Abstract
First-order (contrast) surround suppression has been well characterized both psychophysically and physiologically, but relatively little is known as to whether the perception of second-order visual stimuli exhibits analogous center-surround interactions. Second-order surround suppression was characterized by requiring subjects to detect second-order modulation in stimuli presented alone or embedded in a surround. Both contrast-(CM) and orientation-modulated (OM) stimuli were used. For most subjects and both OM and CM stimuli, second-order surrounds caused thresholds to be higher, indicative of second-order suppression. For CM stimuli, suppression was orientation-specific, i.e., higher thresholds for parallel than for orthogonal surrounds. However, the evidence for orientation specificity of suppression for OM stimuli was weaker. These results suggest that normalization, leading to surround suppression, operates at multiple stages in cortical processing.
Keywords: texture, 2nd-order, surround suppression, spatial vision
1. Introduction
An abundance of evidence suggests that the early visual system analyzes visual information using relatively independent “channels” selective for orientation and spatial frequency (Blakemore & Campbell, 1969; Campbell & Robson, 1968; Campbell, Carpenter, & Levinson, 1969; De Valois & De Valois, 1988; Graham, 1989; Graham & Nachmias, 1971). Each channel is composed of a set of spatially localized linear filters that together tile the visual field. In particular, psychophysical sensitivity to luminance modulations (a type of “first-order” cue in the visual image) is adequately captured by a computational model involving linear filtering followed by rectification; these linear filters are in turn represented neurophysiologically by the classical receptive fields of neurons in the primary visual cortex. While linearity and independence provide a good first approximation to the filter responses, complex, nonlinear spatial interactions among filters have also been well documented.
One such nonlinear spatial interaction is surround suppression. Psychophysically, when a target stimulus is embedded in a high-contrast mask or placed in the vicinity of high-contrast flankers, it becomes harder to detect or discriminate (Petrov, Carandini, & McKee, 2005; Polat & Sagi, 1993; Snowden & Hammett, 1998; Wilkinson, Wilson, & Ellemberg, 1997; Zenger-Landolt & Heeger, 2003) and its perceived contrast is lower (Cannon & Fullenkamp, 1991; Chubb, Sperling, & Solomon, 1989; Ejima & Takahashi, 1985; Olzak & Laurinen, 1999; Snowden & Hammett, 1998; Solomon, Sperling, & Chubb, 1993; Xing & Heeger, 2000; 2001). This is known as surround suppression. Suppression is maximal when the target and surround stimuli have matching spatial frequency and orientation (Cannon & Fullenkamp, 1991; Chubb et al., 1989; Olzak & Laurinen, 1999; Solomon et al., 1993; Xing & Heeger, 2001) and increases with increasing contrast of the surround (Olzak & Laurinen, 1999; Snowden & Hammett, 1998; Solomon et al., 1993).
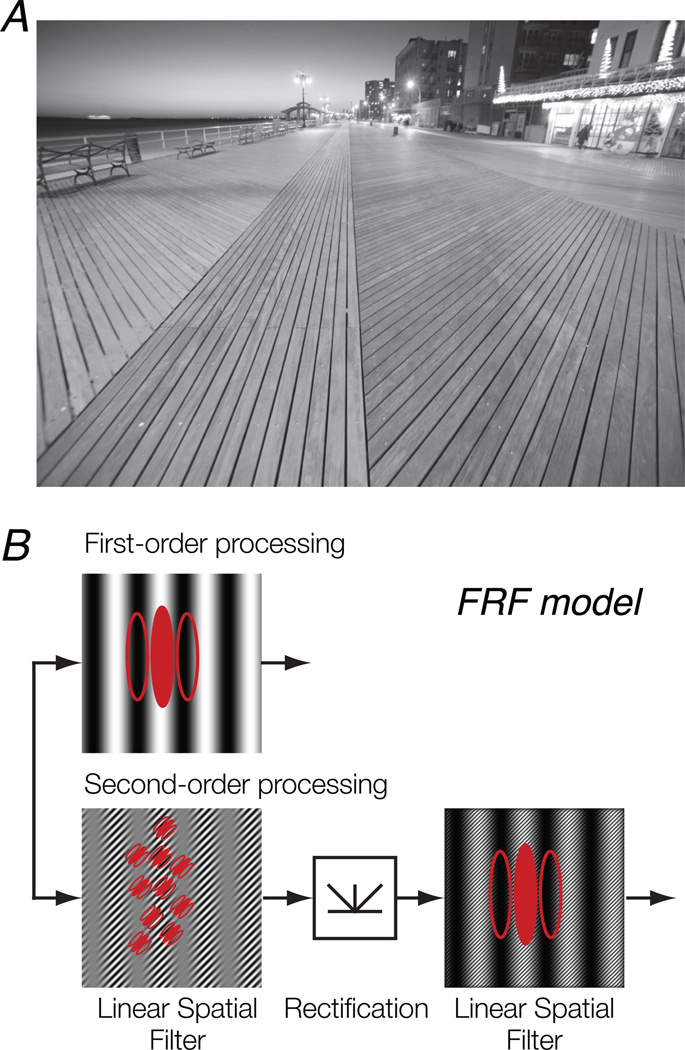
The human visual system is also able to detect image attributes other than luminance modulations. Spatial variations of texture properties (e.g., local orientation, spatial frequency, or contrast) in the visual image are called “second-order.” These kinds of patterns are distinct from first-order, luminance-defined patterns in that they cannot be detected by a simple linear mechanism since there is no variation in mean luminance across the image. The boardwalk in Fig. 1A is an example of a texture-defined pattern that contains modulations of local orientation. The computational models typically used to explain human sensitivity to second-order image structure are called “filter-rectify-filter” (FRF) or “back-pocket” models (Fig. 1B; Chubb & Landy, 1991; Chubb, Econopouly, & Landy, 1994; see Landy & Graham, 2004, for a review). An initial stage of linear filtering is selective for a constituent texture. The output from the first stage is subjected to a static nonlinearity (e.g., full-wave rectification). A second-stage linear filter at a coarser spatial scale is then applied to the rectified, first-stage responses. This results in selectivity for the orientation and spatial frequency of second-order texture modulation. The detection of second-order image structure is thought to operate independently of that of first-order structure.
Figure 1.
Models of second-order processing. A. A natural scene containing second-order patterns. The boardwalk contains modulations of texture (defined by local orientation), which cannot be detected by a simple linear mechanism. B. A typical model of visual processing depicting the parallel pathways for first- and second-order stimuli. Top, first-order, luminance-defined stimuli are processed by a linear filter. Bottom, second-order, texture-defined stimuli are processed via a filter-rectify-filter (FRF) cascade.
Relatively little is known as to whether the perception of second-order stimuli exhibits analogous center-surround interactions observed for first-order stimuli. One psychophysical study provided evidence for second-order surround suppression based on the appearance of texture stimuli, in particular the perceived modulation depth of contrast-modulated stimuli (Ellemberg, Allen, & Hess, 2004). If a surround suppresses the response to a central, second-order stimulus, then its perceived modulation depth would be reduced. The authors found that, analogous to first-order suppression, second-order suppression was selective for orientation and spatial frequency, but the tuning was more broadband (i.e., the suppression effect was evident for greater differences in relative orientations or spatial frequencies between the target and the surround, as compared to first-order suppression).
