Abstract
Background
Malignant pleural mesothelioma (MPM) tumors express VEGF and VEGF receptors. MPM patients have high circulating levels of VEGF, suggesting that tumor angiogenesis is a relevant therapeutic target for MPM. We conducted a phase 2 trial of the VEGFR1/2 TKI AZD2171 in patients with MPM after platinum-based systemic chemotherapy.
Patients and Methods
Patients (pts) with MPM previously treated with a platinum-containing chemotherapy regimen, good performance status, measureable disease and no significant bleeding diathesis or thrombotic history were eligible for enrollment. Pts were treated with AZD2171 45 mg/day on days 1-28 of each 28 day dosing cycle. Tumor measurements were made by RECIST criteria, with a subset analysis of modified RECIST. A two-stage design with a stopping rule based on number of patients with complete or partial response was utilized.
Results
Fifty-four patients were enrolled on study between 11/05 and 4/08. For 47 evaluable patients, 4 patients (9%) had objective responses, including two patients who had 91% and 56% responses in bulky pleural tumor; 16 patients (34%) had stable disease, 20 pts (43%) had disease progression; 2 pts (4%) had symptomatic deterioration and 1 pt (2%) had early death. Four were not assessable for response and assumed to be non-responders. The most common AZD2171-attributable toxicities were fatigue (64%), diarrhea (64%) and hypertension (70%); the majority of patients required a dose reduction. Median overall survival was 9.5 months, 1-year survival was 36% and median progression-free survival was 2.6 months.
Conclusions
The anti-angiogenic AZD2171 has modest single agent activity in MPM. However, several patient tumors were exquisitely sensitive to AZD2171. The starting dose was not well tolerated in this setting. Tumor response by Modified RECIST available for a small subset of patients correlated well with tumor response by RECIST. This study provides a rationale for further testing of AZD2171 with chemotherapy in MPM.
Keywords: Phase 2, angiogenesis inhibitor, pleural mesothelioma
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a neoplasm that originates in the parietal pleura that is strongly associated with asbestos exposure through chronic environmental or occupational exposure. The lag time between exposure and presentation of the disease ranges between 5 and 20 years, although cases have been diagnosed more than 40 years after asbestos exposure.1 MPM is a rare cancer, with between two and three thousand cases diagnosed in the United States per year; it is expected that the incidence of MPM will peak around 2010.2 Globally, however, less stringent regulation of occupational asbestos has resulted in the more recent or continued exposure of large populations asbestos and to continued increase in new cases of MPM in Australia, Britain, Europe, and Asia.3-5
MPM is a relatively chemotherapy- and radiation-resistant tumor. The median survival for untreated patients is in the range of 4 - 12 months.6 Currently, platinum-based therapy in combination with newer generation antifolate drugs such as raltitrexed and pemetrexed remains a benchmark for standard front-line therapy for MPM, with median survival times of 11.4 and 12.1 months, respectively, reported in Phase III trials in combination with cisplatin.7 There currently are no well accepted standard therapies in the setting of second line MPM, and no targeted therapies have been approved for clinical application in MPM.
There is evidence supporting the existence of an autocrine growth loop involving VEGF and its cognate receptors for MPM. VEGF and its cognate receptors VEGFR-1 (flt) and R-2 (KDR) are expressed in human MPM cell lines, with higher levels of VEGF detected in MPM cell line cultures than in normal mesothelial cell cultures. 8 The addition of rhVEGF to MPM cell line cultures stimulates cell proliferation in a dose-dependent manner, while inhibitors of VEGF and VEGF-C inhibit mesothelioma cell growth.9,10Human MPM tumors express the angiogenic cytokines VEGF, FGF-1 and -2, and TGFβ, 11,12; additionally, the co-expression of VEGF and VEGF-R1 in human MPM has been reported.13 MPM patients have high circulating levels of VEGF14. Some studies have reported tumor VEGF expression and tumor microvessel density to be independent prognostic markers.15,16 These data suggest that tumor angiogenesis may be a relevant therapeutic target in MPM.
