Abstract
We performed a phase II study of oral vorinostat (200 mg twice daily, days 1–14 of a 21-day cycle), to examine efficacy and tolerability in patients with relapsed/refractory hodgkin lymphoma (HL) with ≤ 5 prior therapies. The primary endpoint was objective response rate (ORR), with secondary endpoints of progression-free survival (PFS), overall survival (OS), safety and tolerability. A two-stage design was used for patient accrual. Twenty-five eligible patients were accrued in the first stage. Median time on treatment was 3.8 months. ORR was 4% (1 partial response). Median PFS was 4.8 months. The drug was well tolerated. The second stage of accrual was not opened due to few objective responses. Oral vorinostat has limited single agent activity in relapsed/refractory HL. There was one partial response, while seven other patients had stable disease for > 1 year, including 2 with stable disease for nearly 3 years, suggesting that further studies in combination with other active agents in this setting may be warranted.
INTRODUCTION
With use of radiotherapy and regimens such as ABVD, approximately 60% of Hodgkin’s Lymphoma patients may achieve cure, whereas the remaining 40% will either fail up-front treatment or relapse within three years1. These patients can be expected to do poorly with current salvage regimens. The best current salvage regimens such as ESHAP or ICE incorporate platinum compounds; use of ESHAP, for example, a regimen containing cis-platinum, can lead to a good initial response rate, but disease free survival is roughly only 30% at three years2. Patients with better performance status may opt for high-dose chemotherapy with autologous stem cell transplant. However, less than half of transplanted patients achieve five year disease free survival3. A strategy becoming more widely used is to identify potential chemosensitive patients who may respond to autologous transplant by use of protocols such as ICE. These selected patients at Memorial Sloan Kettering have a reported three year disease free survival of 70%, however, non-responders to salvage therapy do poorly4. Thus, newer, more effective agents are necessary.
Vorinostat (suberoylanilide hydroxamic acid), is an orally bioavailable synthetic hydroxamic acid class histone deacetylase inhibitor (HDACi) with both histone and protein deacetylase activity5. In clinical trials, responses among patients with lymphoid malignancies are prominent. In the initial phase I study using intravenous vorinostat, of the four responders, one was an HL patient, one of whom had prolonged stable disease6. In a phase I study of oral vorinostat in Japanese patients with lymphoma, responses were seen in 4 of 6 patients with follicular and mantle cell lymphoma7, whereas in a recently reported phase II study of vorinostat as a single agent, responses were seen in follicular and marginal zone lymphoma8.
We report results of a Phase II study of vorinostat at 200 mg orally, twice-daily, on a two-week-on, one-week-off schedule. Included in this cohort are patients with HL, who have recurred after up to five lines of chemotherapy, including transplant.
PATIENTS AND METHODS
Patients were informed of the investigational nature of the study and signed a written informed consent in accordance with institutional, federal and Declaration of Helsinki guidelines. The trial is registered with ClinicalTrials.gov Identifier as NCT00132028. The study was activated on September, 2005 and closed August, 2007.
Patient Eligibility
Patients over the age of 18 were eligible if they had relapsed/refractory Hodgkin lymphoma with bi-dimensionally measurable disease. Patients may have had up to five prior chemotherapeutic regimens; patients must have been at least three months post autologous or one year post allogeneic transplant. Patients who had had chemotherapy within 4 weeks, radiotherapy within 2 weeks, valproic acid for non-seizure purposes or other histone deacetylase inhibitors within 14 days, or those who had not recovered from adverse events due to agents administered more than 4 weeks prior to registration, were excluded. Patients with clinical evidence of central nervous system involvement were also excluded. Demonstration of recurrent disease, progression or persistence after the most recent line of therapy was required for eligibility. Patients with Zubrod performance status 0– 2, ANC greater than 1000/µL, platelet counts over 100,000 /µL, creatinine ≤ 2 mg/dL, and liver function tests < 2.5 times normal were eligible.
