening negative mood (previous night) | 1.13* | 0.12 | 0.53 | ||||||
| R 2 | 0.03 | 0.12 | 0.13 | ||||||
p < .05.
p < .01.
p < .001.
Figure 2.
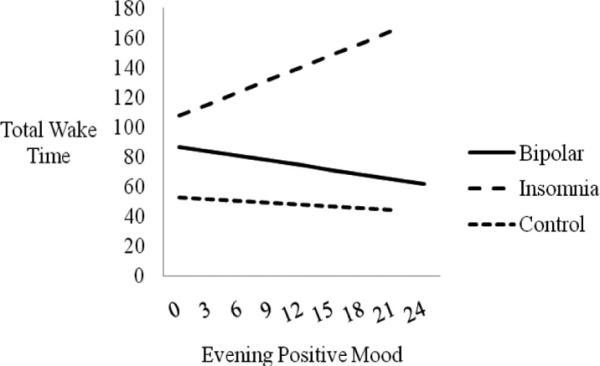
Evening positive mood predicting subsequent total wake time (in minutes), moderated by group status. The estimated sleep–mood relationships based on the regression equations for each group are presented. Lines are depicted only to the maximum value for the independent variable in each group. Only the insomnia × evening positive mood interaction is significant (p < .01).
When total wake time was regressed on evening negative mood for the full sample, more evening negative mood was associated with more subsequent total wake time (p < .001). When bipolar and insomnia diagnostic group status were added as predictors, both bipolar (p < .001) and insomnia (p < .001) group status predicted total wake time. Hence, both evening negative mood and group status were significant predictors of subsequent total wake time.
To examine whether the evening negative mood–total wake time relationship differed by diagnostic group, two interaction terms were added to the regression analysis (bipolar × evening negative mood and insomnia × evening negative mood). Both interaction terms were significant (bipolar, p < .01; insomnia, p < .05), indicating that group status moderated the evening negative mood-total wake time relationship. See Table 5 and Figure 3.
Figure 3.
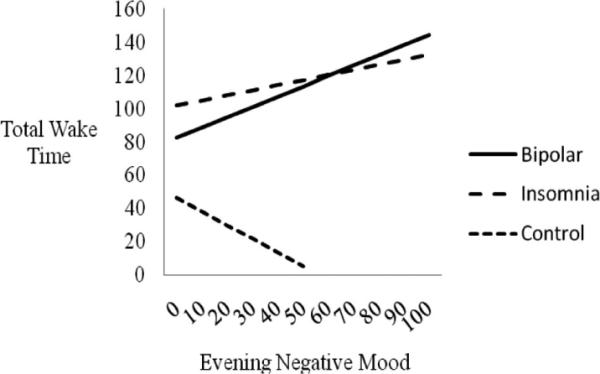
Evening negative mood predicting subsequent total wake time (in minutes), moderated by group status. The estimated sleep–mood relationships based on the regression equations for each group are presented. Lines are depicted only to the maximum value for the independent variable in each group. The bipolar × evening negative mood (p < .01) and insomnia × evening negative mood (p < .05) interactions are significant.
Discussion
The present study was designed to clarify and extend the current literature on sleep and mood, and their relationship, in bipolar disorder and insomnia. The first prediction was that the bipolar and insomnia groups would exhibit greater sleep disturbance than the control group. In support, both the insomnia and interepisode bipolar groups demonstrated more awakenings, longer total wake time, and reduced sleep efficiency compared with the control group but did not differ from each other. Moving on to the other sleep variables, the insomnia group exhibited longer sleep onset latency and more wake after sleep onset than the bipolar and control groups; the bipolar group in turn exhibited longer sleep onset latency and more wake after sleep onset than the control group. Combined, these results replicate previous research indicating that individuals with interepisode bipolar disorder experience sleep that is often as disturbed as those with insomnia (Harvey et al., 2005) and also draw attention to the severity of sleep disturbance in insomnia (Ohayon, 2002). In contrast, there were no differences across the three groups in terminal wakefulness, total sleep time, bedtime, waketime, final arising time, or time in bed, providing support for the current insomnia diagnostic criteria which emphasize difficulty initiating and maintaining sleep (American Psychiatric Association, 2000; Edinger et al., 2004). Moreover, the data extend the transdiagnostic two-factor structure of sleep complaints (Koffel & Watson, 2009) to interepisode bipolar disorder and insomnia, suggesting sleep disturbance in these disorders is characterized by the insomnia factor (i.e., waketime). The relevance of the lassitude factor (i.e., hypersomnia, fatigue, and sleepiness) in these populations should be further assessed.