AZD2171 (Cediranib; AstraZeneca Pharmaceuticals,Wilmington, DE) is an orally active, potent, tyrosine kinase inhibitor (TKI) of VEGFR-1,-2 and -3 at nanomolar concentrations, as well as c-Kit and PDGFR-β.17,18 In phase I studies the maximum tolerated dose was defined as 20-45 mg daily, with reported dose limiting toxicities of proteinuria, diarrhea, thrombocytopenia, hypertension and hypertensive crisis. 19-21 AZD2171 has single agent anti-tumor activity (partial responses and disease stabilization) in a number of solid tumors including ovarian cancer, alveolar soft tissue sarcoma, gastric cancer, biliary cancer, pancreatic cancer, CRC, prostate, RCC, breast cancer, thyroid cancer and NSCLC.21-24
Given that tumor angiogenesis is a potential therapeutic target in MPM and that AZD2171 has shown tolerability and anti-tumor efficacy in a number of solid tumors, we performed a Phase II trial of AZD2171 in patients with advanced or recurrent MPM after platinum-based chemotherapy. The primary objective of the trial was to assess the objective response rate of AZD2171 in second line MPM. Secondary objectives were to measure survival outcomes as well as to define the toxicity profile of AZD2171 in this setting. A protocol amendment was made to also capture tumor response by modified RECIST for pleural tumors.25
PATIENTS AND METHODS
Inclusion Criteria
Patients at least 18 years of age with histologically proven epithelial, sarcomatous or biphasic mesothelioma (by pathology evaluation at local institutions) whose disease was not respectable were eligible for enrollment. Patients were required to have had prior platinum-based chemotherapy and only one prior chemotherapy regimen was allowed. Patients with measurable disease by RECIST criteria were eligible. Prior radiation or surgical procedures for MPM for diagnostic or therapeutic purposes were allowed. A Zubrod performance status of 0-2 and adequate hematologic function (absolute neutrophil count ≥ 1500/ml, platelets ≥ 100,000/ml), hepatic function (serum bilirubin ≤ upper limit of normal and transaminases ≤ 1.5 times upper limit of normal) and renal function (serum creatinine ≤ 1.5 times upper limit of normal or a measured creatinine clearance ≥ 50 mL/min) were required.
Patients were not eligible if they had severe systemic co-morbid disease or a significant cardiac history, uncontrolled hypertension, significant proteinuria, a prolonged QTc interval, were either pregnant or breastfeeding, or had gastrointestinal tract disease resulting in inability to take oral medication or altered gastrointestinal absorption.
The protocol and informed consent document were approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute and the institutional review boards of participating SWOG member sites. Written informed consent was obtained from all patients prior to enrollment. This study was monitored by the Data and Safety Monitoring Committee of the Southwest Oncology Group.
Study Design
The S0509 treatment protocol (ClinicalTrials.gov_Identifier: NCT0024307) consisted of single-agent AZD2171 administered orally at 45 mg/day on days 1-28 of each 28 day dosing cycle. No a priori limit on number of treatment cycles per patient was made. Adverse events were graded according to the CTC Version 3.0. Sequential dose modifications to 30 mg and 20 mg daily were allowed, although a further dose reduction to 10 mg daily was allowed for patients on study ≥ 3 months who were benefiting from treatment. Dose delay until toxicity resolved to ≤ Grade 1 and dose modification were made for ≥ Grade 3 non-hematologic and/or Grade 4 hematologic toxicities, and < Grade 3 but moderate hypertension, and proteinuria).
Patient history, physical examination, hematologic and chemical laboratory analyses were performed prior to beginning cycle 1 of therapy and prior to each subsequent treatment cycle. Tumor measurements were performed after every 2 treatment cycles. Tumor responses were judged by the response evaluation criteria in solid tumors (RECIST) guidelines.26 A protocol amendment was made to capture tumor measurements using the Modified RECIST measurement system.27 Patients were withdrawn from study for disease progression or symptomatic deterioration, unacceptable toxicity as assessed by the study physician, treatment delay of greater than 3 weeks or if more than the prescribed dose modifications were required. A 2-stage design was employed such that 20 patients would be enrolled in the first stage, with an evaluation of response rate and safety performed. A response rate of 20% or higher would be sufficient to declare this regimen worthy of further study, while a response rate of 5% or lower would be indicate no further interest in this setting. If at least one confirmed partial response was noted, the study would continue accrual to a total of 40 patients. This design has a significance level (probability of false declaring an agent with a true 5% response probability to warrant further study) of 5% and a power (probability of correctly declaring an agent with a 20% response probability to warrant further study) of 92%.
Results
Patients
Patient recruitment was undertaken from November, 2005 through April, 2008, with a 3-month temporary closure for first stage evaluation. A total of 54 patients were registered, with 48 eligible for enrollment, but 1 patient not analyzable due to refusal of treatment. Patient characteristics for the 47 patients are presented in Table 1. The median patient age was 66.8 years (range 43 to 84 years). The majority of patients were male. Racial composition was white (94%), not available (6%); ethnic composition was Hispanic (4%), Non-Hispanic (87%) and unknown 9%. Patient performance status was 0 in 34%, 1 in 53% and 2 in 13% of patients. The majority of patients had < 5% loss of body weight within the 6 months prior to enrollment.