Treatment Plan
One cycle of therapy was defined as: vorinostat 200 mg twice daily administered orally for 14 days followed by a seven-day break on a 21-day cycle. Radiological assessment by CT scan was performed at baseline and after every three cycles. PET scanning was performed at the discretion of the treating institution. Response was assessed by standard criteria9. Patients with partial response or stable disease were treated with vorinostat until progression. Patients who achieved CR were treated with two further cycles of vorinostat and then followed after discontinuation. Toxicity was assessed on days 1 and 8 of every cycle, and graded using the NCI CTCAE version 3.0. Treatment was held for patients who presented with non-hematologic toxicity of grade 3 or 4, until toxicity resolved to below grade 2. These patients were restarted at 100 mg daily less than their previous dose. If grade 3 or greater toxicity occurred at that dose, the patient was taken off the drug.
Dose was decreased by 100 mg daily less than their previous dose for patients who had ANC 500–1000/µL, or platelet counts 50–75K /µL. This dose reduction was reversible with normalization of counts. For patients with hematologic toxicity of ANC < 500/µL, or platelet count < 50K/µL, dose was held until recovery to ANC > 1000/µL or platelet count > 50K/µL; if it lasted more than one week, drug would be restarted at 100 mg daily less than their previous dose. If this dose also led to ANC< 500/µL or platelet count < 50K/µL, then patient was removed from study.
Patients already on erythropoeitin or aranesp for lymphoma related anemia were allowed to continue use of these agents, but these were not permitted to be started during therapy. Use of G-CSF or GM-CSF was not allowed during this study.
Patient Evaluation
Pre-treatment evaluation consisted of history and physical examination (H&P), assessment of performance status (PS), complete blood count (CBC), hepatic and renal function tests. Laboratory tests, H&P, and PS assessment were repeated on day 1 of each cycle. CT was obtained at baseline and every three cycles to assess response. Bone marrow biopsy for presence of marrow involvement was done at baseline and every three cycles if baseline exam revealed marrow involvement. Responses were assessed according to the International Working Group criteria9.
Study Design
Confirmed response rate (CR+PR) was the primary endpoint. It was judged that this therapy would be of further interest if the confirmed response rate was 30% or greater, while further testing would not be pursued if the confirmed response rate were 10% or lower. The two-stage design of Green and Dahlberg10 was used. In the first stage, 20 patients were enrolled. If 2 or more patients achieved CR or PR, accrual would continue to a total of 35 patients, with 8 or more responses overall regarded as evidence of sufficient activity to warrant further investigation. This design has a significance level of 2% and a power of 87%. Because of a surge in accrual just before temporary closure of the first stage of accrual, in order to maintain the same significance level, the threshold for continuation to the second stage of accrual was revised up to 3 or more patients achieving CR or PR. All eligible patients beginning vorinostat therapy were included in the analysis. Overall survival (OS) and progression-free survival (PFS) were secondary endpoints. Survival curves were plotted using the Kaplan-Meier product-limit method OS was measured from the day of registration to death from any cause. PFS was defined as time from registration to first instance of progression, relapse, or death from any cause.
RESULTS
Patient Characteristics
A total of 27 patients were enrolled in the study between 9/05 and 1/08 (Table 1). Two patients were ineligible, one due to insufficient documentation of baseline laboratory measures and one due to lack of documentation of HL. In the 25 eligible patients (11 female, 14 male), the median age at registration was 41.6 (range 19.9–71.1) years. Previous treatments included bone marrow transplant in 11 patients, limited radiation (<50% of body) in 16 patients, and extensive radiation (≥50% of body) in 2 patients. All patients received at least 2 lines of prior chemotherapy, with the breakdown as follows: 2 lines (n=6), 3 lines (n=8), 4 lines (n=7), and 5 lines (n=4).
Table1.
