Abstract
Background/Objectives
Osteoporosis and fractures are common in long term care residents but little data are available on screening strategies for treatment of such patients.
Design
Cross-sectional analysis to examine screening strategies for treatment.
Setting
Assisted living or skilled care facilities.
Participants
Two hundred and two frail elderly women age 65 and older, excluding those on bisphosphonates.
Measurements
Treatment eligibility criteria included 1) clinical fractures of the hip or spine (Clin Fx), 2) clinical fractures or bone mineral density (BMD), 3) Clin Fx, BMD, or vertebral fractures (VF assessed by DXA based vertebral fracture assessments), 4) fracture risk algorithm using femoral neck BMD (FRAX-FN), 5) fracture risk algorithm using BMI (FRAX-BMI) or 6) Clin Fx or heel ultrasound (heel US).
Results
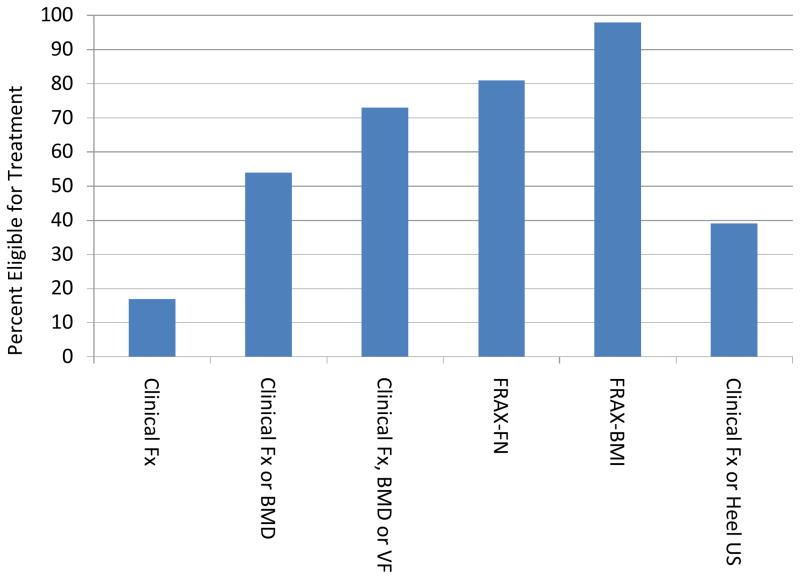
Mean age was 85 years. Treatment eligibility ranged from 17% (Clin Fx) to 98% (FRAX-BMI). VFs were found in 47%, of whom 74% were silent. Criteria with Clin Fx, BMD or VF identified 73% of study participants for treatment. FRAX-FN suggested treatment in 81% but would have missed about 10% of patients with silent vertebral fractures. Clin Fx or heel ultrasound suggested 39% of participants treatment eligible.
Conclusion
In long term care residents, those eligible for osteoporosis treatment ranged from less than 20% to roughly all patients depending on screening criteria. Vertebral fractures are common and identify a subset of patients missed by conventional bone density or FRAX-FN. A reasonable clinical approach could consider treatment for those with clinical fractures of the hip or spine, radiologic evidence for a vertebral fracture or osteoporosis by bone density classification. Prospective studies are needed to determine optimal screening strategies for treatment in this cohort.
Keywords: osteoporosis, long term care, FRAX
INTRODUCTION
Although osteoporosis can be a debilitating condition for adults of all ages, its impact is most devastating for the frail elderly. One out of three individuals over the age of 90 will sustain a hip fracture (1), an event associated with up to 20% chance of death in the subsequent year (2). One quarter of patients with hip fractures are ultimately institutionalized and less than half of them fully recover. Because the fastest growing segment of the United States population are adults over the age of 85, the number of patients with hip fractures is expected to double in the next 15 years (3;4).
The impact of osteoporosis for residents in the nursing home is even more dramatic. Nearly 2 million people live in long term care facilities throughout the United States. Previous studies suggest a prevalence of osteoporosis of 85% in long term care facilities (5–7). The rate of non-vertebral fractures may be 2–3% per year in the community while up to 11% in the nursing home (7). Nursing home residents who fracture at any site are hospitalized more than 15 times as often as those that did not (8). Therefore, it is not unexpected that a panel of experts from the March 2000 NIH Consensus Development Conference on Osteoporosis Prevention, Diagnosis and Treatment identified frail nursing home patients as one of the 5 critical areas that need our immediate attention (9) and this was recently echoed by the NIH/ASBMR 2011 Forum on Aging and Skeletal Health (10).