Table 1. Patient Characteristics.
| N=47 | |
|---|---|
| Median age (years) | 66.8 (range 43-84) |
| Male:Female (%) | 38:9 (81%: 19%) |
| Zubrod Performance Status | |
| 0: 1: 34% 53% |
|
| 2: 13% | |
| Six month prior weight loss: | |
| <5%: 70% | |
| 5-<20%: 17% | |
| >20%: 4% | |
| Not reported: 9% |
Tumor histologic subtype was classified as epithelial (60%), biphasic (6%); and not otherwise specified (23%) by local institution pathology review. No patient had a sarcomatous histology tumor. A formal central pathology review was not performed.
Response and Survival
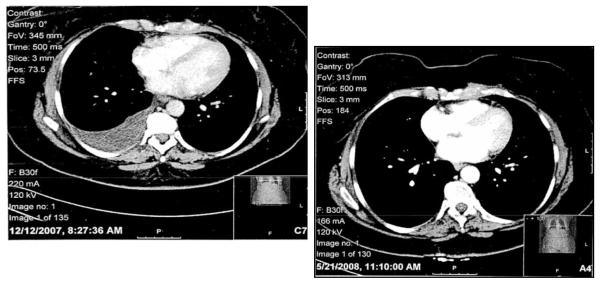
For 4 patients, response was not able to be determined due to inadequate assessment. These four patients are assumed to be non-responders and are included in calculations of response rate. For 43 evaluable patients, response data are summarized in Table 2. There were no complete responses. Four patients (9%) had partial responses, including two patients with bulky disease who had 91% and 56% tumor shrinkage (See Figures 1a-d). Sixteen patients (34%) had stable disease, documented at least 6 weeks after registration and before progression or symptomatic deterioration range 2.4 to 13.7 months). Twenty patients (43%) had progressive disease, 2 patients (4%) had symptomatic deterioration, and 1 patient (2%) had early death.
Table 2. Antitumor Activity – Best Overall Response.
| N=43 | |
|---|---|
| Complete Response (CR) | 0 |
| Partial Response (PR) | 4 (9%) |
| Stable Disease (SD) | 16 (34%) |
| Progressive Disease (PD) | 20 (43%) |
| Symptomatic Deterioration | 2 (4%) |
| Early Death | 1 (2%) |
Figure 1. Tumor response after 5 weeks of AZD2171 treatment by CT imaging.
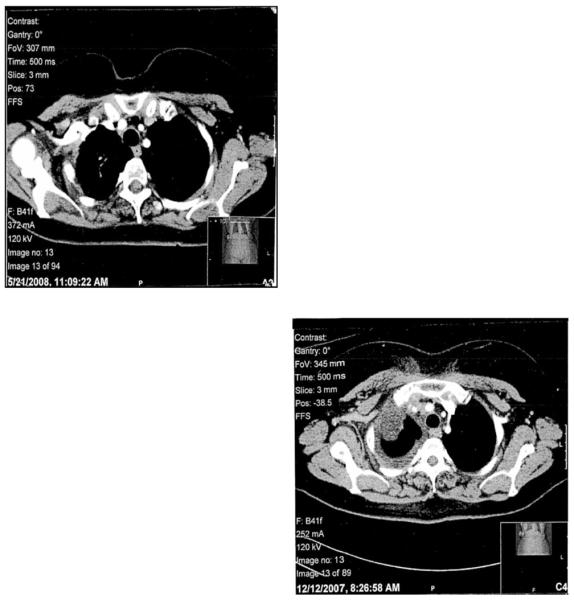
1a: Pre-treatment, upper hemithorax
1b:Post-treatment, upper hemithorax
1c: Pre-treatment, lower hemithorax
1d: Post-treatment, lower hemithorax
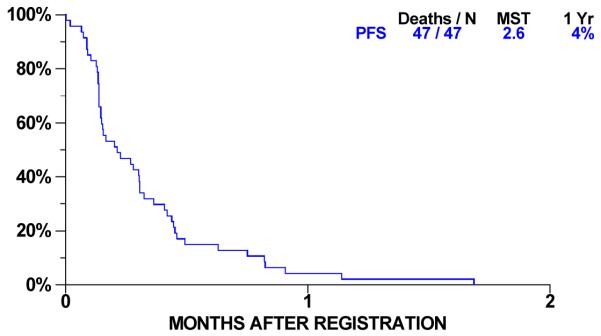
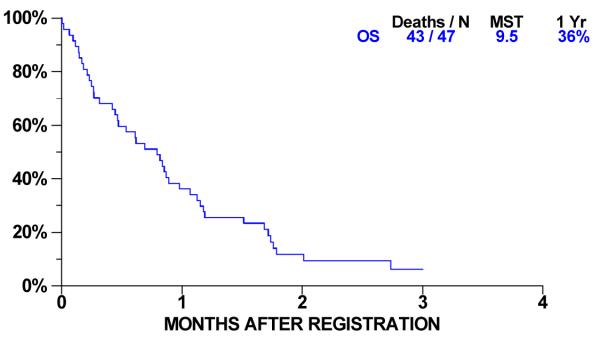
Overall survival for 47 patients is shown in Figure 2. Forty-three had died at the time of analysis; the median overall survival was 9.5 months (95% C.I. 5.6-10.7 months) and one year survival was 36% (95% C.I. 23-50%). Progression-free survival (PFS) is shown in Figure 3. Forty-seven patients have progressed or died. Median PFS was 2.56 months (95% C.I 1.74-3.68 months) and one-year progression-free survival was 4% (95% C.I. 1-13 %). For 16 patients with stable disease, median time to progression is 4.9 months (95% C. I. 3.6 to 5.4 months).
