Abstract
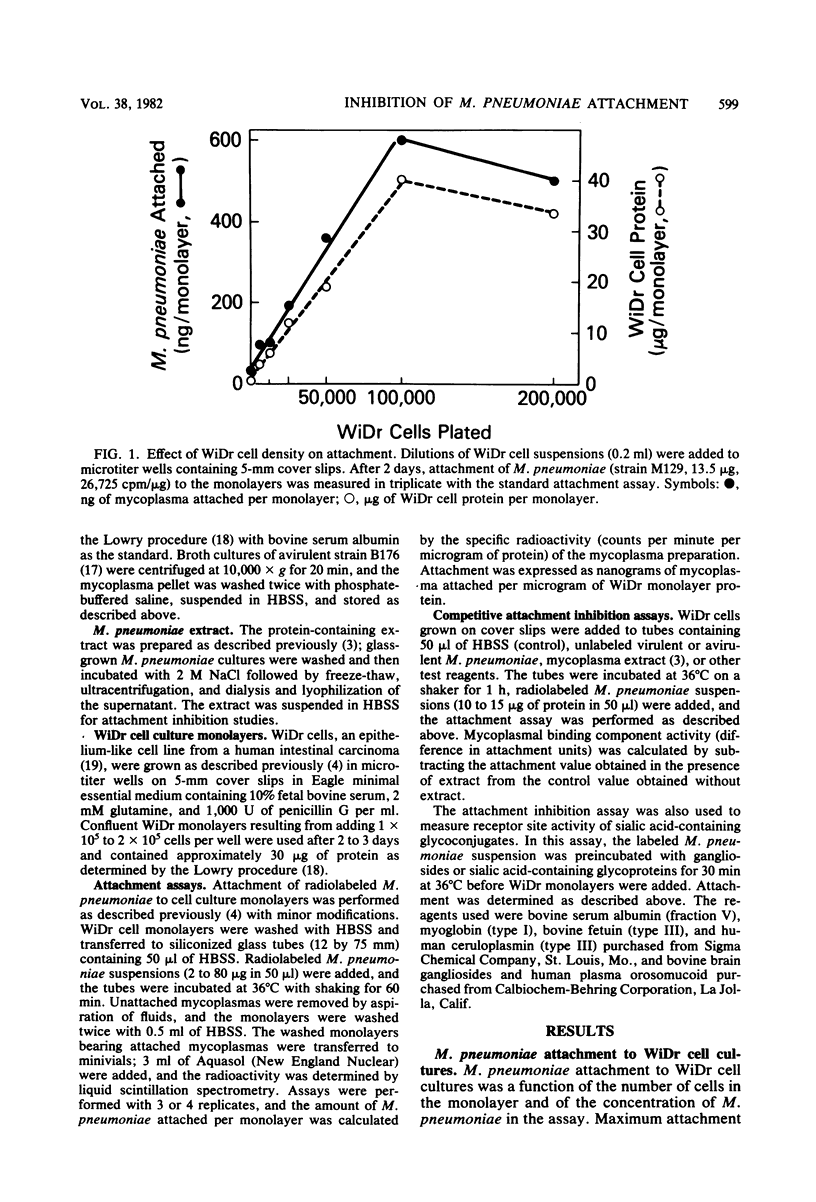
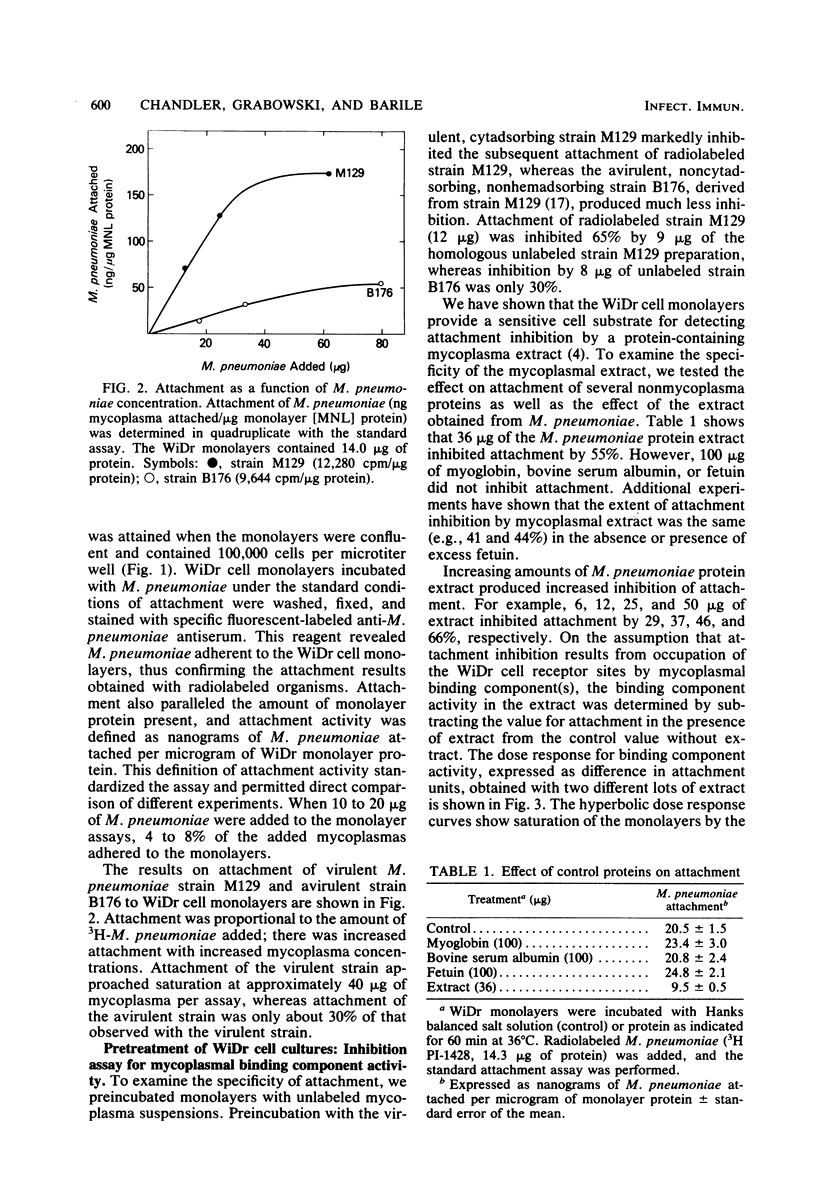
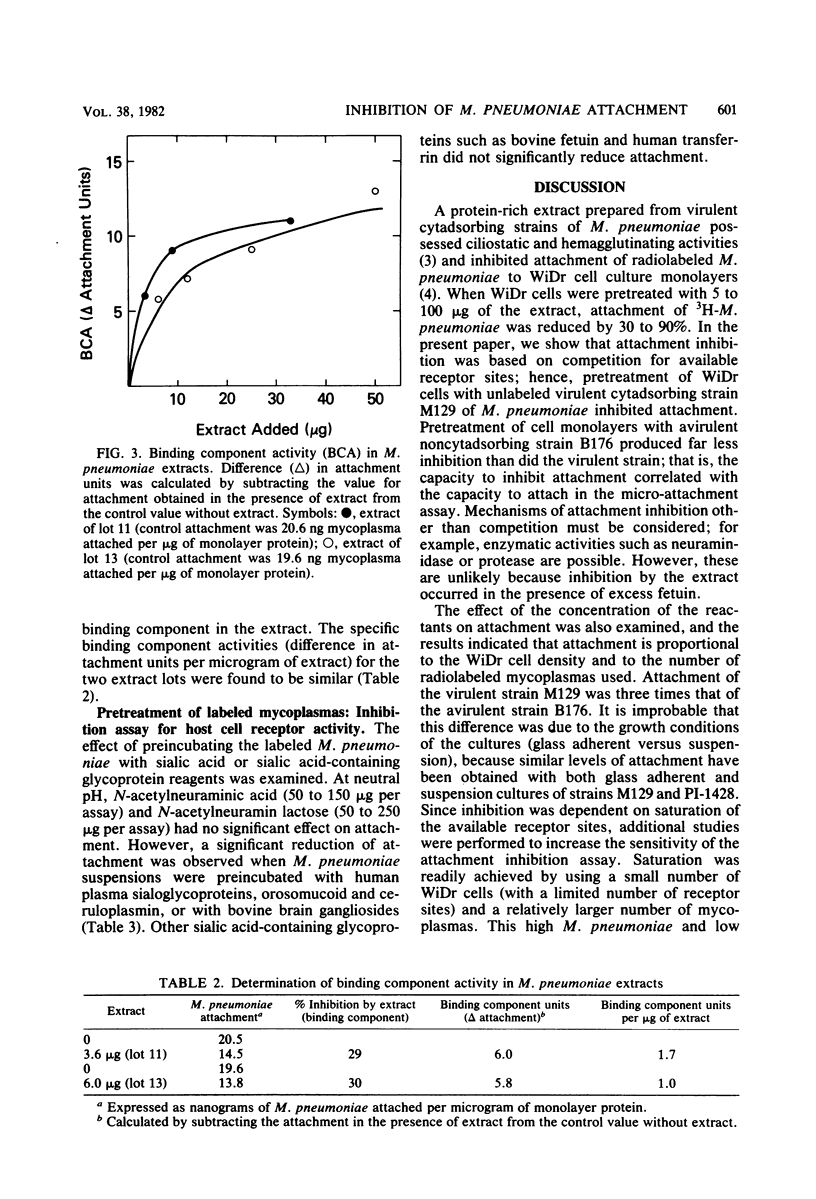
Attachment of Mycoplasma pneumoniae to human WiDr cell culture monolayers was examined by using radiolabeled M. pneumoniae. The amount of attachment was proportional to the density of the WiDr cells and to the concentration of M. pneumoniae in the assay. Saturation of the monolayers was achieved with 40 micrograms of virulent strain M129 per assay, whereas binding of avirulent strain B176 was 70% less than that of strain M129. A competitive attachment inhibition assay was used to measure specific binding component activity. Attachment was inhibited when WiDr cells were pretreated with unlabeled virulent strain M129, whereas avirulent noncytadsorbing strain B176 did not inhibit attachment as well as the virulent strain. A protein-rich extract prepared from virulent, cytadsorbing strains of M. pneumoniae also inhibited attachment. The amount of inhibition was dependent on the amount of extract used, and units for binding component activity in the extract were calculated from the competitive attachment inhibition assays. The competitive attachment inhibition assay was also used to investigate the nature of the receptor site on the WiDr cells. Attachment was inhibited when the radiolabeled M. pneumoniae suspensions were pretreated with human sialoglycoproteins, such as