Similar to most diseases and comorbid conditions in frail elderly, a key issue is whether there are treatment benefits that justify screening for osteoporosis. Unfortunately except for hip protectors (equivocal efficacy) and calcium and vitamin D, there are limited studies for osteoporosis therapy in this cohort. Post hoc sub-analysis of pivotal trials have suggested that many FDA approved therapies are efficacious in women over the age of 75 (11–14). However the older postmenopausal women included in pivotal trials are cognitively intact, independent, ambulatory, and have limited medications and comorbidities. These are not the frail women who reside in nursing homes or assisted living.
The vast majority of patients in long term care are not treated (15–17). Estimates of any bone related treatment in long term care, including hip protectors, range from 7 to 36% (15–18). The reasons for lack of treatment appear to be multifactorial and include lack of evidence supporting efficacy in long term care residents, concerns for side effects, cost of medications, lack of staff time to administer medications and patients with high comorbidity who die before they obtain benefits from fracture reduction therapy (19;20). However, the mean life expectancy in a nursing home is 2.8 years (21) and 3–5 years in assisted living. Since many potent osteoporosis therapy result in fracture reduction in 1 year or less, residents could obtain benefit (22–24).
The other central issue is there is no gold standard test for determining who should be treated for osteoporosis in long term care facilities. The National Osteoporosis Foundation (NOF) has developed a series of consensus guidelines for who should be treated. These guidelines were derived from data of postmenopausal women and men age 50 and older living in the community. These recommendations include treatment for 1) those with a hip or vertebral fracture, 2) a bone mineral density T-score by dual x-ray absorptiometry (DXA) at conventional sites (total hip, femoral neck or spine) ≤ −2.5 (osteoporosis range) or 3) individuals who have low bone mass or osteopenia (T-score between −1 and −2.5) having a FRAX (World Health Organization fracture risk algorithm) score (using femoral neck bone mineral density) resulting in a 10 year probability of hip fracture ≥ 3% or a10 year probability of a major osteoporotic fracture ≥ 20% (25). In the absence of a clinical osteoporotic fracture of the hip or spine, the current NOF guidelines do not specify treatment without a bone density.
The FRAX algorithm is based on a set of specific risk factors, incorporates age, height, weight, hip bone mineral density and is country, gender and race specific. The age range includes women 40 to 90 years. While these guidelines are useful for those in the community with easy access to bone mineral density testing, they may be less helpful for women in long term care with limited access to conventional DXA. However, FRAX also includes a score using the body mass index instead of bone mineral density. This would be a potential option for those in long term care who do not have access to bone mineral density testing by DXA. Guidelines also suggest treatment for those women with vertebral fractures. Although clinical vertebral fractures may be observed in up to 30% of patients living in the community (26), prevalence/incidence of vertebral fractures is not known in long term care residents. Knowledge of a vertebral fracture could significantly alter the decision to treat a resident in long-term care since those residents have 4 times the risk of another future vertebral fracture and more than double the risk of hip fracture (27).
Therefore, this study was designed to examine different screening strategies for osteoporosis treatment eligibility in long term care residents. Treatment would include approved pharmacological therapies including bisphosphonates, estrogen agoniststs/antagonists, calcitonin, denosumab and teriparatide. We sought to describe the proportion of nursing home and assisted living residents that would be recommended for pharmacologic treatment of osteoporosis using different screening strategies.
METHODS
Study Design
This was a cross-sectional analysis of subjects undergoing screening for a clinical trial designed to examine prevention and treatment of bone loss in residents in long term care. We enrolled female residents of long term care facilities in the Pittsburgh area if they were age 65 or older, were residing in a nursing home or assisted living facility, and were currently not on bisphosphonate therapy. Patients with a life expectancy of less than 2 years were not included.
Following administrative meetings in long term care facilities, letters were sent to the affiliated physicians and family members letting them know of the research program. Recruitment was done in partnership with the Activity Directors and Directors of Nursing at each facility. Informational sessions highlighted the specifics of the study process. Per the regulations for skilled facilities in Pennsylvania, permission was obtained from the state before recruitment could begin along with obtaining the Institiutional Review Board approval from the University of Pittsburgh. In addition to the patient’s assent, if the patient had cognitive impairment, their responsible party or power of attorney provided consent.
Measurements
We examined bone mineral density of the hip (total hip, femoral neck) and PA spine (L1–L4) using a mobile DXA unit with an onboard Hologic Discovery A densitometer (Hologic Inc, Bedford, MA). The precision error of scans on elderly patients using the DXA scanning technique is 1.2% for the total hip, 1.9% for the femoral neck, 1.5% for the PA spine (28). The presence of vertebral fractures in the thoracic or lumbar spine were determined by vertebral fracture assessment (VFA) performed by DXA. This assesses vertebral fractures from T6-L4 and classifies them according to the Genant criteria of mild, moderate to severe vertebral fractures (29;30). The sensitivity and specificity of VFA versus conventional x-rays was 100% and 95% respectively in our hands (31). The overall Kappa statistic was 0.92 (31). Vertebral fractures were also identified by review of medical records for lateral thoracic and lumbar x-rays. Ultrasound of the heel was performed with a Hologic Sahara Clinical Bone Sonometer (Hologic Inc, Bedford, MA).