Figure 2. Progression Free Survival.
Figure 3. Overall Survival.
Adverse Events
Forty-seven patients were assessable for adverse events. Hematological adverse events attributed to AZD2171 were infrequent and of grades 1-2 only (Table 3).
Table 3. Hematological Toxicity.
| N=47 | Grade | ||
|---|---|---|---|
| 1 | 2 | % Pts | |
| Anemia | 5 | 1 | 13% |
| Leukopenia | 1 | 0 | 2% |
| Neutropenia | 1 | 0 | 2% |
| Thrombocytopenia | 7 | 0 | 15% |
Grade 1-4 non-hematological adverse events noted in ≥10% of patients attributable to AZD2171 are listed in Table 4; the most common adverse events were fatigue, hypertension, and diarrhea. Rare (<10%) grade 3 and grade 4 events included apnea, ataxia, cognitive disturbance, intestinal pain, colitis, dizziness, encephalopathy, esophageal necrosis, ileal perforation, hand-foot syndrome, hypokalemia, hyponatremia, hypotension, chest wall pain, memory impairment, elevated serum ammonia, generalized muscle weakness, pain NOS, tumor pain, renal failure, speech impairment, and thrombosis/embolism. Biochemical abnormalities were infrequent and of grades 1-2 only (Table 5). There were no treatment-related deaths.
Table 4. Grade 1-4 Non-Hematological Toxicity ( ≥10% Frequency).
| N=47 | Grade | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | % Pts | |
| Constitutional | |||||
| Anorexia | 7 | 5 | 3 | 0 | 32% |
| Weight Loss | 7 | 5 | 2 | 0 | 30% |
| Fatigue | 13 | 10 | 7 | 0 | 64% |
| Dehydration | 0 | 3 | 3 | 0 | 13% |
| Gastrointestinal | |||||
| Nausea | 10 | 4 | 2 | 0 | 34% |
| Vomiting | 5 | 3 | 1 | 0 | 19% |
| Diarrhea | 14 | 12 | 4 | 0 | 64% |
| Constipation | 4 | 1 | 1 | 0 | 13% |
| Heartburn | 3 | 2 | 0 | 0 | 11% |
| ENT | |||||
| Dry mouth | 4 | 1 | 0 | 0 | 11% |
| Voice Changes | 14 | 3 | 0 | 0 | 36% |
| Cardiac | |||||
| Hypertension | 5 | 14 | 14 | 1 | 70% |
| Respiratory | |||||
| Cough | 5 | 1 | 0 | 0 | 13% |
| Renal | |||||
| Proteinuria | 8 | 4 | 2 | 0 | 30% |
| Musculoskeletal | |||||
| Muscle pain | 3 | 1 | 0 | 0 | 9% |
| Neurologic | |||||
| Headache | 4 | 1 | 1 | 0 | 13% |
| Sensory neuropathy | 3 | 0 | 1 | 0 | 9% |
| Endocrine | |||||
| Hypothyroidism | 2 | 4 | 0 | 0 | 13% |
Table 5. Biochemical Toxicity.
| N=47 | Grade | ||
|---|---|---|---|
| 1 | 2 | % Pts | |
| Creatinine | 3 | 2 | 11% |
| Alkaline phosphatase | 4 | 1 | 11% |
| ALT | 7 | 1 | 17% |
| AST | 6 | 2 | 17% |
| Hyperglycemia | 5 | 1 | 13% |
| Hypoalbuminemia | 4 | 1 | 11% |
Dose de-escalation was made in 43 of 47 patients. Six patients were removed from the study due to adverse events attributed to treatment with AZD217. Two patients withdrew from the study for reasons not related to adverse events. Thirty-six patients were withdrawn for disease progression, two patients were withdrawn due to early death, and one patient was withdrawn for unspecified reasons.