We wondered whether the same suppressive effects generalized across different types of second-order stimuli. Here, we used a psychophysical protocol involving the detection of both contrast and orientation modulation to test for and characterize second-order surround suppression. This mirrors analogous experiments on first-order suppression that measured perceived contrast or detection/discrimination sensitivity (Cannon & Fullenkamp, 1991; Chubb et al., 1989; Ejima & Takahashi, 1985; Olzak & Laurinen, 1999; Petrov et al., 2005; Polat & Sagi, 1993; Snowden & Hammett, 1998; Solomon et al., 1993; Wilkinson et al., 1997; Xing & Heeger, 2000; 2001; Zenger-Landolt & Heeger, 2003), though there is no simple way to relate appearance and discrimination measures (see, e.g., Snowden & Hammett, 1998). Furthermore, the use of orientation-modulated stimuli also helped to put aside concerns about potential artifacts present in contrast-modulated stimuli, such as distortion products caused by nonlinearities in the display or early luminance nonlinearities in the visual system (Schofield & Georgeson, 1999; Smith & Ledgeway, 1997). We measured thresholds for second-order target stimuli in the presence of surround stimuli with varying depth and orientation of modulation, and found that target thresholds were greater when the surround comprised a second-order modulation. Furthermore, to our surprise, suppression was only consistently orientation-specific for contrast-modulated stimuli, while support for orientation-specific suppression in orientation-modulated stimuli was weaker. These results are consistent with the idea that there is a plethora of distinct second-order mechanisms, with different second-stage suppression mechanisms, and that the goal of second-order vision is not only to detect boundaries, but also to extract and characterize image statistics, as required by models of texture appearance (e.g., Portilla & Simoncelli, 2000).
2. Methods
2.1. Subjects
Six subjects (two females, aged 25–52) with normal or corrected-to-normal vision participated in the study. Subjects included two of the co-authors. All subjects were experienced psychophysical observers. Subjects provided written informed consent, and the experimental protocol was approved by the University Committee on Activities involving Human Subjects at New York University.
2.2. Visual stimuli
Stimuli were generated using MATLAB (MathWorks, MA) and displayed on a 22’’ flat-screen CRT monitor (Hewlett-Packard p1230; resolution: 1152 × 870; refresh rate: 75 Hz) at a distance of 57 cm. The monitor provided approximately 39.1 × 30.0 deg viewing angle. The display was calibrated and gamma-corrected using a linearized lookup table.
The second-order stimuli were contrast-modulated (CM) or orientation-modulated (OM) horizontal and vertical grating patterns (Fig. 2A,B). A CM grating LCM (Fig. 2A) was generated by sinusoidally modulating the luminance contrast of a noise carrier image N(x,y),
| (1) |
where L0 is the background luminance, AM is the modulation amplitude, and M(x,y) is the modulator image of a two-dimensional vertical or horizontal sine wave grating with spatial frequency (SF) f and phase ϕ. M(x,y) = sin(2π f x + ϕ) (vertical) or M(x,y) = sin(2π f y + ϕ) (horizontal). The carrier image N(x,y) was white noise filtered with an isotropic bandpass filter. The filter was a cosine-ramped annulus in the Fourier domain, with a center SF of 8 cyc/deg and a bandwidth of 1 octave (i.e., the annulus extended from 5.7 – 11.3 cyc/deg). N(x,y) was normalized so that 99.5% of the pixels had values within the range of [−1,1]; the small number of pixels with values outside of that range were clipped to −1 or 1.
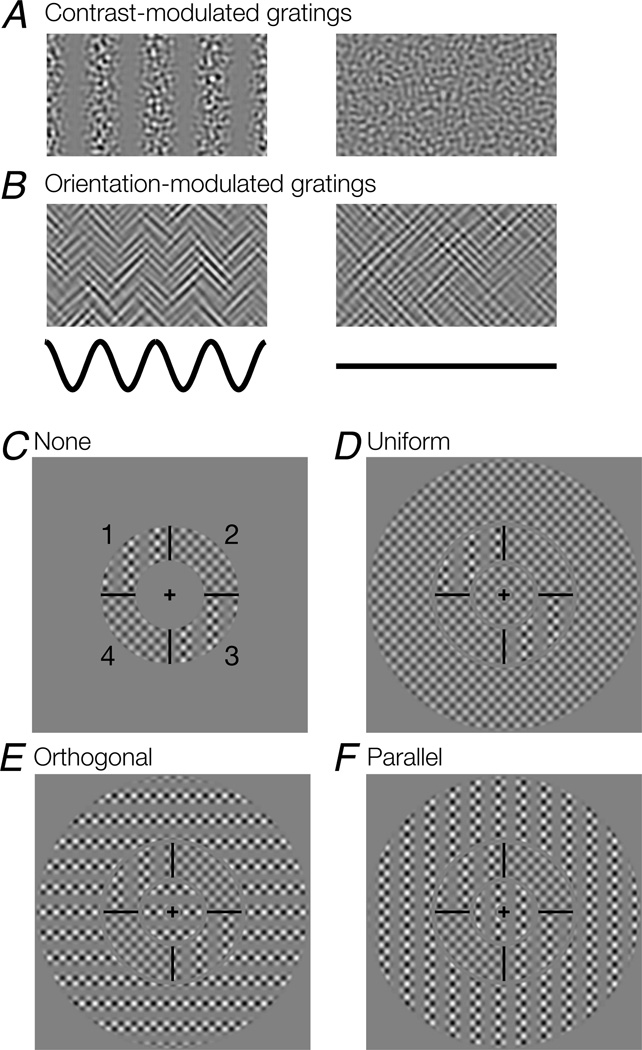
Figure 2.
Stimuli and task. A. Contrast-modulated grating. Left, the luminance contrast of a noise carrier varies sinusoidally in space (Eq. 1, AM = 1). Right, noise carrier only, with no contrast modulation (Eq. 1, AM = 0). B. Orientation-modulated grating. Left, the relative contribution of two oriented noise carriers (45° and 135°) varies sinusoidally (Eq. 2, AM = 1). Right, an orientation-defined pattern in which the two carriers have equal contrast (Eq. 2, AM = 0). Black curves indicate modulation over space for the patterns above them. C–F, Surround conditions; second-order stimuli are shown schematically to make the figure legible. Actual stimuli contained noise rather than plaid carriers as in A–B. C. Target-only (“none”) condition. The target stimulus was defined in an annular region (2 – 3.5 deg in eccentricity) and divided into four quadrant segments by black lines. In each trial, two diagonally opposed segments (either 1 & 3, or 2 & 4; shown here as 1 and 3) contained a modulated second-order grating, while the other two segments contained zero second-order modulation (noise carrier only for CM; an equal mixture of the two noise carriers for OM). The orientation of modulation was either horizontal or vertical (shown here as vertical). Numbers did not appear on the actual stimulus. D. The surround stimulus contained zero second-order modulation (“uniform” condition). The target was embedded in a surround inside and outside of the target annulus. The entire stimulus covered a circular region (diam: 15.1 deg). E. The surround stimulus was fully modulated (AM = 1 in Eqs. 1 and 2), with the orientation of modulation orthogonal to the target modulation (“orthogonal” condition). F. The surround stimulus was fully modulated, with the orientation of modulation parallel to the target modulation (“parallel” condition).
An OM grating (Fig. 2B) was generated by sinusoidally modulating between two orthogonally oriented noise carrier patterns N1 and N2 (Landy & Oruç, 2002; Larsson, Landy, & Heeger, 2006),
| (2) |
where L0, AM and M were as defined earlier. The noise carriers N1 and N2 were generated similarly to N for CM gratings above, but were instead filtered with bandpass filters oriented at 45° and 135° respectively. The filters were sharp-edged annular wedges in the Fourier domain (orientation bandwidth: 10°; center SF: 6 cyc/deg; SF bandwidth: 1 octave). The square root ensured that expected contrast power was constant across the stimuli (Landy & Oruç, 2002). We used a modulator SF f = 1.5 cyc/deg for both CM and OM stimuli, and the modulator phase was randomized from trial to trial.
2.3. Experimental procedure
Experiments were performed separately for CM and OM stimuli. The target stimulus was an annular region that extended between 2 and 3.5 deg eccentricity (Fig. 2C–F). The annulus was divided into four quadrant segments separated by black lines. During each trial, two diagonally positioned segments (either 1 and 3, or 2 and 4, Fig 2C) contained a (horizontally or vertically) modulated second-order grating, while the other two segments contained a pattern with no modulation (AM = 0). In the case of CM stimuli, this was simply the noise carrier (Fig. 2A, right); in the case of OM stimuli, this was an equal mixture of the two oriented noise carriers (Fig. 2B, right). The target was either presented alone (“none”, Fig. 2C) or embedded in a surround stimulus inside and outside of the annulus (Fig. 2D–F). All annular envelopes that contained the target or the surround had raised cosine edges (raised-cosine widths for inner and outermost edges of the surround: 0.5 deg; for the edges at which the target and the surround bordered each other: ~0.1 deg). The annular surround outside of the target region extended the entire stimulus to 15.1 deg in diameter. Three types of surround stimuli were used: no second-order modulation (“uniform”, AM = 0; Fig. 2D), full modulation (AM = 1) with the modulator orientation orthogonal to the target modulation (“orthogonal”, Fig. 2E), and full modulation with the modulator orientation parallel to the target modulation (“parallel”, Fig. 2F). In all, this yielded four possible trial types corresponding to the four surround conditions.