Clinical fragility fracture data of the thoracic or lumbar spine or hip were collected at baseline. A fragility fracture was defined as a fracture following a fall from standing or sitting height or lesser trauma as many vertebral fractures occur without a fall. Subjects were queried if they had a clinical fragility fracture as an adult to use in the National Osteoporosis Foundation (NOF) clinical guidelines for treatment eligibility. Fractures were verified with medical records.
Clinical Risk Factors
To characterize the frailty of the cohort, because there is no conventional assessment for frailty in residents in long term care (32–34), we examined the Physical Activities of Daily Living [eat, dress, groom hair, walk, transfer, bathe, get to bathroom (35)], the Instrumental Activities of Daily Living [able to use phone, shop, prepare meals, housework, take medication, handle money (36)], gait speed [<0.6 m/sec considered frail, (37)] and Duke comorbidity Index, (38). For the FRAX score, in addition to height and weight assessed at baseline, we queried patients about previous fracture at any site (except fingers, toes and skull), parental hip fracture, current smoking, use of glucocorticoids, diagnosis of rheumatoid arthritis, questions regarding secondary osteoporosis (disorder strongly associated with osteoporosis) and alcohol intake. Using these variables, a FRAX score (web version 3.4) was calculated using bone mineral density of the femoral neck (FRAX-FN) (39). Because the FRAX algorithm only goes to age 90, those above 90 were considered 90 years old and included in the analysis. In addition, we calculated FRAX using only body mass index (FRAX-BMI).
Screening Strategies
In order to determine which residents would be treated for osteoporosis we examined several different screening strategies including patients with 1) clinical fractures at baseline (Clin Fx), either at the spine or hip verified by medical record or radiologic examination; 2) clinical fracture or bone mineral density (BMD) osteoporosis classification by the spine, total hip or femoral neck T-scores ≤ −2.5 (Clin Fx or BMD); 3) clinical fracture, osteoporosis by bone mineral density criteria, or those who had a vertebral fracture (VF) identified by VFA [graded as mild, moderate or severe by Genant criteria (29)] or conventional lateral thoracic or lumbar x-ray (Clin Fx, BMD or VF); 4) FRAX score using femoral neck bone mineral density that demonstrated a major osteoporotic fracture risk in 10 years ≥ 20% or hip fracture risk ≥3% (FRAX-FN); 5) FRAX calculation using only height and weight for BMI (no bone mineral density) with a 10 year risk of a major fracture ≥20% or hip fracture risk ≥3% (FRAX-BMI); and 6) clinical fractures or osteoporosis classification by heel ultrasound with T-score ≤ −2.5 (ClinFx or heel US) (Table 1).
Table 1.
Screening Strategies for Pharmacologic Treatment
| Strategy | Category | Criteria for Positive Screen | Origin of Guideline/Strategy |
|---|---|---|---|
| Clinical Fracture | Clin Fx | Major osteoporotic fracture of the hip or vertebrae | NOF (25) |
| Clinical Fracture or Bone Mineral Density | Clin Fx or BMD | Major osteoporotic fracture of the hip or vertebrae or Bone mineral density (by Dual X-ray Absorptiometry, DXA): hip (femoral neck or total hip) or spine T-score ≤ −2.5 (osteoporosis range) |
NOF (25) |
| Clinical Fracture, Bone Mineral Density or Vertebral Fracture | Clin Fx, BMD or VF | Major osteoporotic fracture of the hip or vertebrae or Bone mineral density (by Dual X-ray Absorptiometry, DXA): hip (femoral neck or total hip) or spine T-score ≤ −2.5 (osteoporosis range) or Vertebral fracture determined by vertebral fracture assessment (DXA derived) |
NOF (25) |
| FRAX using Femoral Neck Bone Mineral Density | FRAX-FN | FRAX calculation using femoral neck BMD: Low bone mass and a WHO 10 year probability of hip fracture ≥ 3% or 10 year probability of any major osteoporotic related fracture ≥ 20% | NOF (25) WHO (39) |
| FRAX using Body Mass Index | FRAX-BMI | FRAX calculation using only height and weight: with WHO 10 year probability of hip fracture ≥ 3% or 10 year probability of any major osteoporotic related fracture ≥ 20% | NOF (25) WHO (39) |
| Clinical Fracture or Heel Ultrasound | Clin Fx Or Heel US |
Major osteoporotic fracture of hip or vertebrae or Heel ultrasound T-score ≤ −2.5 |
NOF (25) NOF-Ultrasound predicts fracture risk, T- scores not equivalent to DXA (25) |
RESULTS
Seven hundred and thirty-three contacts were made in 11 long term care facilities in the Pittsburgh, Pennsylvania area. Two hundred and fifty-two women consented to screening, and of those women, BMD, VFA, historical information were available in 202 patients who were included in the study. Ultrasound was available in 182 of these residents.