Modified RECIST correlation with RECIST
For 11 patients, modified RECIST data were reported. There was 100% correlation in response assessment for modified RECIST with RECIST measurements.
DISCUSSION
Interest in studying the anti-angiogenic agent AZD2171 in MPM was based on a significant body of evidence suggesting that angiogenesis is an important growth signaling pathway for MPM. In the second line setting, AZD2171 showed modest clinical activity as a single agent, with 9% of patients having had objective tumor responses, including 2 with marked shrinkage of bulky tumors. Because anti-angiogenic agents may induce a cytostatic rather than cytoreductive tumor response, stable disease is considered an important outcome measure in clinical trials of anti-angiogenics. In this uncontrolled trial, 34% of patients had stable disease, yielding a clinical benefit rate (CR, PR and SD) of 43%, which compares favorably to the clinical benefit rate of 46.6% for second line pemetrexed in patients enrolled in an expanded access program, but less favorably to that reported for second line pemetrexed (clinical benefit rate of 59.3%) reported in a phase III study of second line pemetrexed vs. BSC.28,29 There are, however, distinct limitations in using clinical benefit rate derived from single arm phase 2 studies in MPM due to confounding by the quite variable natural history of untreated MPM. Thus, there is great interest in the development and validation of functional imaging studies and surrogate biomarkers of antitumor activity to augment the assessment of anti-angiogenics and other potentially cytostatic agents in MPM.
For the entire study population the median PFS was short (2.56 months), similar to time to progression reported for some targeted agents that have been deemed inactive in MPM.30-32 Looking at response rate and survival outcome measures together, this study’s efficacy data are in concert with those for other agents that target multiple receptor tyrosine kinases including the VEGF receptors. In single agent studies, front-line Vatalanib for pleural and peritoneal mesothelioma yielded a response rate of 11% and a 3-month PFS of 55%, front-line/second-line Sorafanib therapy for pleural and peritoneal mesothelioma yielded a response rate of 4% and a median failure-free survival of 3.7 months, and second-line Sunitinib in MPM yielded a response rate of 23% with a median time to progression of 3.5 months.33-35In contrast, bevacizumab, a monoclonal antibody that blocks VEGF ligand binding to the VEGF receptors, did not have meaningful clinical activity when combined with the EGFR inhibitor erlotinib in previously treated MPM, nor did bevacizumab enhance the activity of cytotoxic chemotherapy for front-line treatment of pleural/peritoneal mesothelioma, for reasons that are not clear. 36,37
PDGF-mediated signaling by AZD2171 may have played a role in the anti-tumor activity noted in the current study. PDGF receptor β is highly expressed in mesothelioma cell lines but not in normal human mesothelioma cells, and PDGF appears to be a paracrine growth factor for mesothelioma.38,39 In vitro, imatinib mesylate, a potent inhibitor of PDGF α and β receptors and c-Kit, synergized with gemcitabine and pemetrexed in PDGFRβ-positive mesothelioma cells.40 In a xenograft model of MPM, imatinib mesylate enhanced the activity of gemcitabine.41 In the clinical setting, however, no significant efficacy was reported for single agent imatinib mesylate in unselected MPM patients.42 We did not study the expression of PDGF receptor β in patient tumors and therefore, we were unable to determine a role for PDGF signaling inhibition by AZD2171 in this study.
In this study cohort of patients with a good performance status after one platinum-based prior systemic therapy, AZD2171 was not well tolerated at the starting dose of 45 mg daily, and the majority of patients required a dose reduction. Several phase I studies of AZD2171 in solid tumor patients determined the MTD to be lower than 45 mg.20,21 The safety profile of AZD2171 as a second line agent in MPM was similar to that already reported for this and other VEGF receptor tyrosine kinase inhibitors.
MPM is a difficult tumor to measure accurately, as are other pleural-based tumor types, given the non-spherical pattern of growth. Limitations of RECIST in assessing response and outcome measures such as progression-free survival have been documented.43 Other measurement systems for pleural tumors have been devised, including modified RECIST, in which pleural thickness is measured perpendicular to the chest wall and mediastinum.27 To further investigate modified RECIST as a measurement system, we captured modified RECIST measurements via an amendment once the study had already been initiated and 27 patients had already been enrolled. We were only able to capture modified RECIST measurements for a small subset of patients, where tumor response assessment by modified RECIST correlated well with response by RECIST. Presumably, unfamiliarity with modified RECIST as well as difficulties in obtaining retrospective tumor measurements impacted on the collection of modified RECIST measurements. Modified RECIST and other means of assessing pleural tumor response such as PET imaging and volumetric measurement systems should be routinely incorporated into large clinical MPM trials in order to validate these measurement systems.