Subjects performed a 2-alternative, forced-choice (2AFC) task in which they indicated with a key press which segments of the target region (1&3 or 2&4) contained patterns with second-order modulation. The subject maintained fixation on a central 0.4-deg crosshair throughout the experiment, and received feedback after each trial through a change in the color of the fixation cross. The stimulus was displayed for 250 ms, and the subject had unlimited time to respond. The next trial began 750 ms after the response. All trial types were counterbalanced and presented in pseudorandom order using two interleaved adaptive staircases per surround condition (1-up-3 down and 1-up-2-down; Levitt, 1971), resulting in a total of 8 staircases per experimental session. Each session typically consisted of 4 blocks of 200 – 256 trials. Different sessions were held on separate days. Within each session the staircases carried over from block to block. For the CM experiments, each of three subjects completed 1600 trials in total (from two sessions); one subject completed 2520 trials in total (from three sessions). For the OM experiments, each of the four subjects completed 2048 trials (from two sessions).
2.4. Data analysis
For each subject, we computed the percentage correct as a function of the modulation amplitude of the target for each of the four surround conditions, pooling across target locations (1&3 or 2&4), target modulator orientations (horizontal and vertical), and staircases.
For each subject, we fit a modified Weibull function (Quick, 1974) to the data for each surround condition,
| (3) |
where Ps was the probability of a correct choice for condition s, and mj was the jth target modulation. The “lapse rate” parameter λ was introduced to avoid biased parameter estimates (Wichmann & Hill, 2001). We fit the four psychometric functions for all surround conditions simultaneously, using a single slope parameter β and lapse-rate parameter λ across conditions, while allowing for a condition-specific threshold parameter αs. Values for the six free parameters (β, λ, and α1–α4) were estimated using a maximum-likelihood procedure. We estimated a discrimination threshold for each condition as the target modulation needed to obtain 75% performance accuracy. Overall goodness-of-fit for each subject did not differ significantly between psychometric functions fit separately to each surround condition and those fit simultaneously to all conditions with shared parameters β and λ (p > 0.05 for all subjects; likelihood ratio test; Hoel, 1971); we therefore opted to use the latter model, which contained fewer parameters.
We used bootstrapping to obtain confidence intervals for the threshold estimates and p values for differences in thresholds across conditions. For each subject and each condition, we non-parametrically bootstrapped the trials at each tested target modulation amplitude (equivalent to drawing from a binomial distribution with parameters indicated by the measured number of trials and probability of success), obtained a new psychometric function, and recalculated the fit on the bootstrapped data to obtain a threshold. We repeated this procedure 1000 times, and the 2.5th and 97.5th percentiles of the resulting distribution of threshold values provided a 95% confidence interval. To determine whether the threshold for one condition was significantly higher than the threshold for another condition in a single subject (i.e., whether their ratio was significantly greater than 1), we computed the ratio between two randomly selected bootstrapped values corresponding to the two thresholds. We repeated this procedure 5000 times and determined the p value as the fraction of times that the bootstrapped ratios were less than 1. To determine whether thresholds differed significantly between two conditions across all subjects, we performed a similar analysis by randomly sampling bootstrapped thresholds from those computed above for individual subjects. The sampled thresholds for each condition were averaged across subjects and then the ratio of average thresholds for the two conditions was computed. This procedure was repeated for 5000 times and the p value corresponded to the fraction of these bootstrapped ratios that was less than 1.
3. Results
We tested for second-order surround suppression by measuring the modulation sensitivity for second-order target stimuli as a function of the type of surround modulation. A higher discrimination threshold when surround modulation is present would suggest that second-order modulation of the surround exerts a suppressive effect on the sensitivity for target stimuli. Comparing thresholds for parallel versus orthogonal surround modulation would indicate whether second-order surround suppression is orientation-selective. Any potential confounding, first-order suppression was accounted for by comparing the thresholds for the target when it was presented in isolation versus embedded in a uniform surround with no second-order modulation.
3.1. Contrast-modulated stimuli
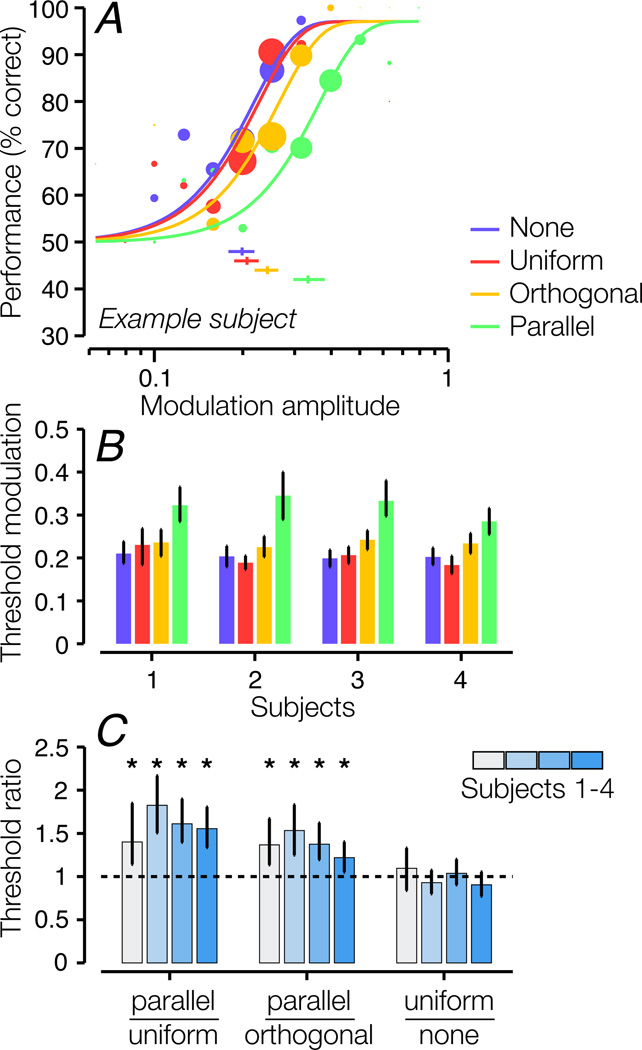
Contrast modulation sensitivity was lower for the target stimulus when the target was viewed in the presence of a modulated surround stimulus, indicative of second-order surround suppression (Fig. 3). Compared to the uniform-contrast surround condition, psychometric functions for the full-modulation surround conditions were systematically shifted to the right, reflecting higher discrimination thresholds (Fig. 3A). In addition, threshold was greater for the parallel-surround condition than for the orthogonal-surround condition, indicating that the suppressive effect was orientation-selective. Second-order suppression was robust across all four tested subjects (Fig. 3B; Fig 3C, left bar group) as well as when thresholds were averaged across subjects (see Section 2.4; p < 0.0002). Furthermore, all subjects showed an orientation-specific effect (Fig. 3B; Fig. 3C, middle bar group; p = 0.003 across all subjects). None of the subjects showed significant change in threshold between the no-surround and uniform-surround conditions (Fig. 3C, right bar group; p = 0.62 across all subjects), confirming that the observed suppression of target modulation sensitivity was not due to first-order features (overall contrast) of the surround stimulus.
Figure 3.