The mean age of patients was 85.4 ± 0.4 (mean ± standard error) years with a range of 68 to 99 years (Table 2). Forty patients (19.8%) were over age 90 years (the cut off for FRAX). Ninety-seven percent were Caucasian with 3% African American. By the Physical Activities of Daily Living (35), 74% had at least 1 disability and 31% had at least 3. By the Instrumental Activities of Daily Living (36), 93% had at least 1 disability, 70% had at least 3. Gait speed was <0.6 m/sec in 70% (37). Fifty-five percent could not transfer independently without the aid of a device or person. One hundred percent had at least 1 comorbid condition, 82% had at least 3 [Duke comorbidity Index, (38)]. The average body mass index was 27.6 ± 0.4 kg/m2. The average T-scores were −0.7 ± 0.1 for the spine, −2.0 ± 0.1 for the total hip, −2.1 ± 0.1 for the femoral neck and −1.4 ± 0.1 for the heel ultrasound.
Table 2.
Patients Eligible for Treatment Based on Screening Strategy (Mean ± SEM unless otherwise indicated)
| Total | Clinical Fracture ┼ | Clinical Fracture or BMD ⧧ | Clinical Fracture, BMD or Vertebral Fracture & | FRAX-FNΔ | FRAX-BMI# | Clinical Fracture or Heel Ultrasound γ | |
|---|---|---|---|---|---|---|---|
| Age (Years) | 85.4±0.4 | 85.8±0.8 | 86.5±0.4 | 86.6±0.4 | 86.1±0.4 | 85.7±0.4 | 87.7±0.6 |
| BMI (Kg/m2) | 27.6±0.4 | 27.4±0.9 | 26.5±0.4 | 26.8±0.4 | 26.8±0.4 | 27.4±0.4 | 25.8±0.7 |
| Spine T-score | −0.7±0.1 | −1.3±0.3 | −1.5±0.2 | −1.0±0.2 | −1.1±0.1 | −0.8±0.1 | −1.6±0.3 |
| Total Hip T-Score | −2.0±0.1 | −2.3±0.2 | −2.7±0.1 | −2.3±0.1 | −2.3±0.1 | −2.0±0.1 | −2.9±0.1 |
| Femoral Neck T-Score | −2.1±0.1 | −2.5±0.2 | −2.8±0.1 | −2.3±0.1 | −2.4±0.1 | −2.1±0.1 | −2.8±0.2 |
BMD (bone mineral density) classified by spine, total hip or femoral neck, T-score ≤ −2.5
Clinical fractures: spine or hip fragility fracture
Vertebral fracture identified by DXA-derived by vertebral fracture assessment or x-ray
FRAX-FN: uses femoral neck BMD with 10 year major osteoporotic fracture risk ≥ 20%, hip fracture risk ≥ 3%
FRAX-BMI: uses BMI without femoral neck BMD with 10 year major osteoporotic fracture risk ≥ 20%, hip fracture risk ≥ 3%
Heel ultrasound, T-score ≤ −2.5
Figure 1 demonstrates the percentage of patients eligible for treatment based on each screening strategy. Seventeen percent of patients had a clinical fracture (spine or hip) and were eligible for treatment. Based on BMD criteria with a T-score ≤ −2.5 or lower at the spine, total hip or femoral neck or clinical fractures, 54% would be treated. If we substituted heel ultrasound for BMD, the combination of a clinical fracture or heel ultrasound would result in pharmacologic treatment of 39%. Vertebral fractures assessed by VFA or clinical vertebral fractures would suggest treatment in half of the patients. Of these 101 patients, 74% were identified by VFA (silent vertebral fractures) and 26% were clinical with historical x-rays. If we included women meeting any of those 3 criteria (clinical fracture, osteoporosis by BMD or VF), 73% would have been treated. FRAX computed with BMD of the femoral neck (FRAX-FN) suggested, 81% would be treated; FRAX-BMI based on BMI (without BMD), would recommend treatment in 98%.
Figure 1.
The percent of residents eligible for treatment based on clinical fractures (Clinical Fx); Clinical fractures or bone mineral density osteoporosis classification: (Clinical Fx or BMD); Clinical fractures, BMD or vertebral fractures (Clinical Fx, BMD or VF); FRAX algorithm using femoral neck BMD (FRAX-FN); FRAX using body mass index (FRAX-BMI); or clinical fractures or heel ultrasound osteoporosis classification (Clinical Fx or Heel US).