Our second prediction was that the bipolar and insomnia groups would exhibit greater negative mood than the control group and further that the bipolar group would exhibit greater negative and positive mood than the insomnia and control groups. Consistent with the first part of this hypothesis, the bipolar and insomnia groups experienced more morning and evening negative mood compared with the control group. These results align with previous research that has observed heightened negative effect in the interepisode period in bipolar disorder (Yurgelun-Todd et al,, 2000) and more negative mood in insomnia (Bonnet & Arand, 1997). Clinically, the findings raise the possibility that mood disturbance may be an important target for the development of novel treatments for insomnia (Harvey et al., 2010). Interestingly, and contrary to our hypothesis, there were no differences in negative mood between the bipolar and insomnia groups, consistent with the proposed importance of transdiagnostic or universal processes (Barlow, Allen, & Choate, 2004; Fairburn, Cooper, & Shafran, 2003; Harvey, Watkins, Mansell, & Shafran, 2004). Importantly, mood in the insomnia group was not influenced by comorbid psychiatric disorders; mood parameter analyses comparing the subgroups without comorbidities (n = 15) and with comorbidities (n = 19) yielded no differences. With regard to positive mood, also contrary to our hypothesis, the bipolar group did not demonstrate more positive mood than the insomnia and control groups, suggesting that in a naturalistic context negative mood may be more prevalent than positive mood in interpisode bipolar disorder. It is possible that the POMS vigor scale does not adequately measure the type of positive mood disturbance characteristic of interepisode bipolar disorder. It is also possible that the one-week length of the study may not have been sufficient to observe elevated positive mood in bipolar participants, as such elevations may not be demonstrated at a trait level (for review see Watson & Naragon-Gainey, 2010) but rather may be linked to goal-attainment life events (Johnson et al., 2008). Indeed, in laboratory studies interepisode bipolar participants demonstrate elevated affective response to positive mood inductions (Farmer et al., 2006) but in the absence of a mood induction exhibit reduced attentional biases toward positive stimuli compared to healthy participants (Jongen, Smulders, Ranson, Arts, & Krabbendam, 2007). Hence, the growing literature reveals nuanced positive affect patterns that a one-week diary may not adequately capture.
The final aim was to examine the proposed bidirectional relationship between sleep and mood in bipolar disorder and insomnia. Sleep disturbance was operationalized as total wake time. We predicted that sleep disturbance would be associated with greater subsequent morning negative mood in the bipolar and insomnia groups compared with the control group. Consistently, bipolar group status moderated the effect of total wake time on subsequent morning negative mood. These results align with previous research showing that negative mood in bipolar disorder is adversely affected by sleep disturbance (Perlman et al., 2006). Neurobiological pathways may explain this effect of sleep on next morning mood. For example, healthy individuals who were sleep deprived exhibited increased amygdala activity and decreased activity in the medial–prefrontal cortex, a region known to exert top-down control on the limbic area (Yoo, Gujar, Hu, Jolesz, & Walker, 2007). It is possible that in bipolar disorder the threshold at which this effect occurs is lower (Leibenluft, Charney, & Pine, 2003). Cognitive–behavioral mechanisms may also underlie the effect of sleep on subsequent morning mood. For example, learned helplessness may occur when individuals with chronic sleep disturbance attempt to make “helpful” behavioral changes (e.g., extend time in bed) which actually perpetuate the sleep disturbance, resulting in a feeling of powerlessness to improve sleep (e.g., Riemann et al., 2010). Accordingly, the data from the present study suggest that two of the most empirically supported treatments for insomnia, stimulus control and sleep restriction, may be relevant for bipolar disorder given the emphasis within these treatment approaches on de-conditioning and consolidated sleep (e.g., Morin et al., 2006). These interventions should be applied moderately and cautiously to avoid a shift toward hypomania or mania (e.g., Harvey, 2008b; Smith, Huang, & Manber, 2005).
For the insomnia group, the finding that sleep disturbance does not predict morning negative mood adds to the accruing evidence that the daytime impairments that are reported by patients with insomnia may be, at least in part, independent of poor sleep (Neitzert Semler & Harvey, 2005). Hence, this finding adds to the calls within the literature for a detailed examination of daytime functioning among insomnia patients (Espie, 2002; Harvey, 2005). Clinically, if replicated, interventions may be needed to reduce expectations of negative consequences among amp;insomnia patients after poor sleep. One cognitive model of insomnia posits that such expectations may exacerbate actual consequences (Harvey, 2005).