Discrimination performance for contrast-modulated stimuli. A. Psychometric functions for an example subject. A psychometric function was computed for each of the four surround conditions (see Fig. 2C–F). Data points show percentage correct as a function of the modulation amplitude of the target; larger sizes indicate more trials. The size of each data point corresponds to the number of trials presented for that target modulation amplitude. The curves show best-fitting Weibull functions. We estimated the modulation threshold as the target modulation corresponding to 75% correct. Error bars beneath the curves indicate 95% confidence intervals for the threshold estimates, obtained via bootstrapping. B. Modulation threshold for each surround condition and each subject. Error bars: 95% bootstrapped confidence intervals. C. Ratios between thresholds for pairs of conditions. Error bars: 95% bootstrapped confidence intervals. Dashed line indicates a ratio of 1 (identical thresholds). Asterisks indicate statistical significance for threshold ratios > 1 (* p < 0.01).
3.2. Orientation-modulated stimuli
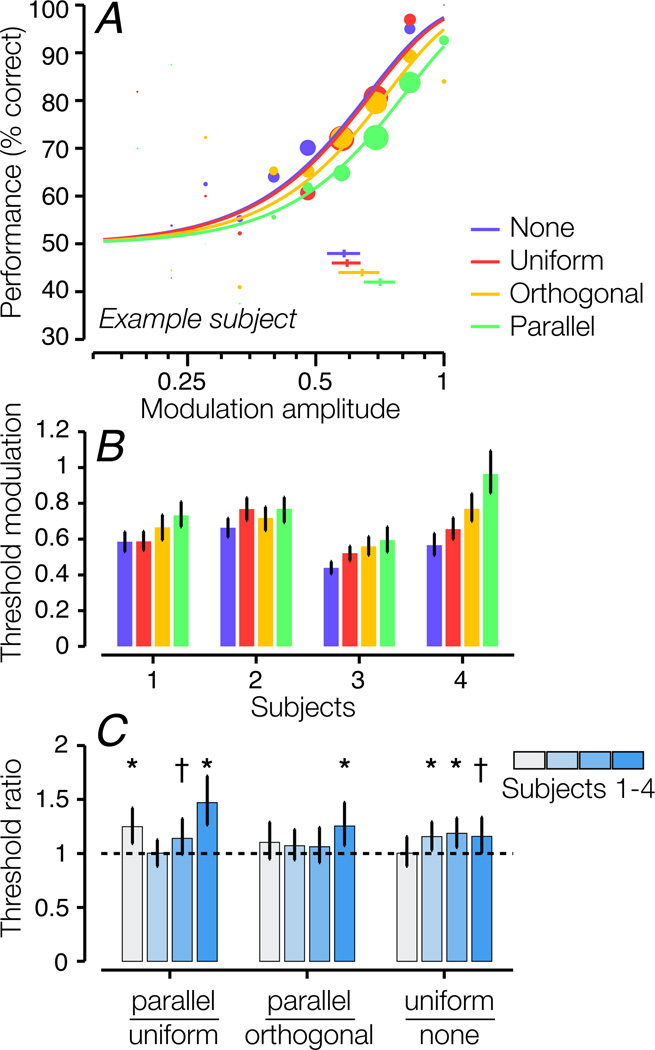
Three out of four subjects showed higher target modulation thresholds for the parallel surround compared to the uniform surround (Fig. 4B; Fig. 4C, left bar group; p < 0.0002 across all subjects). Most subjects did not show a statistically significant difference in thresholds for parallel versus orthogonal surrounds, suggesting that second-order suppression for OM stimuli is weakly or not orientation-selective (Fig. 4C, middle bar group). However, when thresholds were averaged across subjects, the threshold difference between parallel and orthogonal surrounds was statistically significant (p = 0.012). Three out of four subjects also showed significant first-order suppression, that is, their thresholds were lower in the no-surround than the uniform-surround condition (Fig. 4C, right bar group; p = 0.014 across all subjects). The strength of second-order suppression was weaker for OM stimuli than for CM stimuli, and fewer subjects exhibited statistically significant second-order surround suppression.
Figure 4.
Discrimination performance for orientation-modulated stimuli. Same conventions as Fig. 3. A. Psychometric functions for an example subject. B. Modulation threshold for each surround condition and each subject. C. Ratios between thresholds for pairs of conditions. Asterisks indicate statistical significance for threshold ratios > 1 (* p < 0.01, † p < 0.05).
We also measured OM detection in five subjects with a carrier that was broader-band and higher spatial frequency (orientation bandwidth: 30°; center SF: 8 cyc/deg). In those data, psychometric functions were shallower and, in some conditions, only reached 70–80% by AM = 1. The small range of usable modulation amplitude resulted in noisier measurements and poorer fits; we therefore opted to use the narrow-band carrier stimuli (Landy & Oruç, 2002). Nonetheless, we obtained results (data not shown) that were qualitatively similar. Four out of five subjects showed significant second-order suppression, exhibiting higher modulation thresholds for parallel than uniform surrounds (p < 0.05).
4. Discussion
4.1. Normalization and the cascade model of visual processing
First-order (contrast) surround suppression has been closely linked to neurophysiological suppression in V1 cells. The responses of a V1 neuron are smaller in the presence of a surrounding stimulus placed outside of its classical receptive field, which is ineffective in driving the cell when presented alone (Bair, Cavanaugh, & Movshon, 2003; Cavanaugh, Bair, & Movshon, 2002a; 2002b; DeAngelis, Freeman, & Ohzawa, 1994; Heeger, 1992a; Hubel & Wiesel, 1968; J. B. Levitt & Lund, 1997; Maffei & Fiorentini, 1976). Analogous to psychophysical suppression, the suppression is largest when the surround stimulus is at the neuron’s preferred orientation and spatial frequency (Cavanaugh, Bair, & Movshon, 2002b; DeAngelis et al., 1994; Knierim & Van Essen, 1992).
V1 surround suppression is well described by divisive normalization, a functional model introduced to explain a variety of suppressive phenomena evident in the responses of V1 neurons (Albrecht & Geisler, 1991; Carandini & Heeger, 1994; 2012; Carandini, Heeger, & Movshon, 1997; Heeger, 1991; 1992b; 1992a; 1993; 1994; Nestares & Heeger, 1997; Tolhurst & Heeger, 1997a; 1997b). The normalization model posits that the rectified responses of a neuron to a preferred stimulus are suppressed divisively (normalized) by the summed responses across a population of neurons. During psychophysical surround suppression, perceptual sensitivity for a target stimulus is degraded by a high-contrast surround stimulus. Normalization implies that the excitatory drive from the target stimulus is suppressed divisively by neural responses selective to the surround stimulus, and the suppression is stronger when surround contrast is higher. The target must then have a higher contrast to evoke a criterion response for detection or discrimination.
While nonlinearities in neural responses such as surround suppression have been studied most extensively in V1, analogous suppressive effects have been reported in some extrastriate areas as well: divisive normalization has been used to account for suppressive effects in dorsal-stream visual cortical areas MT and MST (Britten & Heuer, 1999; Heuer & Britten, 2002; Recanzone, Wurtz, & Schwarz, 1997; Snowden, Treue, Erickson, & Andersen, 1991) and in ventral stream areas V4 and IT (Miller, Gochin, & Gross, 1993; Missal, Vogels, & Orban, 1997; Reynolds & Desimone, 2003; Reynolds, Chelazzi, & Desimone, 1999; Richmond, Wurtz, & Sato, 1983; Rolls & Tovee, 1995; Sato, 1989; Zoccolan, Cox, & DiCarlo, 2005). Therefore, it has been proposed that, akin to linear filtering and rectification, normalization may be a “canonical” operation carried out by neural populations at multiple stages of cortical computation (Carandini & Heeger, 2012; Grossberg, 1973; Heeger, Simoncelli, & Movshon, 1996; Kouh & Poggio, 2008; Luo, Axel, & Abbott, 2010; Olsen, Bhandawat, & Wilson, 2010; Simoncelli & Heeger, 1998).