Of the 101 patients that had a vertebral fracture by VFA or x-ray, 16 (16%) had a normal bone density and 35 (35%) had low bone mass (osteopenia). This means that 51 of 101 (roughly half) of patients with vertebral fractures would have been missed by a screening strategy that uses bone density criterion only.
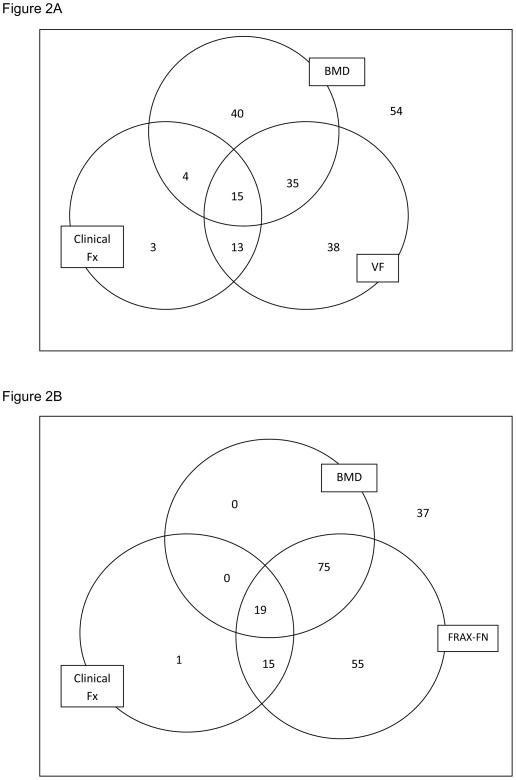
We examined the overlap of the screening methods to identify subsets of patients included or excluded for treatment, and present results graphically with three criteria at a time for clarity (Figures 2–3). The patients who would be treated based on BMD alone (N=94), overlapped with those eligible by VF (N=101) by only 50 patients (Figure 2A). Approximately two-thirds of the patients that should be treated by either of these two criteria were in one group or the other, and would have been missed if only one method was utilized. When the clinical fracture criterion was included in the analysis only 3 additional patients with clinical fractures would have been included for treatment.
Figure 2.
A.) Venn diagram illustrating number of patients eligible for treatment based on criteria for bone mineral density (BMD), vertebral fractures (VF) or clinical fractures (Clinical Fx) and overlap of the 3 criteria. Fifty-four patients were not eligible for treatment. B.) Venn diagram illustrating number of patients eligible for treatment based on criteria for bone mineral density (BMD), clinical fractures (Clinical Fx) or fracture risk algorithm (FRAX-FN, using femoral neck BMD) and overlap of the 3 criteria. Thirty-seven patients were not eligible for treatment.
Figure 3.
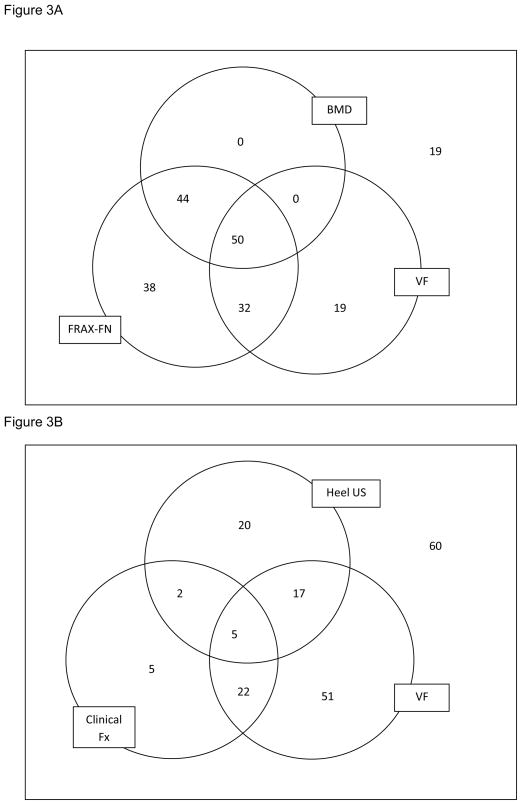
A.) Venn diagram illustrating number of patients eligible for treatment based on criteria for bone mineral density (BMD), vertebral fractures (VF) or fracture risk algorithm (FRAX-FN, using femoral neck BMD) and overlap of the 3 criteria. Nineteen patients were not eligible for treatment. B.) Venn diagram illustrating number of patients eligible for treatment based on criteria for clinical fractures (Clinical Fx); vertebral fractures (VF) or heel ultrasound (Heel US) and overlap of the 3 criteria. Sixty patients were not eligible for treatment.