Baseline Characteristics (N=25)
| Median age (range) | 41.6 (19.9–71.1) |
|
Gender Male Female |
14 (56%) 11 (44%) |
|
Race White Other Missing |
20 (83%) 4 (17%) 1 |
|
Performance status 0/1 2 |
12/12 (48%/48%) 1 (4%) |
|
Disease Status Recurrent Refractory |
13 (52%) 12 (48%) |
|
Prior Treatment Bone Marrow Transplant Radiation Therapy Extensive (≥50% of body) Limited (<50% of body) Median # Chemotherapy Regimens (range) |
11 (44%) 2 (8%) 16 (64%) 3 (2–5) |
Treatment and Toxicities
The median duration of treatment was 3.8 months (range 0.2–35.8 months). Reasons for discontinuation of therapy included disease progression (n=11 patients), toxicities (n=7), physician preference (n=4), patient death (n=2), and patient refusal (n=1). Four patients experienced Grade 4 hematologic toxicities, and 12 more patients experienced Grade 3 toxicities, primarily hematologic (see Table 2).
Table 2.
Adverse Events
| Toxicity | Grade 3 (%) | Grade 4 (%) |
|---|---|---|
| Anemia | 7 (28%) | 1 (4%) |
| Anorexia | 1 (4%) | 0 |
| Dehydration | 2 (8%) | 0 |
| Diarrhea | 1 (4%) | 0 |
| Elevated alk phos | 1 (4%) | 0 |
| Elevated creatinine | 1 (4%) | 0 |
| Elevated INR | 2 (8%) | 0 |
| Fatigue | 1 (4%) | 0 |
| Increased PTT | 1 (4%) | 0 |
| Infection: bladder/urinary tract | 1 (4%) | 0 |
| Hyperglycemia | 1 (4%) | 0 |
| Hypotension | 1 (4%) | 0 |
| Leukopenia | 1 (4%) | 0 |
| Lymphopenia | 3 (12%) | 0 |
| Muscle weakness | 1 (4%) | 0 |
| Musculoskeletal pain | 1 (4%) | 0 |
| Nausea | 1 (4%) | 0 |
| Neutropenia | 1 (4%) | 0 |
| Pneumonitis | 1 (4%) | 0 |
| Thrombocytopenia | 1 (4%) | 3 (12%) |
Efficacy
The breakdown of best response observed for the study population is presented in Table 3. One of the 25 eligible patients had a partial response, giving an overall confirmed response rate of 4% (95% CI 0–20%), which, according to the study design, was too low to initiate a second stage of accrual. Four patients for whom response assessment was inadequate are considered non-responders.
Table 3.
Best Response
| Best Response | N (%) |
|---|---|
| Complete response | 0 |
| Partial response | 1 (4%) |
| Stable disease | 12 (48%) |
| Progressive disease | 8 (32%) |
| Assessment inadequate | 4 (16%) |
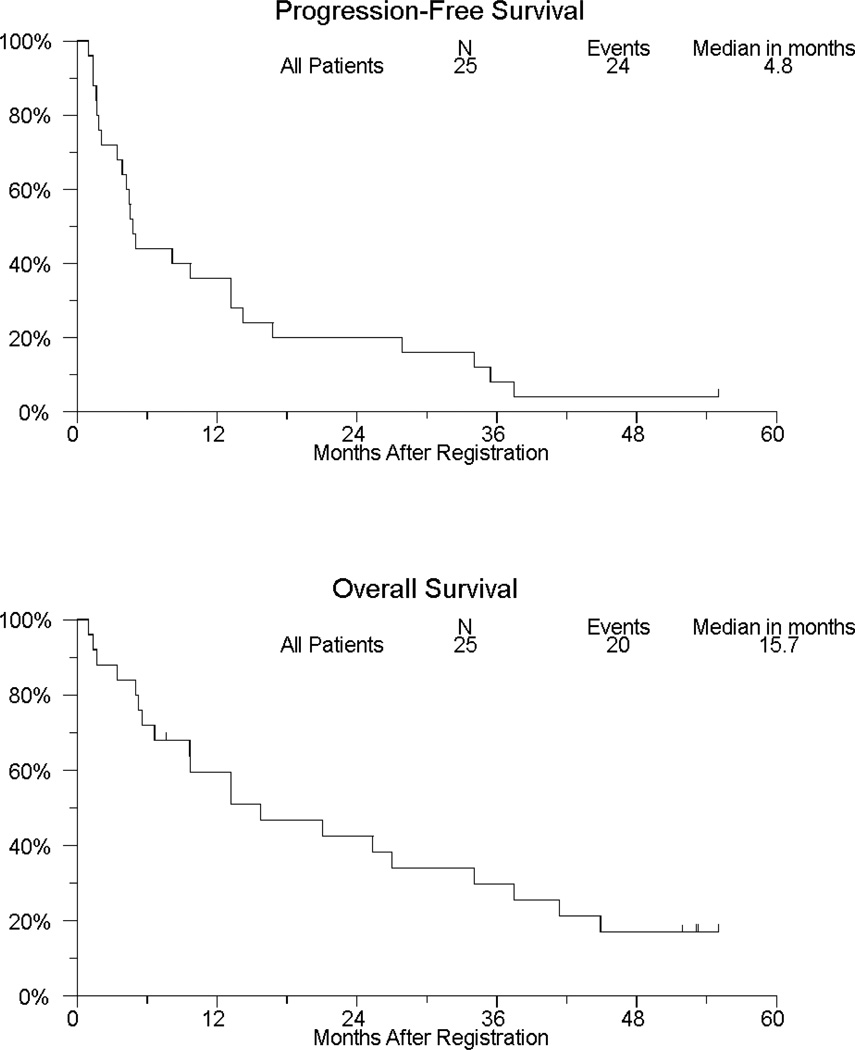
Figure 1 shows PFS and OS for the study population. There have been 20 patient deaths, with median OS (95% CI) of 15.7 months (6.6, 34.1). Twenty-four patients have either progressed or died, with median PFS (95% CI) of 4.8 months (3.4, 13.2). Among 12 patients whose best response was stable disease, 7 remained progression-free for more than a year, 2 of whom were on protocol treatment for more than a year.
Fig.1.
DISCUSSION
The outcome for patients with relapsed/refractory Hodgkin lymphoma on standard therapy, for those who progress after standard salvage regimens, and particularly for those who progress after stem cell transplant, is poor; new approaches are needed for these patients.