We also predicted that sleep disturbance would be associated with greater subsequent morning positive mood in the bipolar group compared with the insomnia and control groups. This hypothesis was not supported. Bipolar disorder group status did not moderate the relationship between total wake time and subsequent morning positive mood (nor did insomnia group status). It is possible that many participants did not experience severe enough sleep disturbance within the one-week reporting period to influence morning positive mood. We note that the average total wake time for the bipolar group was 1.25 hours and that total sleep time averaged 7.5 hours. Perhaps moderate sleep disturbance is associated with subsequent morning negative mood, while more severe sleep disturbance is associated with subsequent morning positive mood disturbance (e.g., Wehr et al., 1987).
Moving on to the other direction of the sleep–mood cycle, our hypothesis was that evening negative mood disturbance would be associated with greater subsequent sleep disturbance in the insomnia group, compared with the bipolar and control groups, and that evening positive mood would be associated with greater subsequent sleep disturbance in the bipolar group, compared with the insomnia and control groups. Results indicated that both the bipolar and insomnia groups moderated the relationship between evening negative mood and subsequent sleep disturbance. This is consistent with the proposal that negative mood is likely to impact subsequent sleep (Espie, 2002). That this pattern of results held for both the bipolar and insomnia groups lends support for a transdiagnostic account (e.g., Barlow et al., 2004). Hence, theories of insomnia emphasizing physiologic activation, cognitive arousal, and failure to de-arouse before sleep (e.g., Bonnet & Arand, 2010; Espie, Broomfield, MacMahon, Macphee, & Taylor, 2006; Harvey, 2005) may also be relevant to interepisode bipolar disorder. It will be important for future research to delineate which of these potential contributors comprise universal processes and which are disorder-specific features (Harvey et al., 2004).
Interestingly, the association between evening positive mood and increased total wake time was moderated by the insomnia, but not bipolar disorder, group. The potential cognitive and physiological arousal associated with a positive mood state may have been antithetical to sleep in the insomnia group (Espie, 2002). However, it is surprising that this effect was not observed in the bipolar group, in contrast to previous findings (Talbot et al., 2009). While the already-highlighted concern about the measurement of positive mood remains an issue, it is also possible that the content of the positive mood in the present study differed from the previous study. The previous study included a mood induction with a positive autobiographical recall component, perhaps leading individuals to consider past experiences of success and reward (Johnson, 2005) with a consequent arousing effect before sleep. In contrast, the naturalistic aspect of the present study may have contributed to a less intense positive mood, with a lower likelihood of impacting sleep. Future research could elicit descriptions of mood content from participants to examine these potential differences.
Several limitations are important to consider. First, the relatively small sample size may have limited statistical power. Second, the overall predictive power of the significant interactions was low, explaining 13–14% of the variance, and the significant interactions explained only .5–1% of the variance beyond the group effects. Hence, it will be important to continue to examine additional factors affecting sleep and mood in bipolar disorder and insomnia. Third, to maximize the likelihood of attaining a response from participants, a range of time was available for participants to complete the evening mood measure (between 4 p.m. and bedtime). The majority of participants telephoned their entries and completion within this window of time was recorded. However, future research should (a) consider the use of mobile devices for sleep and mood measures with monetary incentives to encourage timely entries, and (b) more precisely record the time of completion to evaluate the impact of earlier versus later evening moods on subsequent sleep. We further recommend that future studies include an assessment of chronotype to control for the impact of circadian factors on sleep and mood (e.g., Hasler, Mehl, Bootzin, & Vazire, 2008).
In addition, the present study used subjective measures because insomnia is currently defined based on self-report (Edinger et al., 2004). However, previous research has sometimes demonstrated a discrepancy between objectively- and subjectively estimated sleep both in good sleepers and those with psychiatric disorders (Morin, 2000; Tang & Harvey, 2006). Hence, we recommend that future research include an objective estimate of sleep to help establish the biological and perceptual contributions to the sleep–mood cycle. We also note that previous research suggests that 2–3 weeks are necessary for some sleep variable averages because of their within-subject variability (Wolgemuth, Edinger, Fins, & Sullivan, 1999). We recommend that future studies collect data for a longer period of time. Similarly, it would be valuable to gather more frequent daytime mood and sleep assessments (e.g., napping) to more fully assess the relationship between sleep and mood. The latter two recommendations need to be balanced with a consideration of participant burden and compliance.