We examined the overlap among clinical fractures, BMD and FRAX-FN. Because FRAX-FN includes femoral neck BMD and clinical fractures, only one additional patient would not have been missed by FRAX-FN criterion (Figure 2B) that would have been identified by fractures. In comparison, if we compared overlap of BMD, FRAX-FN and VF (not necessarily included in the FRAX calculation due to silent nature of VF), VF identifies an additional 19 patients to be treated compared to FRAX by itself (Figure 3A).
Heel ultrasound by itself only identified 24% of patients for treatment. However, these 44 women would have been largely missed by clinical fracture criterion. Heel ultrasound, identified an additional 37 women eligible for treatment (20%) above and beyond those identified by clinical fractures. If we consider both clinical fractures and vertebral fractures, heel ultrasound added an additional 20 patients (11%) for treatment (3B).
DISCUSSION
We observed that the eligibility of treatment for older women in long term care with a mean age of 85 ranged from 17 to 98%, depending on the screening strategy. Overall, treatment based on clinical fractures of the spine and hip alone would suggest treatment in less than 20%. Assessing vertebral fractures by vertebral fracture assessment DXA technology or x-ray identified an additional cohort of patients requiring treatment missed by bone mineral density. Three quarters of the vertebral fractures identified were silent. The combination of bone mineral density (able to detect both spine and hip osteoporosis criteria), clinical vertebral and hip fractures and vertebral fractures by VFA suggested treatment in close to three quarters of the patients. This combination supports the NOF clinical guidelines; however, the NOF guidelines also include those with appropriate FRAX criteria. Surprisingly, FRAX based on femoral neck bone density alone identified 81% of participants for treatment but missed almost 10% of women with silent vertebral fractures that might benefit from treatment.
The findings of this study need to be put in context of the clinical relevance and ramifications. First there are limited data on the efficacy and safety of osteoporosis treatment in residents in long term care except for studies on calcium and vitamin D. Second, the guidelines of the NOF were based on community dwelling post menopausal women - - a cohort very different from the cognitively impaired, mobility challenged and dependent residents in long term care. Finally, although most women in long term care facilities live long enough to appreciate a benefit of fracture reduction from therapy for osteoporosis, it would be reasonable to target women who would gain the greatest benefit from screening and treatment.
This study demonstrates the importance of considering different screening strategies for optimal osteoporosis treatment. Seventeen percent of patients had clinical fractures of the spine and hip. These patients have clinical osteoporosis and should be the first who are evaluated for secondary causes of osteoporosis (for example vitamin D deficiency) and treated. While many physicians rely on bone mineral density alone, over half of frail elderly patients would not be treated based on BMD alone. FRAX with femoral neck BMD takes into account age and other risk factors important for aging. In this analysis, FRAX did not omit any patients that would have been treated by BMD alone. The substitution for BMI instead of BMD changed FRAX treatment eligibility from 81 to 98% or nearly all of the residents. Unfortunately, most pivotal treatment studies have examined entry criteria utilizing bone mineral density T-score osteoporosis classification and/or history of vertebral or hip fractures. Because FRAX has not been used as a clinical trial entry criteria, especially with BMI alone, it is not clear if treatment would be justified by BMI criteria alone. Furthermore, FRAX, while validated in the community cohorts, has not been validated in the long term care setting.
Vertebral fractures were observed in half of the cohort and the majority were silent. In our population, roughly half of patients with a normal or low bone mineral density (osteopenia) had a vertebral fracture. This means that approximately half of patients would have been misclassified with respect to treatment eligibility. Although radiology is available in some long term care facilities, a lateral thoracic and lumbar film are needed to diagnose a vertebral fracture and these more specialized films are often not available on site. We have previously demonstrated the importance of the identification of vertebral fractures in community dwelling elderly women and in men with prostate cancer on androgen deprivation therapy (31;40). This study further emphasizes the importance of identification of vertebral fracture in the diagnosis of osteoporosis and screening strategy for treatment.
Previous studies have looked at ultrasound as a tool for screening. Our study agrees with others that have found that heel ultrasound screening with a T-score threshold of ≤ −2.5 only identifies the tip of the iceberg of patients with osteoporosis. The National Health and Nutrition Examination Survey (NHANES III, 1988–1991) that included 1558 women age 50 and older, estimated 20% had osteoporosis by femoral neck BMD (41). The National Osteoporosis Risk Assessment (NORA) study examined peripheral measures in 200,160 ambulatory women age 50 and older with no previous diagnosis of osteoporosis. In their cohort, heel ultrasound identified 3.4% with osteoporosis (42). This means that roughly 83% with osteoporosis by femoral neck BMD were missed by heel ultrasound. We found that heel ultrasound alone identified only 24% for treatment but increased treatment criteria up to 39% when added to clinical fractures.