FRF models provide a relatively simple mechanism to account for second-order pattern perception that could be implemented in neuronal circuitry. Neurons with properties consistent with the second-stage filter have been found in extrastriate visual areas of cat and monkey (see Section 4.2 below). Variants of the FRF model also include first-order normalization, in which the rectified outputs of first-stage filters are inhibited by the pooled responses of other first-stage filters, before providing input to the second-stage filters (Graham, 1991; Graham & Sutter, 1998; Graham, Beck, & Sutter, 1992; Olzak & Thomas, 1999). This is consistent with the cross-channel nonlinear interactions (i.e., first-order normalization) observed both psychophysically and neurophysiologically.
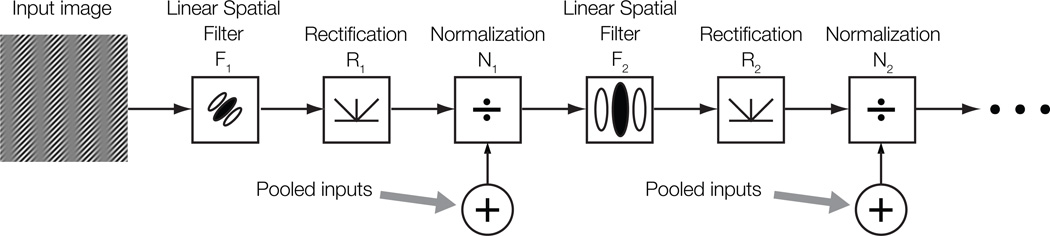
An extension of the FRF models is to combine them with the normalization model, thus treating normalization as a stage in a canonical series of computations. In this “cascade model”, the three operations—linear filtering, rectification, and normalization—are cascaded across sequential stages: F1R1N1F2R2N2 (Fig. 5). The cascade model thus bridges theories inferred from both psychophysics and single-cell physiology. A key prediction of this model is that the rectified outputs of second-stage filters undergo normalization in a manner similar to those of first-stage filters. This model therefore predicts that the strength of second-order suppression should increase with the overall outputs of second-order filters, which depend on the overall second-order modulation of the image.
Figure 5.
The cascade model of visual processing. The cascade model combines the normalization model and the FRF model, and posits that the three operations—filtering, rectification, and normalization—are repeated and cascaded across sequential stages, leading to a series of stages F1R1N1, F2R2N2, etc.
We used the detection of contrast and orientation modulation to measure observers’ perceptual sensitivity for second-order features and infer possible underlying neural suppression associated with second-order visual processing. We found significant threshold elevation when the surround stimulus contained high modulation depth compared to when it contained no modulation, which indicated second-order surround suppression. For CM stimuli, suppression was orientation-specific, i.e., subjects exhibited higher thresholds when the orientation of second-order surround modulation was parallel to that of the target. Suppression was not consistently orientation-specific for OM stimuli. For both OM and CM stimuli, space-averaged responses of first-stage filters (F1) were independent of the modulation depth of the surround, and therefore first-stage suppression (N1) was constant across all surround-present conditions. In sum, our results provided evidence for the existence of second-order suppression computed independently of N1. While these results do not demonstrate whether the nature of suppression is divisive as predicted by normalization, they are consistent with the cascade model of cortical processing, and suggest that a general gain-control mechanism may contribute to both first-and second-order visual processing.
4.2. Second-order visual processing and its neural substrates
The cascade model provides a functional rather than a mechanistic description of visual computation, and does not make specific predictions about where each of the component operations might occur in visual cortex. Although drawn as a feedforward process in Fig. 5, the operations of linear summation and division can be computed with either feedforward (Carandini, Heeger, & Senn, 2002; Olsen & Wilson, 2008; Reichardt, Poggio, & Hausen, 1983) or feedback mechanisms (Angelucci, Levitt, & Lund, 2002; Carandini et al., 1997; Carandini & Heeger, 1994; Heeger, 1992a; 1993).
While psychophysical models adequately capture human sensitivity to a variety of second-order modulations, relatively little is known about how second-order vision is represented in visual cortex. Theoretical and psychophysical results show that the detection of first and second-order patterns require separate mechanisms, as their interactions are weak or absent (Ellemberg et al., 2004; Lin & Wilson, 1996; Morgan, Mason, & Baldassi, 2000; Schofield & Georgeson, 1999; Scott-Samuel & Georgeson, 1999). Like first-order channels, second-order channels are tuned for orientation and spatial frequency (Arsenault, Wilkinson, & Kingdom, 1999; Dakin, Williams, & Hess, 1999; Landy & Oruç, 2002; Schofield & Georgeson, 1999; Scott-Samuel & Georgeson, 1999; Sutter, Sperling, & Chubb, 1995), but have wider bandwidth (Landy & Oruç, 2002). Some neurons in area 18 of the anesthetized cat (Mareschal & Baker, 1998; Song & Baker, 2007; Zhou & Baker, 1993), and in macaque areas V2 (Leventhal et al., 1998; Rossi et al., 2001; but see El-Shamayleh & Movshon, 2011) and MT (O'Keefe & Movshon, 1998; Olavarria et al., 1992), have been reported to show selectivity for second-order cues. fMRI-adaptation studies demonstrated selectivity for second-order modulation of orientation (Larsson et al., 2006) and spatial frequency (Hallum, Landy, & Heeger, 2011) in human visual cortex. Progressively stronger adaption for second-order modulation was reported in higher-order visual areas, providing support for the idea that second-order processing takes places outside of V1. However, the presence of orientation and spatial-frequency selective adaption in V1 suggests that subpopulations of V1 neurons may also perform second-order processing (Hallum et al., 2011).
4.3. Previous evidence for higher-order suppression
Several physiology studies have found divisive suppression in extrastriate areas (e.g., Britten & Heuer, 1999; Heuer & Britten, 2002; Recanzone et al., 1997; Snowden et al., 1991), but evidence for multiple stages of divisive suppression has been largely inconclusive because it is difficult to determine if the suppression is computed de novo or inherited from the inputs (Rust, Mante, Simoncelli, & Movshon, 2006). For instance, one study (Britten & Heuer, 1999) examined the spatial interaction of multiple small Gabor stimuli within the receptive fields of single MT neurons, and found that the responses of MT neurons were less than those predicted by linear summation, and were instead well described by divisive normalization. It is known that neurons in area MT receives direct inputs from V1. While this presents some evidence for N2 in the dorsal stream, it is unclear to what extent the measured suppression included normalization computed in and inherited from V1 versus that computed in MT.
4.4. Strong evidence of orientation-dependent suppression for CM but not for OM stimuli
Analogous to N1, we found that N2 for CM stimuli was orientation-selective, but N2 was not consistently orientation-selective for OM stimuli. According to some implementations of the FRF model, F2 does not discriminate between inputs from different types of carriers (“carrier-invariance”). However, some classes of FRF models detect different texture-modulation types with separate FRF mechanisms (e.g., Kingdom, Prins, & Hayes, 2003). This might seem computationally inefficient because a large number of neurons would be required for representing different types of second-order stimuli. However, it makes sense if the goal of second-order vision is not only to detect boundaries, but also to extract and characterize image statistics, as required by models of texture appearance (e.g., Portilla & Simoncelli, 2000). Following that logic, signals from different carrier types would need to be kept separate rather than recombined for characterizing the textures, and second-order suppression for different carrier types would be mediated by different normalization pools.
4.5. Lack of first-order suppression for CM stimuli
We did not observe significant first-order suppression for CM stimuli, as thresholds for no-surround and uniform-surround conditions did not differ. This might be seen as surprising because the responses of the first-stage filters should be suppressed (due to normalization) by the contrast of a surround stimulus (e.g., the first-order contrast might be perceived to decrease from 100% to 80%). As the outputs of first-stage filters provide input to second-stage filters, weaker inputs would predict lower modulation sensitivity. However, second-order sensitivity for the target stimulus may remain the same despite modest attenuation of its first-order inputs, consistent with a number of studies that found that detectability of second-order contrast modulation is only weakly dependent on carrier contrast (for a variety of carriers) once the contrast was sufficiently above detection threshold (Cropper, 1998; Dakin & Mareschal, 2000; Jamar & Koenderink, 1985; Schofield & Georgeson, 1999). Functionally, this could be due to an invariance mechanism that ensures that the second-stage of the model maintains sensitivity to second-order features despite variations in first-order contrast. In addition, this lack of dependence on carrier contrast is more pronounced for high-spatial-frequency carriers (Dakin & Mareschal, 2000). This is consistent with our measurements, which were conducted at a relatively high carrier frequency (center SF = 8 cyc/deg) and 100% carrier contrast. Lastly, similar to our results, Ellemberg et al. (2004) also found no reduction in the apparent modulation depth of a contrast-modulated target when it was flanked by unmodulated carrier flanks compared to when it was presented alone.