A final limitation is that sleep-related side effects of the psychotropic medications are a potential confound. We acknowledge that medication effects could contribute to increased or decreased wake time and may also influence morning and evening mood. However, research on medication-free bipolar samples is unrepresentative and lacks generalizability (Philips, Travis, Fagiolini, & Kupfer, 2008) and would severely limit progress on understanding serious mental illness across many areas including sleep, neuropsychology (Clark & Goodwin, 2004), and neuroimaging (Philips et al., 2008). Following previous research (Talbot et al., 2009), we elected to not control for possible medication effects for several reasons. Nearly all bipolar participants were medicated, and they were taking a heterogeneous group of medications. As such, there would be minimal statistical power to examine the effects of individual medications through subgroup analyses. Additionally, numerous medications can have either sedating or alerting side effects (e.g., aripiprazole, risperidone, venlafaxine, sertraline, zonisamide; Physicians' Desk Reference Staff, 2007), making it difficult to create sedating-medication and alerting-medication subgroups. Finally, the majority of bipolar participants were taking several psychotropic medications, potentially creating unknown interaction effects. We note, however, that the prevalence of sleep-related side effects are relatively low (e.g., between 4 and 37% for the medications taken in the present study's bipolar sample; Physicians' Desk Reference Staff, 2007). Additionally, many individuals experience side effects only early in treatment (Ketter & Wang, 2002). Nonetheless, there is a need for future research to either adapt research designs or develop statistical approaches to allow for continued progress. Finally, we note that for consistency across groups we did not control for medications in the insomnia group, though this issue applied to only two of the 34 participants.
In sum, consistent with the proposed importance of transdiagnostic or universal processes, the current study provides evidence that in both interepisode bipolar disorder and insomnia sleep is significantly disturbed, negative mood is heightened, and there is a relationship between negative mood and subsequent sleep disturbance. Together, these results are congruent with the theory that disruptions in nighttime sleep and daytime mood may be mutually maintaining (Benca et al., 1992; Harvey, 2008b) and extend the current literature by suggesting that negative mood and sleep disturbance are bidirectionally related in interepisode bipolar disorder.
Acknowledgments
We thank Howard Liu for his assistance with data collection and coding. This research was supported by the National Institute of Mental Health Grant R34 MH080958 (awarded to A.G.H.) and a dissertation grant from The Society for a Science of Clinical Psychology (SSCP) and the National Institute of Mental Health Grant 5T32MH020006 (awarded to L.S.T.).
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 2000. text revision. [Google Scholar]
- Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behavior Therapy. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. doi:10.1016/S0005-7894(04)80036-4. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disorders. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. doi:10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Archives of General Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, Obermeyer WH. Sleep and mood disorders. Sleep Medicine Reviews. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. doi:10.1016/S1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Medicine Reviews. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. doi:10.1016/S1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Medicine Reviews. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. doi:10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall M, Kupfer DJ. Daytime symptoms in primary insomnia: A prospective analysis using ecologicla momentary assessment. Sleep Medicine. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. doi:10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DJ, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chengappa KNR, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, Stapf DA. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. American Journal of Psychiatry. 2003;60:1636–1642. doi: 10.1176/appi.ajp.160.9.1636. doi:10.1176/appi.ajp.160.9.1636. [DOI] [PubMed] [Google Scholar]
- Clark L, Goodwin GM. State- and trait-related deficits in sustained attention in bipolar disorder. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:61–68. doi: 10.1007/s00406-004-0460-y. doi:10.1007/s00406-004-0460-y. [DOI] [PubMed] [Google Scholar]