There are several limitations to this study. FRAX was designed to include patients age 40–90; however, close to 20% of our patients were over age 90 and were included in the age 90 bracket although they were older. Second, bone mineral density assessment may have been falsely elevated due to osteoarthritis, degenerative joint disease, osteophytes, scoliosis and even arthritis of the hip which are all common in this population. This would underestimate the number of older patients in our analysis who should be treated based on bone density criteria. Moreover, we did not include forearm bone density. A third limitation is that we did not verify all vertebral fractures by conventional x-rays. Our VFA fracture ascertainment was DXA-based. However, we have previously shown in a population of older ambulatory men, the kappa statistics for vertebral fracture assessment with conventional radiology was 0.92 (31). Finally, we did not include men.
Despite the limitations, there are several important strengths of the study. We specifically gathered information to identify patients with rheumatoid arthritis, those who were smoking, on glucocorticoids, and who had a personal fracture or family history of fracture as patients were enrolled in the study to be included in FRAX. Because of the mobile DXA unit, we were able to include femoral neck bone mineral density to be included in the FRAX calculation. This allowed us to look at the difference between FRAX based on BMI alone and bone density. Furthermore, our findings extend those of NHANES III to a more frail population and are of comparable precision. NHANES III included 212 women age 80 and older living in the community and reported 52% with osteoporosis (41) while our analysis included 202 in long-term care. Finally, we were able to examine ultrasound which potentially could have been a useful and feasible assessment tool to screen for treatment for osteoporosis in this population.
In summary, we found that alternative screening strategies identified widely varying percentages of eligible patients for osteoporosis treatment ranging from 17 to 98%. Because vertebral fractures are common in this population and often silent, some assessment for vertebral fractures should be performed. While FRAX using BMI appears to be a feasible assessment tool in this population, it appears to have over-estimated who should be treated. In contrast, heel ultrasound using a T-score threshold of ≤−2.5, another feasible tool, appears to identify too few. Furthermore, femoral neck bone density used in FRAX may under diagnose patients who should be treated because of missing knowledge regarding silent vertebral fractures.
Overall, our results suggest that some assessment for vertebral fractures is appropriate. A reasonable clinical approach might consider treatment for those with a clinical hip or spine fracture, radiologic evidence of a vertebral fracture or bone density osteoporosis classification if available. Moreover, since most osteoporosis therapies have demonstrated fracture reduction in approximately 1 year, residents expected to live less than 1 year may not receive a benefit. Future studies are needed to follow these parameters prospectively along with pharmacologic therapy to more definitively determine which specific strategies are most appropriate to identify individuals in the frail elderly population for treatment.
Acknowledgments
Funding for this project provided by a generous donation from the Holleran family and NIH awards R01 AG028068, University of Pittsburgh Clinical Translational Research Center RFA-RM-06-002, Clinical Translational Science Institute Ul1 RR024153 NIH/NCRR, Pittsburgh Older American’s Independence Center P30 AG024827, and 2K24DK062895.
Sponsor’s Role: The sponsors had no involvement in the study design, methods, subject recruitment; collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: The editor inchief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors had access to the study data, and participated in subject recruitment, analysis or interpretation of the data (or both), and preparation of the manuscript. Editorial support for this article, comprising language editing, content checking, formatting, and referencing was provided by Megan Miller and Dory Adams.
References
- 1.Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178–208. doi: 10.1093/oxfordjournals.epirev.a036281. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR. Should perimenopausal women be screened for osteoporosis? Ann Intern Med. 1986;104:817–23. doi: 10.7326/0003-4819-104-6-817. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, III, O’Fallon WM, Riggs BL. Secular trends in the incidence of hip fractures. Calcif Tissue Int. 1987;41:57–64. doi: 10.1007/BF02555245. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey JL, Hoffman S. Risk factors for hip fracture. N Engl J Med. 1987;316:404–406. doi: 10.1056/NEJM198702123160709. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman SI, Girman CJ, Buie VC, et al. The prevalence of osteoporosis in nursing home residents. Osteoporos Int. 1999;9:151–157. doi: 10.1007/s001980050129. [DOI] [PubMed] [Google Scholar]
- 6.Komar L, Nieves J, Cosman F, et al. Calcium homeostasis of an elderly population upon admission to a nursing home. J Am Geriatr Soc. 1993;41:1057–1064. doi: 10.1111/j.1532-5415.1993.tb06452.x. [DOI] [PubMed] [Google Scholar]