We did observe significant first-order suppression in 3 out of 4 subjects for OM stimuli. This result is consistent with the idea that the detection of OM and CM may be mediated by separate FRF mechanisms. One previous study, however, suggested that discriminability of OM did not depend on carrier contrast for a carrier contrast range of 60–80% (Barbot, Landy, & Carrasco, 2011). This would predict that the first-order surround in our experiment would have little effect on the second-order modulation sensitivity of the target, even when the surround lowers the effective first-order target contrast (due to first-order suppression). However, this previous study was conducted using different parameters (e.g., lower carrier and modulator spatial frequencies and a larger eccentricity than those tested here), and it is unknown whether any of those variables could have contributed to the differences.
4.6. Center-surround configuration
Our results, using a discrimination task, agree with those of Ellemberg et al. (2004), which were based on the appearance of second-order stimuli. However, one difference between our study and that of Ellemberg et al (2004) is that we used a large surround stimulus, while they used localized Gabor stimuli. Previous first-order suppression experiments have used designs in which the target was flanked by Gabors or Gabor-like elements (Wilkinson et al., 1997; Williams & Hess, 1998; Zenger-Landolt & Koch, 2001) or was embedded in a large surround (Chubb et al., 1989; Petrov et al., 2005; Snowden & Hammett, 1998; Solomon et al., 1993; Xing & Heeger, 2000), generally producing similar patterns of results. Therefore, we do not expect the size of target and surround stimuli to contribute to any substantial differences in results. In addition, in some first-order experiments, suppression also depends on the relative phase between target and surround stimuli (Ejima & Takahashi, 1985; Olzak & Laurinen, 1999; Polat & Sagi, 1993; Williams & Hess, 1998; Zenger & Sagi, 1996), though these experiments were mostly performed for foveal targets. It has been found that first-order surround suppression in the periphery does not depend on the relative phase between the target and the surround (Petrov & McKee, 2006). Here we randomized the relative phase between the target and the surround for peripheral target stimuli, and did not systematically examine how second-order surround suppression depends on relative target-surround configuration.
4.7. Surround facilitation versus suppression
In reports of first-order center-surround interactions, the effect of a surround is typically suppressive, but in some manipulations, it has been found to be facilitative, i.e., enhanced detection or greater apparent contrast of the target stimulus in the presence of a surround (Cannon & Fullenkamp, 1993; Ejima & Takahashi, 1985; Polat & Sagi, 1993; Xing & Heeger, 2001). How do these findings relate to our results? First-order facilitation is mainly evident in low surround contrasts (Cannon & Fullenkamp, 1993; Ejima & Takahashi, 1985; Yu, Klein, & Levi, 2002; Zenger & Sagi, 1996; Zenger-Landolt & Koch, 2001), which has been hypothesized to be due to signal-to-noise enhancement from spatial summation. For our manipulation we only used surround modulation depths of 0% or 100%, and did not use any weak surround modulations. In addition, first-order facilitation has mainly been reported for foveal target stimuli (Cannon & Fullenkamp, 1993; Ejima & Takahashi, 1985; Polat & Sagi, 1993; Zenger & Sagi, 1996); facilitation switches to suppression when the target stimuli are placed in the periphery (Chubb et al., 1989; Petrov et al., 2005; Snowden & Hammett, 1998; Solomon et al., 1993; Wilkinson et al., 1997; Williams & Hess, 1998; Xing & Heeger, 2000; Zenger-Landolt & Koch, 2001). Our target stimuli were presented 2 – 3.5 degrees in the periphery (Fig. 2C–F). The mainly suppressive effects that we found are therefore consistent with those observed for first-order target stimuli presented in the periphery. But it remains an open question how second-order suppression depends on variables such as target eccentricity. The strength of N2 might increase as a function of eccentricity, analogous to first-order data. In addition, facilitation might also occur with a low modulation depth of the surround or with a foveal target.
Highlights.
We tested for second-order surround suppression by measuring modulation thresholds.
Thresholds were higher with than without second-order surrounds.
Orientation-specific suppression for contrast- but not orientation-modulated stimuli.
Normalization may underlie surround suppression at multiple visual processing stages.
Acknowledgements
This work was supported by NIH Grant EY16165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albrecht DG, Geisler WS. Motion selectivity and the contrast-response function of simple cells in the visual cortex. Visual Neuroscience. 1991;7(6):531–546. doi: 10.1017/s0952523800010336. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Progress in Brain Research. 2002;136:373–388. doi: 10.1016/s0079-6123(02)36031-x. [DOI] [PubMed] [Google Scholar]
- Arsenault AS, Wilkinson F, Kingdom FA. Modulation frequency and orientation tuning of second-order texture mechanisms. Journal of the Optical Society of America A, Optics, Image science, and Vision. 1999;16(3):427–435. doi: 10.1364/josaa.16.000427. [DOI] [PubMed] [Google Scholar]
- Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. Journal of Neuroscience. 2003;23(20):7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Landy MS, Carrasco M. Exogenous attention enhances 2nd-order contrast sensitivity. Vision Research. 2011;51(9):1086–1098. doi: 10.1016/j.visres.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Campbell FW. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. The Journal of Physiology. 1969;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Heuer HW. Spatial summation in the receptive fields of MT neurons. Journal of Neuroscience. 1999;19(12):5074–5084. doi: 10.1523/JNEUROSCI.19-12-05074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. The Journal of Physiology. 1968;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Carpenter RH, Levinson JZ. Visibility of aperiodic patterns compared with that of sinusoidal gratings. The Journal of Physiology. 1969;204(2):283–298. doi: 10.1113/jphysiol.1969.sp008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MW, Fullenkamp SC. Spatial interactions in apparent contrast: inhibitory effects among grating patterns of different spatial frequencies, spatial positions and orientations. Vision Research. 1991;31(11):1985–1998. doi: 10.1016/0042-6989(91)90193-9. [DOI] [PubMed] [Google Scholar]
- Cannon MW, Fullenkamp SC. Spatial interactions in apparent contrast: individual differences in enhancement and suppression effects. Vision Research. 1993;33(12):1685–1695. doi: 10.1016/0042-6989(93)90034-t. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Summation and division by neurons in primate visual cortex. Science. 1994;264(5163):1333–1336. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nature Reviews Neuroscience. 2012;13(1):51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Movshon JA. Linearity and normalization in simple cells of the macaque primary visual cortex. Journal of Neuroscience. 1997;17(21):8621–8644. doi: 10.1523/JNEUROSCI.17-21-08621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. Journal of Neuroscience. 2002;22(22):10053–10065. doi: 10.1523/JNEUROSCI.22-22-10053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. Journal of Neurophysiology. 2002a;88(5):2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. Journal of Neurophysiology. 2002b;88(5):2547–2556. doi: 10.1152/jn.00693.2001. [DOI] [PubMed] [Google Scholar]
- Chubb C, Landy MS. Orthogonal distribution analysis: A new approach to the study of texture perception. In: Landy MS, Movshon JA, editors. Computational Models of Visual Processing. Cambridge, MA: MIT Press; 1991. pp. 253–271. [Google Scholar]