Anti-angiogenic agents can augment the activity of cytotoxic chemotherapy; one proposed mechanism is through the modulation of tumor interstitial fluid pressure (TIFP) as in vivo, elevated TIFP is a barrier to drug delivery to the tumor.44 Both VEGF and PDGF contribute to increased TIFP, while inhibitors of VEGF and/or PDGF signaling can reduce TIFP and augment chemotherapy effects in vivo.45-50 Given that MPM is relatively resistant to standard cytotoxic chemotherapy, there is great interest in pursuing strategies that would improve the chemosensitivity of MPM. The addition of AZD2171 to cytotoxic chemotherapy in solid tumors is feasible as shown in a number of phase I studies51-53, and an augmentation of the effect of chemotherapy by AZD2171 was reported in a randomized phase II study in NSCLC, where the addition of AZD2171 45 mg daily to Taxol plus Carboplatin resulted in improved tumor responses and PFS for AZD2171 compared with placebo with chemotherapy. Toxicities in the experimental arm led to a significant number of dose reductions, thus highlighting the challenges of adding targeted anti-angiogenics to standard chemotherapy.54
In summary, there appears to be a small subset of MPM patients whose tumors are highly driven by angiogenic signaling, thus conferring moderate to exquisite sensitivity to single agent tyrosine kinase inhibition of the VEGF receptors. It remains of paramount importance to develop strategies that would enable the selection of MPM patients for treatment with targeted anti-angiogenic therapies. While a large number of potentially predictive biomarkers for response to anti-angiogenic therapy have been studied, none have been validated for routine clinical use. The small number of responding patients in this study set did not allow us to investigate, with appropriate statistical power, potentially predictive tumor and surrogate serum-based markers of response to AZD2171. Given the signal of activity of single agent AZD2171 in second line MPM reported here, and the potential of anti-angiogenics to augment the activity of cytotoxic chemotherapy, the Southwest Oncology Group has initiated a Phase I/randomized Phase II clinical trial of pemetrexed plus cisplatin with or without AZD2171 for first-line treatment of MPM. Both tumor-based and surrogate biomarkers of angiogenesis will be collected in the context of this study, in order to attempt to elucidate biomarkers that may allow for the personalization of anti-angiogenic therapy in MPM.
ACKNOWLEDGEMENTS
This investigation supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA14028, CA46441, CA105409, CA13612, CA45808, CA67575, CA20319, CA86780, CA35090, CA67663, CA46282, CA42777, CA76448, CA04919, CA35176, CA63848, CA27057, CA16385. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute. We thank Connie Ballon-Almanza, Katy Robert and Gretchen Goetz for their assistance as protocol coordinators of this study
REFERENCES
- 1.Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J.Occup.Med. 1992;34:718–721. [PubMed] [Google Scholar]
- 2.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev.Toxicol. 2009;39:576–588. doi: 10.1080/10408440903044928. [DOI] [PubMed] [Google Scholar]
- 3.Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br.J.Cancer. 1999;79:666–672. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao SC, Reid G, Lee K, Vardy J, Clarke S, van Zandwijk N. Malignant Mesothelioma. Intern.Med.J. 2010 doi: 10.1111/j.1445-5994.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br.J Cancer. 2005;92:587–593. doi: 10.1038/sj.bjc.6602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martini N, McCormack PM, Bains MS, Kaiser LR, Burt ME, Hilaris BS. Pleural mesothelioma. Ann.Thorac.Surg. 1987;43:113–120. doi: 10.1016/s0003-4975(10)60182-8. [DOI] [PubMed] [Google Scholar]
- 7.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 8.Ohta Y, Shridhar V, Bright RK, Kalemkerian GP, Du W, Carbone M, Watanabe Y, Pass HI. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br.J Cancer. 1999;81:54–61. doi: 10.1038/sj.bjc.6690650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strizzi L, Catalano A, Vianale G, Orecchia S, Casalini A, Tassi G, Puntoni R, Mutti L, Procopio A. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 10.Masood R, Kundra A, Zhu S, Xia G, Scalia P, Smith DL, Gill PS. Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops. Int.J Cancer. 2003;104:603–610. doi: 10.1002/ijc.10996. [DOI] [PubMed] [Google Scholar]
- 11.Demirag F, Unsal E, Yilmaz A, Caglar A. Prognostic significance of vascular endothelial growth factor, tumor necrosis, and mitotic activity index in malignant pleural mesothelioma. Chest. 2005;128:3382–3387. doi: 10.1378/chest.128.5.3382. [DOI] [PubMed] [Google Scholar]