Vorinostat is an oral HDACi with activity against class I and II histone deacetylases, approved by the US FDA in 2006 for the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma (CTCL) who have progressive, persistent or recurrent disease on or following two systemic therapies.. There is good preclinical evidence to support the use of histone deacetylase inhibitors (HDACi) in lymphoid malignancies. HDACi have been shown to affect a host of cellular targets and processes that might lead to antitumor activity in lymphoid malignancies, including angiogenesis, apoptosis, the cell cycle, and tumor immunology11–14. Relevant specific targets whose levels or acetylation are affected by this class of agents include p53, GADD45, p21waf-1/cip1, hsp90, as well as various pro-inflammatory cytokines15–17. In preclinical work, vorinostat has demonstrated significant activity against Hodgkin lymphoma cell lines, in which vorinostat, through inhibition of both class I and II deacetylases, leads to a pro-apoptotic cell type, through effects upon Myc, Bcl-XL, hTERT, Bad, Bid, and miRNAs, leading directly to apoptosis as well as sensitizing to other molecular agents18,19. Given the interesting preclinical and clinical activity of this class of drugs in lymphoid malignancy, we conducted a phase II study of the oral HDAC inhibitor, vorinostat as a single agent in HL.
In this trial, toxicities were tolerable and consistent with the now well recognized syndrome seen with HDAC inhibitor treatment, of fatigue, anorexia and thrombocytopenia, and were manageable in this group of heavily treated patients. Formal PR was documented in one patient. For another patient, the treating institution reported shrinkage of involved nodes, which remained FDG-PET negative for over three years, however, due to inconsistencies in scanning techniques used over time, this did not qualify as a response. Among the patients that did not achieve CR or PR, seven patients maintained stable disease for at least one year. A patient with progressive disease after autologous transplant had resolution of symptoms from lung involvement and stable disease for a period of three years, after which she was enrolled upon the SGN-35 study and entered CR. It is of interest to compare the results of this early vorinostat study with subsequent studies using vorinostat or similar agents. Younes, et al, described activity with single agent MGCD0013, however, with enough toxicity to hinder development of the agent20. A large phase II study was recently completed with panobinostat (LBH589), in which 2 CR’s and 15 PR’s were reported out of 127 patients21. In these studies, prolonged stable disease and partial responses were much more common than CR. This prolongation of stable disease confirms the original phase I observations in which PR and prolonged stable disease responses were seen in HL patients treated with vorinostat22. It is becoming clear that the prolonged states of stable disease seen with this class of agents are of clinical importance. Further study of these agents, particularly in combination with chemotherapy or other molecular agents, is warranted.