- Chubb C, Econopouly J, Landy MS. Histogram contrast analysis and the visual segregation of IID textures. Journal of the Optical Society of America A, Optics, Image science, and Vision. 1994;11(9):2350–2374. doi: 10.1364/josaa.11.002350. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(23):9631–9635. doi: 10.1073/pnas.86.23.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper SJ. Detection of chromatic and luminance contrast modulation by the visual system. Journal of the Optical Society of America A, Optics, Image science, and Vision. 1998;15(8):1969–1986. doi: 10.1364/josaa.15.001969. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Mareschal I. Sensitivity to contrast modulation depends on carrier spatial frequency and orientation. Vision Research. 2000;40(3):311–329. doi: 10.1016/s0042-6989(99)00179-0. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Williams CB, Hess RF. The interaction of first- and second-order cues to orientation. Vision Research. 1999;39(17):2867–2884. doi: 10.1016/s0042-6989(98)00307-1. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. Spatial Vision. New York: Oxford University Press; 1988. [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. Journal of Neurophysiology. 1994;71(1):347–374. doi: 10.1152/jn.1994.71.1.347. [DOI] [PubMed] [Google Scholar]
- Ejima Y, Takahashi S. Apparent contrast of a sinusoidal grating in the simultaneous presence of peripheral gratings. Vision Research. 1985;25(9):1223–1232. doi: 10.1016/0042-6989(85)90036-7. [DOI] [PubMed] [Google Scholar]
- El-Shamayleh Y, Movshon JA. Neuronal Responses to Texture-Defined Form in Macaque Visual Area V2. Journal of Neuroscience. 2011;31(23):8543–8555. doi: 10.1523/JNEUROSCI.5974-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellemberg D, Allen HA, Hess RF. Investigating local network interactions underlying first- and second-order processing. Vision Research. 2004;44(15):1787–1797. doi: 10.1016/j.visres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Graham N. Visual Pattern Analyzers. New York: Oxford University Press; 1989. [Google Scholar]
- Graham N. Complex channels, early local nonlinearities, and normalization in texture segregation. In: Landy MS, Movshon JA, editors. Computational Models of Visual Processing. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- Graham N, Nachmias J. Detection of grating patterns containing two spatial frequencies: a comparison of single-channel and multiple-channels models. Vision Research. 1971;11(3):251–259. doi: 10.1016/0042-6989(71)90189-1. [DOI] [PubMed] [Google Scholar]
- Graham N, Sutter A. Spatial summation in simple (Fourier) and complex (non-Fourier) texture channels. Vision Research. 1998;38(2):231–257. doi: 10.1016/s0042-6989(97)00154-5. [DOI] [PubMed] [Google Scholar]
- Graham N, Beck J, Sutter A. Nonlinear processes in spatial-frequency channel models of perceived texture segregation: effects of sign and amount of contrast. Vision Research. 1992;32(4):719–743. doi: 10.1016/0042-6989(92)90188-o. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Contour enhancement, short term memory, and constancies in reverberating neural networks. Studies in applied Mathematics. 1973;52(3):213–257. [Google Scholar]
- Hallum LE, Landy MS, Heeger DJ. Human primary visual cortex (V1) is selective for second-order spatial frequency. Journal of Neurophysiology. 2011;105(5):2121–2131. doi: 10.1152/jn.01007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ. Nonlinear model of neural responses in cat visual cortex. In: Landy MS, Movshon JA, editors. Computational Models of Visual Processing. Cambridge, MA: MIT Press; 1991. pp. 119–133. [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Visual Neuroscience. 1992a;9(2):181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Half-squaring in responses of cat striate cells. Visual Neuroscience. 1992b;9(5):427–443. doi: 10.1017/s095252380001124x. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Modeling simple-cell direction selectivity with normalized, half-squared, linear operators. Journal of Neurophysiology. 1993;70(5):1885–1898. doi: 10.1152/jn.1993.70.5.1885. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. The representation of visual stimuli in primary visual cortex. Current Directions in Psychological Science. 1994;3(5):159–163. [Google Scholar]
- Heeger DJ, Simoncelli EP, Movshon JA. Computational models of cortical visual processing. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(2):623–627. doi: 10.1073/pnas.93.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer HW, Britten KH. Contrast dependence of response normalization in area MT of the rhesus macaque. Journal of Neurophysiology. 2002;88(6):3398–3408. doi: 10.1152/jn.00255.2002. [DOI] [PubMed] [Google Scholar]
- Hoel PG. Introduction to Mathematical Statistics. 4th ed. New York: John Wiley; 1971. [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. The Journal of Physiology. 1968;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamar JH, Koenderink JJ. Contrast detection and detection of contrast modulation for noise gratings. Vision Research. 1985;25(4):511–521. doi: 10.1016/0042-6989(85)90154-3. [DOI] [PubMed] [Google Scholar]
- Kingdom FAA, Prins N, Hayes A. Mechanism independence for texture-modulation detection is consistent with a filter-rectify-filter mechanism. Visual Neuroscience. 2003;20(1):65–76. doi: 10.1017/s0952523803201073. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Van Essen DC. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. Journal of Neurophysiology. 1992;67(4):961–980. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- Kouh M, Poggio T. A canonical neural circuit for cortical nonlinear operations. Neural Computation. 2008;20(6):1427–1451. doi: 10.1162/neco.2008.02-07-466. [DOI] [PubMed] [Google Scholar]
- Landy MS, Oruç I. Properties of second-order spatial frequency channels. Vision Research. 2002;42(19):2311–2329. doi: 10.1016/s0042-6989(02)00193-1. [DOI] [PubMed] [Google Scholar]
- Landy M, Graham N. Visual perception of texture. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. pp. 1106–1118. [Google Scholar]
- Larsson J, Landy MS, Heeger DJ. Orientation-selective adaptation to first- and second-order patterns in human visual cortex. Journal of Neurophysiology. 2006;95(2):862–881. doi: 10.1152/jn.00668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Schmolesky MT, Zhou Y. Neural correlates of boundary perception. Visual Neuroscience. 1998;15(6):1107–1118. doi: 10.1017/s0952523898156110. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. The Journal of the Acoustical Society of America. 1971;49(2):467–477. [PubMed] [Google Scholar]
- Levitt JB, Lund JS. Contrast dependence of contextual effects in primate visual cortex. Nature. 1997;387(6628):73–76. doi: 10.1038/387073a0. [DOI] [PubMed] [Google Scholar]
- Lin LM, Wilson HR. Fourier and non-Fourier pattern discrimination compared. Vision Research. 1996;36(13):1907–1918. doi: 10.1016/0042-6989(95)00260-x. [DOI] [PubMed] [Google Scholar]
- Luo SX, Axel R, Abbott LF. Generating sparse and selective third-order responses in the olfactory system of the fly. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(23):10713–10718. doi: 10.1073/pnas.1005635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Research. 1976;16(10):1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Mareschal I, Baker CL. A cortical locus for the processing of contrast-defined contours. Nature Neuroscience. 1998;1(2):150–154. doi: 10.1038/401. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Suppression of visual responses of neurons in inferior temporal cortex of the awake macaque by addition of a second stimulus. Brain Research. 1993;616(1–2):25–29. doi: 10.1016/0006-8993(93)90187-r. [DOI] [PubMed] [Google Scholar]
- Missal M, Vogels R, Orban GA. Responses of macaque inferior temporal neurons to overlapping shapes. Cerebral Cortex. 1997;7(8):758–767. doi: 10.1093/cercor/7.8.758. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Mason AJ, Baldassi S. Are there separate first-order and second-order mechanisms for orientation discrimination? Vision Research. 2000;40(13):1751–1763. doi: 10.1016/s0042-6989(00)00015-8. [DOI] [PubMed] [Google Scholar]
- Nestares O, Heeger DJ. Modeling the apparent frequency-specific suppression in simple cell responses. Vision Research. 1997;37(11):1535–1543. doi: 10.1016/s0042-6989(96)00268-4. [DOI] [PubMed] [Google Scholar]
- O'Keefe LP, Movshon JA. Processing of first- and second-order motion signals by neurons in area MT of the macaque monkey. Visual Neuroscience. 1998;15(2):305–317. doi: 10.1017/s0952523898152094. [DOI] [PubMed] [Google Scholar]