orosomucoid and ceruloplasmin, and bovine gangliosides. These findings support the present concept that the mammalian receptor site for M. pneumoniae is a sialic acid-containing glycoprotein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banai M., Kahane I., Razin S., Bredt W. Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect Immun. 1978 Aug;21(2):365–372. doi: 10.1128/iai.21.2.365-372.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., Razin S., Bredt W., Kahane I. Isolation of binding sites to glycophorin from Mycoplasma pneumoniae membranes. Infect Immun. 1980 Dec;30(3):628–634. doi: 10.1128/iai.30.3.628-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Collier A. M., Barile M. F. Attachment of Mycoplasma pneumoniae to hamster tracheal organ cultures, tracheal outgrowth monolayers, human erythrocytes, and WiDr human tissue culture cells. Infect Immun. 1982 Mar;35(3):937–942. doi: 10.1128/iai.35.3.937-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- Feldner J., Bredt W., Kahane I. Adherence of erythrocytes to Mycoplasma pneumoniae. Infect Immun. 1979 Jul;25(1):60–67. doi: 10.1128/iai.25.1.60-67.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Barden-Stahl Y. D., Polisky R. B., Engelhardt J. A. Differences in the attachment of Mycoplasma pneumoniae cells and membranes to tracheal epithelium. Infect Immun. 1977 Jun;16(3):766–772. doi: 10.1128/iai.16.3.766-772.