- Di Nardo PA, Moras K, Barlow DH, Rapee RM, Brown TA. Reliability of the DSM-III-R anxiety disorder categories: Using the Anxiety Disorders Schedule-Revised (ADIS-R) Archives of General Psychiatry. 1993;50:251–256. doi: 10.1001/archpsyc.1993.01820160009001. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, American Academy of Sleep Medicine Work Group Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Olsen MK, Stechuchak KM, Carney CE, Chiang A, et al. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening; Paper presented at the 23rd Annual Meeting of the Associated Professional Sleep Societies, LLC.2009. [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:145–149. doi: 10.1016/j.jbtep.2009.11.007. doi:10.1016/j.jbtep.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie CA. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annual Review of Psychology. 2002;53:215–243. doi: 10.1146/annurev.psych.53.100901.135243. doi:10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: A theoretical review. Sleep Medicine Reviews. 2006;10:215–245. doi: 10.1016/j.smrv.2006.03.002. doi:10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behaviour Research and Therapy. 2003;41:509–528. doi: 10.1016/s0005-7967(02)00088-8. doi:10.1016/S0005-7967(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Farmer A, Lam D, Sahakian B, Roiser J, Burker A, O'Neill N, McGuffin P. A pilot study of positive mood induction in euthymic bipolar subjects compared with healthy controls. Psychological Medicine. 2006;36:1213–1218. doi: 10.1017/S0033291706007835. doi:10.1017/S0033291706007835. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer MB, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders–Patient Edition (SCID-I/P, Version 2.0) Biomedics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fukuda K, Etoh T, Iwadate T, Ishii A. The course and prognosis of manic-depressive psychosis: A quantitative analysis of episodes and intervals. Tohoku Journal of Experimental Medicine. 1983;139:299–307. doi: 10.1620/tjem.139.299. doi:10.1620/tjem.139.299. [DOI] [PubMed] [Google Scholar]
- Gruber J. Can feeling too good be bad? A review and synthesis of positive emotion disturbance in bipolar disorder. Clinical Psychology and Psychotherapy. doi: 10.1002/cpp.776. in press. [DOI] [PubMed] [Google Scholar]
- Harrow M, Goldberg JF, Grossman LS, Meltzer HY. Outcome in manic disorders: A naturalistic follow-up study. Archives of General Psychiatry. 1990;47:665–671. doi: 10.1001/archpsyc.1990.01810190065009. [DOI] [PubMed] [Google Scholar]
- Harvey AG. A cognitive theory of and therapy for chronic insomnia. Journal of Cognitive Psychotherapy An International Quarterly. 2005;19:41–60. [Google Scholar]
- Harvey AG. Insomnia, psychiatric disorders, and the transdiagnostic perspective. Current Directions in Psychological Science. 2008a;17:299–303. doi:10.1111/j.1467-8721.2008.00594.x. [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. American Journal of Psychiatry. 2008b;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. doi:10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, McGlinchey EL, Gruber J. Toward an affective science of insomnia treatments. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. Guilford Press; New York: 2010. [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. doi:10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. Oxford University Press; Oxford: 2004. [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. Journal of Research in Personality. 2008;42:1537–1546. doi:10.1016/j.jrp.2008.07.012. [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of Affective Disorders. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. doi:10.1016/S0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. doi:10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Cuellar AK, Ruggero C, Winett-Perlman C, Goodnick P, White R, Miller I. Life events as predictors of mania and depression in bipolar I disorder. Journal of Abnormal Psychology. 2008;117:268–277. doi: 10.1037/0021-843X.117.2.268. doi:10.1037/0021-843X.117.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen EM, Smulders FT, Ranson SM, Arts BM, Krabbendam L. Attentional bias and general orienting processes in bipolar disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:168–183. doi: 10.1016/j.jbtep.2006.10.007. doi:10.1016/j.jbtep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. doi:10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Wang PW. Psychotropic medications in bipolar disorder: Pharmacodynamics, pharmacokinetics, drug interactions, adverse effects and their management. In: Yatham LM, Kusumakar V, editors. Bipolar disorder: A clinician's guide to biological treatments. Routledge; London: 2002. p. 320. [Google Scholar]
- Koffel E, Watson D. The two-factor structure of sleep constraints and its relation to depression and anxiety. Journal of Abnormal Psychology. 2009;118:183–194. doi: 10.1037/a0013945. doi:10.1037/a0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of bipolar disorder. Society of Biological Psychiatry. 2003;53:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. doi:10.1016/S0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Marriott M, Begin H, Robb J, Joffe RT, Young LT. Subsyndromal symptoms assessed in longitudinal, prospective follow-up of a cohort of patients with bipolar disorder. Bipolar Disorders. 2003;5:349–355. doi: 10.1034/j.1399-5618.2003.00048.x. doi:10.1034/j.1399-5618.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- McCrae CS, McNamara CS, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, Craggs JG. Sleep and affect in older adults: Using multilevel modeling to examine daily associations. Journal of Sleep Research. 2008;17:42–53. doi: 10.1111/j.1365-2869.2008.00621.x. doi:10.1111/j.1365-2869.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Druppleman LF. EITS manual for the profile of mood states. Educational and Industrial Test Services; San Diego: 1971. [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: A controlled naturalistic study using actigraphy. Journal of Affective Disorders. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. doi:10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Monroe LJ. Psychological and physiological differences between good and poor sleepers. Journal of Abnormal Psychology. 1967;72:255–264. doi: 10.1037/h0024563. doi:10.1037/h0024563. [DOI] [PubMed] [Google Scholar]