- Olavarria JF, DeYoe EA, Knierim JJ, Fox JM, Van Essen DC. Neural responses to visual texture patterns in middle temporal area of the macaque monkey. Journal of Neurophysiology. 1992;68(1):164–181. doi: 10.1152/jn.1992.68.1.164. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452(7190):956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66(2):287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzak LA, Laurinen PI. Multiple gain control processes in contrast-contrast phenomena. Vision Research. 1999;39(24):3983–3987. doi: 10.1016/s0042-6989(99)00131-5. [DOI] [PubMed] [Google Scholar]
- Olzak LA, Thomas JP. Neural recoding in human pattern vision: model and mechanisms. Vision Research. 1999;39(2):231–256. doi: 10.1016/s0042-6989(98)00122-9. [DOI] [PubMed] [Google Scholar]
- Petrov Y, McKee SP. The effect of spatial configuration on surround suppression of contrast sensitivity. Journal of Vision. 2006;6(3):224–238. doi: 10.1167/6.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, Carandini M, McKee SP. Two distinct mechanisms of suppression in human vision. Journal of Neuroscience. 2005;25(38):8704–8707. doi: 10.1523/JNEUROSCI.2871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Sagi D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vision Research. 1993;33(7):993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- Portilla J, Simoncelli E. A parametric texture model based on joint statistics of complex wavelet coefficients. International Journal of Computer Vision. 2000;40(1):49–70. [Google Scholar]
- Quick RF. A vector-magnitude model of contrast detection. Kybernetik. 1974;16(2):65–67. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH, Schwarz U. Responses of MT and MST neurons to one and two moving objects in the receptive field. Journal of Neurophysiology. 1997;78(6):2904–2915. doi: 10.1152/jn.1997.78.6.2904. [DOI] [PubMed] [Google Scholar]
- Reichardt W, Poggio T, Hausen K. Figure-Ground Discrimination by Relative Movement in the Visual System of the Fly: Part II: Towards the neural circuitry. Biological Cybernetics. 1983;46(Suppl):1–30. [Google Scholar]
- Reynolds JH, Desimone R. Interacting roles of attention and visual salience in V4. Neuron. 2003;37(5):853–863. doi: 10.1016/s0896-6273(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms sub-serve attention in macaque areas V2 and V4. Journal of Neuroscience. 1999;19(5):1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond BJ, Wurtz RH, Sato T. Visual responses of inferior temporal neurons in awake rhesus monkey. Journal of Neurophysiology. 1983;50(6):1415–1432. doi: 10.1152/jn.1983.50.6.1415. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ. The responses of single neurons in the temporal visual cortical areas of the macaque when more than one stimulus is present in the receptive field. Experimental Brain Research. 1995;103(3):409–420. doi: 10.1007/BF00241500. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Desimone R, Ungerleider LG. Contextual modulation in primary visual cortex of macaques. Journal of Neuroscience. 2001;21(5):1698–1709. doi: 10.1523/JNEUROSCI.21-05-01698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust NC, Mante V, Simoncelli EP, Movshon JA. How MT cells analyze the motion of visual patterns. Nature Neuroscience. 2006;9(11):1421–1431. doi: 10.1038/nn1786. [DOI] [PubMed] [Google Scholar]
- Sato T. Interactions of visual stimuli in the receptive fields of inferior temporal neurons in awake macaques. Experimental Brain Research. 1989;77(1):23–30. doi: 10.1007/BF00250563. [DOI] [PubMed] [Google Scholar]
- Schofield AJ, Georgeson MA. Sensitivity to modulations of luminance and contrast in visual white noise: separate mechanisms with similar behaviour. Vision Research. 1999;39(16):2697–2716. doi: 10.1016/s0042-6989(98)00284-3. [DOI] [PubMed] [Google Scholar]
- Scott-Samuel NE, Georgeson MA. Does early non-linearity account for second-order motion? Vision Research. 1999;39(17):2853–2865. doi: 10.1016/s0042-6989(98)00316-2. [DOI] [PubMed] [Google Scholar]
- Simoncelli E, Heeger D. A model of neuronal responses in visual area MT. Vision Research. 1998;38(5):743–761. doi: 10.1016/s0042-6989(97)00183-1. [DOI] [PubMed] [Google Scholar]
- Smith AT, Ledgeway T. Separate detection of moving luminance and contrast modulations: fact or artifact? Vision Research. 1997;37(1):45–62. doi: 10.1016/s0042-6989(96)00147-2. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Hammett ST. The effects of surround contrast on contrast thresholds, perceived contrast and contrast discrimination. Vision Research. 1998;38(13):1935–1945. doi: 10.1016/s0042-6989(97)00379-9. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Treue S, Erickson RG, Andersen RA. The response of area MT and V1 neurons to transparent motion. Journal of Neuroscience. 1991;11(9):2768–2785. doi: 10.1523/JNEUROSCI.11-09-02768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JA, Sperling G, Chubb C. The lateral inhibition of perceived contrast is indifferent to on-center/off-center segregation, but specific to orientation. Vision Research. 1993;33(18):2671–2683. doi: 10.1016/0042-6989(93)90227-n. [DOI] [PubMed] [Google Scholar]
- Song Y, Baker CL. Neuronal response to texture- and contrast-defined boundaries in early visual cortex. Visual Neuroscience. 2007;24(1):65–77. doi: 10.1017/S0952523807070113. [DOI] [PubMed] [Google Scholar]
- Sutter A, Sperling G, Chubb C. Measuring the spatial frequency selectivity of second-order texture mechanisms. Vision Research. 1995;35(7):915–924. doi: 10.1016/0042-6989(94)00196-s. [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Heeger DJ. Comparison of contrast-normalization and threshold models of the responses of simple cells in cat striate cortex. Visual Neuroscience. 1997a;14(2):293–309. doi: 10.1017/s0952523800011433. [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Heeger DJ. Contrast normalization and a linear model for the directional selectivity of simple cells in cat striate cortex. Visual Neuroscience. 1997b;14(1):19–25. doi: 10.1017/s0952523800008725. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Attention, Perception & Psychophysics. 2001;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wilkinson F, Wilson HR, Ellemberg D. Lateral interactions in peripherally viewed texture arrays. Journal of the Optical Society of America A, Optics, Image science, and Vision. 1997;14(9):2057–2068. doi: 10.1364/josaa.14.002057. [DOI] [PubMed] [Google Scholar]
- Williams CB, Hess RF. Relationship between facilitation at threshold and suprathreshold contour integration. Journal of the Optical Society of America A, Optics, Image science, and Vision. 1998;15(8):2046–2051. doi: 10.1364/josaa.15.002046. [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Center-surround interactions in foveal and peripheral vision. Vision Research. 2000;40(22):3065–3072. doi: 10.1016/s0042-6989(00)00152-8. [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Measurement and modeling of center-surround suppression and enhancement. Vision Research. 2001;41(5):571–583. doi: 10.1016/s0042-6989(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Yu C, Klein SA, Levi DM. Facilitation of contrast detection by cross-oriented surround stimuli and its psychophysical mechanisms. Journal of Vision. 2002;2(3):243–255. doi: 10.1167/2.3.4. [DOI] [PubMed] [Google Scholar]
- Zenger B, Sagi D. Isolating excitatory and inhibitory nonlinear spatial interactions involved in contrast detection. Vision Research. 1996;36(16):2497–2513. doi: 10.1016/0042-6989(95)00303-7. [DOI] [PubMed] [Google Scholar]
- Zenger-Landolt B, Heeger DJ. Response suppression in V1 agrees with psychophysics of surround masking. Journal of Neuroscience. 2003;23(17):6884–6893. doi: 10.1523/JNEUROSCI.23-17-06884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenger-Landolt B, Koch C. Flanker effects in peripheral contrast discrimination--psychophysics and modeling. Vision Research. 2001;41(27):3663–3675. doi: 10.1016/s0042-6989(01)00175-4. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Baker CL. A processing stream in mammalian visual cortex neurons for non-Fourier responses. Science. 1993;261(5117):98–101. doi: 10.1126/science.8316862. [DOI] [PubMed] [Google Scholar]
- Zoccolan D, Cox DD, DiCarlo JJ. Multiple object response normalization in monkey inferotemporal cortex. Journal of Neuroscience. 2005;25(36):8150–8164. doi: 10.1523/JNEUROSCI.2058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]