- 7.Chandler JM, Zimmerman SI, Girman CJ, et al. Low bone mineral density and risk of fracture in white female nursing home residents. JAMA. 2000;284:972–7. doi: 10.1001/jama.284.8.972. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman S, Chandler JM, Hawkes W, et al. Effect of fracture on the health care use of nursing home residents. Arch Intern Med. 2002;162:1502–1508. doi: 10.1001/archinte.162.13.1502. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous. NIH consensus declares osteoporosis a major public health issue. Osteoporosis Report. 2000;16:1. [Google Scholar]
- 10.Khosla S, Bellido TM, Drezner MK, et al. Forum on aging and skeletal health: Summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2011;26:2565–2578. doi: 10.1002/jbmr.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boonen S, Black DM, Colon-Emeric CS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58:292–299. doi: 10.1111/j.1532-5415.2009.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonen S, Marin F, Mellstrom D, et al. Safety and efficay of teraparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54:782–789. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 13.Schneider DL. Management of osteoporosis in geriatric populations. Curr Osteoporos Rep. 2008;6:100–107. doi: 10.1007/s11914-008-0018-4. [DOI] [PubMed] [Google Scholar]
- 14.Boonen S, Dejaeger E, Vanderschueren D, et al. Osteoporosis and osteoporotic fracture occurrence and prevention in the lderly: A geriatric perspective. Best Pract Res Clin Endocrinol Metabol. 2008;22:765–785. doi: 10.1016/j.beem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Parikh S, Mogun H, Avorn J, et al. Osteoporosis medication use in nursing home patients with fractures in 1 US State. Arch Intern Med. 2008;168:1111–1115. doi: 10.1001/archinte.168.10.1111. [DOI] [PubMed] [Google Scholar]
- 16.Colon-Emeric C, Lyles KW, Levine DA, et al. Prevalence and predictors of osteoporosis treatment in nursing home residents with known osteoporosis or recent fracture. Osteoporos Int. 2007;18:553–559. doi: 10.1007/s00198-006-0260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jachna CM, Shireman TI, Whittle J, et al. Differing patterns of antiresorptive pharmacotherapy in nursing facility residents and community dwellers. J Am Geriatr Soc. 2005;53:1275–1281. doi: 10.1111/j.1532-5415.2005.53401.x. [DOI] [PubMed] [Google Scholar]
- 18.Beaupre LA, Majumdar SR, Dieleman S, et al. Diagnosis and treatment of osteoporosis before and after admission to long-term care institutions. Osteoporos Int. 2011 Mar 5; doi: 10.1007/s00198-011-1582-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Sawka AM, Ismaila N, Raina P, et al. Hip fracture prevention strategies in long-term care: A survey of canadian physicians opinions. Can Fam Physician. 2010;56:e392–e397. [PMC free article] [PubMed] [Google Scholar]
- 20.Wright RM. Use of osteoporosis medications in older nursing facility residents. J Am Med Dir Assoc. 2007;8:453–457. doi: 10.1016/j.jamda.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dollard KJ, editor. Facts and Trends: The Nursing Facility Sourcebook. American Health Care Association; 2001. [Google Scholar]
- 22.Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin. 2004;20:433–439. doi: 10.1185/030079903125003125. [DOI] [PubMed] [Google Scholar]
- 23.Harrington JT, Ste-Marie LG, Brandi ML, et al. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 24.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 25.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporsis. Washington, DC: Copyright NOF; 2008. [Google Scholar]
- 26.Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 27.Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 28.Varney LF, Parker RA, Vincelette A, et al. Classification of osteoporosis and osteopenia in postmenopausal women is dependent on site-specific analysis. J Clin Densitom. 1999;2:275–283. doi: 10.1385/jcd:2:3:275. [DOI] [PubMed] [Google Scholar]
- 29.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res. 1996;11:984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 30.Black DM, Palermo L, Nevitt MC, et al. Comparison of methods for defining prevalent vertebral deformities: The Study of Osteoporotic Fractures. J Bone Miner Res. 1995;10:890–902. doi: 10.1002/jbmr.5650100610. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan S, Wagner J, Resnick NM, et al. Vertebral fractures and the misclassification of osteoporosis in men with prostate cancer. J Clin Densitom. 2011 doi: 10.1016/j.jocd.2011.05.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Kan GA, Rolland Y, Houles M, et al. The assessment of frailty in older adults. Clin Geriatr Med. 2010;26:275–286. doi: 10.1016/j.cger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Rockwood K, Abeysundera MJ, Mitnitski A. How should we grade frailty in nursing home patients? J Am Med Dir Assoc. 2007;8:595–603. doi: 10.1016/j.jamda.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 35.Katz SC, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 36.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 37.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 38.Rigler SK, Studenski S, Wallace D, et al. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehab. 2002;16:420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA on behalf of the World Health Organization Scientific Group. Assessment of Osteoporosis at the Primary Health Care Level. University of Sheffield; UK: WHO Collaborating Center; 2008. [Google Scholar]
- 40.Xu W, Perera S, Medich D, et al. Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone. 2011;48:307–311. doi: 10.1016/j.bone.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looker AC, Johnston CC, Jr, Wahner HW, et al. Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res. 1995;10:796–802. doi: 10.1002/jbmr.5650100517. [DOI] [PubMed] [Google Scholar]
- 42.Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]