- Morin CM. The nature of insomnia and the need to redefine our diagnostic criteria. Psychosomatic Medicine. 2000;62:483–485. doi: 10.1097/00006842-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger J, D., Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: An update of recent evidence (1998–2004) Sleep. 2006;29:1396–1406. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. Kluwer Academic/Plenum Press Publishers; New York: 2003. [Google Scholar]
- Morin CM, Kowatch RA, Wade JB. Behavioral management of sleep disturbances secondary to chronic pain. Journal of Behavior Therapy and Experimental Psychiatry. 1989;20:295–302. doi: 10.1016/0005-7916(89)90060-8. doi: 10.1016/0005-7916(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic Medicine. 2003;65:259–267. doi: 10.1097/01.psy.0000030391.09558.a3. doi:10.1097/01.PSY.0000030391.09558.A3. [DOI] [PubMed] [Google Scholar]
- Neitzert Semler C, Harvey AG. Misperception of sleep can adversely affect daytime functioning in insomnia. Behaviour Research and Therapy. 2005;43:843–856. doi: 10.1016/j.brat.2004.06.016. doi:10.1016/j.brat.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: Distinguishing insomnia related to mental disorders from sleep disorders. Journal of Psychiatric Research. 1997;31:333–346. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disorders. 2006;8:271–274. doi: 10.1111/j.1399-5618.2006.00330.x. doi:10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Philips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. doi:10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physicians' Desk Reference Staff . Physicians' desk reference 2008: Hospital/library version. 62nd ed. Thomson Health-care; Stamford: 2007. [Google Scholar]
- Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Medicine Reviews. 2000;4:277–298. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Roth T, Roehrs T, Pies R. Insomnia: Pathophysiology and implications for treatment. Sleep Medicine Reviews. 2007;11:71–79. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ruggero CJ, Johnson SL. Reactivity to a laboratory stressor among individuals with Bipolar I disorder in full or partial remission. Journal of Abnormal Psychology. 2006;115:539–544. doi: 10.1037/0021-843X.115.3.539. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psycho-metric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Shacham S. A shortened version of the Profile of Mood States. Journal of Personality Assessment. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. American Journal of Psychiatry. 1997;154:1417–1423. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the structured clinical interview for DSM-III-R axis I (SCID-I) Acta Psychiatrica Scandinavica. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Smith TM, Huang IM, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clinical Psychology Review. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Johnson SJ. Hypomanic tendencies predict lower startle magnitudes during pleasant pictures. Psychophysiology. 2002;39:S80. [Google Scholar]
- Talbot LS, Hairston IS, Eidelman P, Gruber J, Harvey AG. The effect of mood on sleep onset latency and REM sleep in interepisode bipolar disorder. Journal of Abnormal Psychology. 2009;118:448–458. doi: 10.1037/a0016605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG. Altering misperception of sleep in insomnia: Behavioural experiments versus verbal explanation. Journal of Consulting and Clinical Psychology. 2006;74:767–776. doi: 10.1037/0022-006X.74.4.767. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, Young AH. Neurocognitive impairment in euthymic patients with bipolar affective disorder. British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Woolson RF, Fleming JA. Long-term outcome of major psychoses. I. Schizophrenia and affective disorders compared with psychiatrically symptom-free surgical conditions. Archives of General Psychiatry. 1979;36:1295–1301. doi: 10.1001/archpsyc.1979.01780120025002. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Kales A. Sleep and its disorders. Annual Review of Medicine. 1999;50:387–400. doi: 10.1146/annurev.med.50.1.387. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the positive and negative affect schedule-expanded form. University of Iowa; IA: 1994. Unpublished manuscript. [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: Evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review. 2010;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. American Journal of Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davis M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID): Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Wolgemuth WK, Edinger JD, Fins AI, Sullivan RJ. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–244. [PubMed] [Google Scholar]
- World Health Organization . World Health Report 2001. Mental health: New understanding, new hope. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Yoo S, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep–a prefrontal amygdala disconnect. Current Biology. 2007;17:877–878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]