- 12.Kumar-Singh S, Weyler J, Martin MJ, Vermeulen PB, van Marck E. Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and -2, and TGF beta expression. J Pathol. 1999;189:72–78. doi: 10.1002/(SICI)1096-9896(199909)189:1<72::AID-PATH401>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Konig J, Tolnay E, Wiethege T, Muller K. Co-expression of vascular endothelial growth factor and its receptor flt-1 in malignant pleural mesothelioma. Respiration. 2000;67:36–40. doi: 10.1159/000029460. [DOI] [PubMed] [Google Scholar]
- 14.Linder C, Linder S, Munck-Wikland E, Strander H. Independent expression of serum vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in patients with carcinoma and sarcoma. Anticancer Res. 1998;18:2063–2068. [PubMed] [Google Scholar]
- 15.Demirag F, Unsal E, Yilmaz A, Caglar A. Prognostic significance of vascular endothelial growth factor, tumor necrosis, and mitotic activity index in malignant pleural mesothelioma. Chest. 2005;128:3382–3387. doi: 10.1378/chest.128.5.3382. [DOI] [PubMed] [Google Scholar]
- 16.Edwards JG, Cox G, Andi A, Jones JL, Walker RA, Waller DA, O’Byrne KJ. Angiogenesis is an independent prognostic factor in malignant mesothelioma. Br.J Cancer. 2001;85:863–868. doi: 10.1054/bjoc.2001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Yla-Herttuala S, Wedge SR, Jurgensmeier JM, Alitalo K. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Research. 2008;68:4754–4762. doi: 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- 18.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jurgensmeier JM, Ogilvie DJ. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 19.Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CJ, Stadler WM, Roth B, Hutcheon D, Conry S, Puchalski T, Morris C, Small EJ. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25:445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto N, Tamura T, Yamamoto N, Yamada K, Yamada Y, Nokihara H, Fujiwara Y, Takahashi T, Murakami H, Boku N, Yamazaki K, Puchalski TA, Shin E. Phase I, dose escalation and pharmacokinetic study of cediranib (RECENTIN), a highly potent and selective VEGFR signaling inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother.Pharmacol. 2009;64:1165–1172. doi: 10.1007/s00280-009-0979-8. [DOI] [PubMed] [Google Scholar]
- 22.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, Berkenblit A, Campos S, Horowitz N, Cannistra SA, Lee H, Lee J, Roche M, Hill M, Whalen C, Sullivan L, Tran C, Humphreys BD, Penson RT. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27:5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jurgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 24.Ryan CJ, Stadler WM, Roth B, Hutcheon D, Conry S, Puchalski T, Morris C, Small EJ. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25:445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 25.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann.Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl.Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann.Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 28.Janne PA, Wozniak AJ, Belani CP, Keohan ML, Ross HJ, Polikoff JA, Mintzer DM, Ye Z, Monberg MJ, Obasaju CK. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac.Oncol. 2006;1:506–512. [PubMed] [Google Scholar]
- 29.Jassem J, Ramlau R, Santoro A, Schuette W, Chemaissani A, Hong S, Blatter J, Adachi S, Hanauske A, Manegold C. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol. 2008;26:1698–1704. doi: 10.1200/JCO.2006.09.9887. [DOI] [PubMed] [Google Scholar]
- 30.Garland LL, Rankin C, Gandara DR, Rivkin SE, Scott KM, Nagle RB, Klein-Szanto AJ, Testa JR, Altomare DA, Borden EC. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol. 2007;25:2406–2413. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 31.Jackman DM, Kindler HL, Yeap BY, Fidias P, Salgia R, Lucca J, Morse LK, Ostler PA, Johnson BE, Janne PA. Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer. 2008;113:808–814. doi: 10.1002/cncr.23617. [DOI] [PubMed] [Google Scholar]
- 32.Mathy A, Baas P, Dalesio O, van Zandwijk N. Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer. 2005;50:83–86. doi: 10.1016/j.lungcan.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Jahan TM, Gu L, Wang X, Kratzke RA, Dudek AZ, Green MR, Vokes EE, Kindler HL. Vatalanib (V) for patients with previously untreated advanced malignant mesothelioma (MM): A phase II study by the Cancer and Leukemia Group B (CALGB 30107) J Clin Oncol. 2010;24(18S) Ref Type: Generic. [Google Scholar]