ACKNOWLEDGEMENTS
This work was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services: CA32102, CA38926, CA46368, CA04919, CA67575, CA45808, CA45560, CA37981, CA58882, CA35119 (SWOG) and by grant numbers U01-CA62505 and N01-CM62209 (NCI Cancer Therapy Evaluation Program) and P30-CA-033572 (City of Hope).
Footnotes
POTENTIAL CONFLICTS OF INTEREST
None: Drs. Cook, Fisher, Forman
Grant (money to institution): Dr. Kirschbaum (CTEP/NCI, SWOG); Dr. Rimsza (NIH/NCI); Mr. Goldman (NCI)
Grants (money to institution): Dr. Kirschbaum (research funding from Merck);
Consulting fee/honorarium: Dr. Zain (Merck, honorarium for speaking)
Payment for lecture (money to investigator): Dr. Kirschbaum (Merck, Novartis, Celgene, Millenium; Dr. Zain (Merck, speakers’ bureau)
REFERENCES
- 1.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio J, Segura A, Garcera S, et al. ESHAP is an active regimen for relapsing Hodgkin's disease. Ann Oncol. 1999;10:593–595. doi: 10.1023/a:1026454831340. [DOI] [PubMed] [Google Scholar]
- 3.Stiff PJ, Unger JM, Forman SJ, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biol Blood Marrow Transplant. 2003;9:529–539. doi: 10.1016/s1083-8791(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 5.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007 Feb 26;26(9):1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 6.Kelly WK, Richon VM, O'Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 7.Watanabe T, Kato H, Kobayashi Y. Potential efficacy of the oral histonedeacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschbaum M, Frankel P, Popplewell L, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2011 Mar 20;29(9):1198–1203. doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 10.Green SJ, Dahlberg S. Planned Versus Attained Design in Phase II Clinical Trials. Stat Med. 1992;11:853–862. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Lu S, Wu L, et al. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1) Mol Cell Biol. 2006;26:2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280:145–153. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda T, Towatari M, Kosugi H, et al. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96:3847–3856. [PubMed] [Google Scholar]
- 14.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009;280:125–133. doi: 10.1016/j.canlet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Richon VM, Sandhoff TW, Rifkind RA, et al. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scuto A, Kirschbaum M, Kowolik C, et al. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood. 2008;111:5093–5100. doi: 10.1182/blood-2007-10-117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, Zhao D, Kirschbaum M, et al. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci 2008 USA. 105:4796–4801. doi: 10.1073/pnas.0712051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and micro-RNA levels. Cancer Res. 2011 Apr 18; doi: 10.1158/0008-5472.CAN-10-2259. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008 Aug 15;112(4):1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younes A, Bociek RG, Kuruvilla J, et al. Mocetinostat (MGCD0103), an isotype-selective histone deacetylase (HDAC) inhibitor, produces clinical responses in relapsed/refractory Hodgkin lymphoma (HL): update from a phase II clinical study. Blood (ASH Annual Meeting Abstracts) 2010;116 Abstract 1763. [Google Scholar]
- 21.Sureda A, Engert A, Browett PJ, et al. Interim results for the phase II study of panobinostat (LBH589) in patients (Pts) with relapsed/refractory Hodgkin's lymphoma (HL) after autologous hematopoietic stem cell transplant (AHSCT) J Clin Oncol. 2010;28(suppl):15s. abstr 8007. [Google Scholar]
- 22.O’Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, Curley T, Moskowitz C, Portlock C, Horwitz S, Zelenetz AD, Frankel S, Ricon V, Marks P, Kelly WK. J Clin Oncol. 2006;24(1):166–173. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]