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Gunderson H., Schaeffer S. L., Barden-Stahl Y. D. Ciliated respiratory epithelial monolayers: new model for Mycoplasma pneumoniae infection. Infect Immun. 1978 Jul;21(1):333–336. doi: 10.1128/iai.21.1.333-336.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D., Davies H. A., Dourmashkin R. R. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: characterization of the in vitro model. Infect Immun. 1979 Jul;25(1):446–454. doi: 10.1128/iai.25.1.446-454.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: role of receptor sites. Infect Immun. 1979 Jul;25(1):455–459. doi: 10.1128/iai.25.1.455-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. R., Hill R. L. Interaction of Mycoplasma gallisepticum with sialyl glycoproteins. Infect Immun. 1980 Nov;30(2):353–361. doi: 10.1128/iai.30.2.353-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Clyde W. A., Jr, Baseman J. B. Characterization of hemadsorption-negative mutants of Mycoplasma pneumoniae. Infect Immun. 1981 Apr;32(1):127–136. doi: 10.1128/iai.32.1.127-136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi P., Wallace R., Johnson J., Earley E. M., O'Brien S., Ferrone S., Pellegrino M. A., Milstien J., Needy C., Browne W. Characterization of the WIDR: a human colon carcinoma cell line. In Vitro. 1979 Jun;15(6):401–408. doi: 10.1007/BF02618407. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