- 34.Janne PA, Wang XF, Krug LM, Hodgson L, Vokes EE, Kindler EE. Sorafenib in malignant mesothelioma (MM): A phase II trial of the Cancer and Leukemia Group B (CALGB 30307) J Clin Oncol. 2010;25(18S) Ref Type: Generic. [Google Scholar]
- 35.Nowak AK, Millward MJ, Francis R, van der Schaaf A, Musk AW, Byrne MJ. Phase II study of sunitinib as second-line therapy in malignant pleural mesothelioma (MPM) J Clin Oncol. 2008;26(15S):5–20. doi: 10.1097/JTO.0b013e31825f22ee. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 36.Jackman DM, Kindler HL, Yeap BY, Fidias P, Salgia R, Lucca J, Morse LK, Ostler PA, Johnson BE, Janne PA. Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer. 2008;113:808–814. doi: 10.1002/cncr.23617. [DOI] [PubMed] [Google Scholar]
- 37.Karrison T, Kindler HL, Gandara DR, et al. Final analysis of a multi-center, double-blind, placebo-controlled, randomized phase II trial of gemcitabin/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioms (MM) J Clin Oncol. 2007;25(18S):7526. abstr. Ref Type: Generic. [Google Scholar]
- 38.Langerak AW, Van der Linden-Van Beurden CA, Versnel MA. Regulation of differential expression of platelet-derived growth factor alpha- and beta-receptor mRNA in normal and malignant human mesothelial cell lines. Biochim.Biophys.Acta. 1996;1305:63–70. doi: 10.1016/0167-4781(95)00196-4. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. J Biochem.Mol.Biol. 2003;36:49–59. doi: 10.5483/bmbrep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 40.Bertino P, Porta C, Barbone D, Germano S, Busacca S, Pinato S, Tassi G, Favoni R, Gaudino G, Mutti L. Preliminary data suggestive of a novel translational approach to mesothelioma treatment: imatinib mesylate with gemcitabine or pemetrexed. Thorax. 2007;62:690–695. doi: 10.1136/thx.2006.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertino P, Piccardi F, Porta C, Favoni R, Cilli M, Mutti L, Gaudino G. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin Cancer Res. 2008;14:541–548. doi: 10.1158/1078-0432.CCR-07-1388. [DOI] [PubMed] [Google Scholar]
- 42.Mathy A, Baas P, Dalesio O, van Zandwijk N. Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer. 2005;50:83–86. doi: 10.1016/j.lungcan.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 43.van Klaveren RJ, Aerts JG, de Bruin H, Giaccone G, Manegold C, van Meerbeeck JP. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004;43:63–69. doi: 10.1016/s0169-5002(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann M, McCormack E, Mujic M, Rossberg M, Bernd A, Bereiter-Hahn J, Gjertsen BT, Wiig H, Kippenberger S. Increased plasma colloid osmotic pressure facilitates the uptake of therapeutic macromolecules in a xenograft tumor model. Neoplasia. 2009;11:812–822. doi: 10.1593/neo.09662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klosowska-Wardega A, Hasumi Y, Burmakin M, Ahgren A, Stuhr L, Moen I, Reed RK, Rubin K, Hellberg C, Heldin CH. Combined anti-angiogenic therapy targeting PDGF and VEGF receptors lowers the interstitial fluid pressure in a murine experimental carcinoma. PLoS.One. 2009;4:e8149. doi: 10.1371/journal.pone.0008149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat.Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF, Davidoff AM. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 48.Pietras K, Stumm M, Hubert M, Buchdunger E, Rubin K, Heldin CH, McSheehy P, Wartmann M, Ostman A. STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin Cancer Res. 2003;9:3779–3787. [PubMed] [Google Scholar]
- 49.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- 50.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, Rubin K. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–2934. [PubMed] [Google Scholar]
- 51.Goss G, Shepherd FA, Laurie S, Gauthier I, Leighl N, Chen E, Feld R, Powers J, Seymour L. A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the National Cancer Institute of Canada Clinical Trials Group. Eur.J Cancer. 2009;45:782–788. doi: 10.1016/j.ejca.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Goss G, Shepherd FA, Laurie S, Gauthier I, Leighl N, Chen E, Feld R, Powers J, Seymour L. A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the National Cancer Institute of Canada Clinical Trials Group. Eur.J Cancer. 2009;45:782–788. doi: 10.1016/j.ejca.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D, Robertson J, Puchalski TA, Seymour L. Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2008;26:1871–1878. doi: 10.1200/JCO.2007.14.4741. [DOI] [PubMed] [Google Scholar]
- 54.Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28:49–55. doi: 10.1200/JCO.2009.22.9427. [DOI] [PubMed] [Google Scholar]