Abstract
Background/Aims
Proton pump inhibitors (PPI) are widely used to prevent NSAID-induced peptic ulcers. NSAIDs produce small intestinal injury and some PPIs have been reported to have a protect against NSAID-induced small bowel injury in rats. To compare PPIs, revaprazan, and phosphatidylcholine-associated indomethacin (Indo-PC) for protection against indomethacin (Indo) induced small bowel injury. Methods: Rat intestinal epithelial cells (IEC-6) were pretreated with omeprazole, lansoprazole or revaprazan prior to exposure to Indo or Indo-PC. Cell viability was assessed by methyl thiazolyl tetrazolium assay. Omeprazole, lansoprazole or revaprazan were administered orally to rats prior to vehicle or Indo. Indo-PC was administered alone. After 24h small intestinal erosions were counted; intestinal bleeding was assessed as hemoglobin concentration of small intestinal fluid. Results: Omeprazole, lansoprazole and revaprazan did not protect against Indo-induced IEC-6 cell injury. Indo-PC was less damaging in vitro than Indo alone. In vivo neither omeprazole nor lansoprazole protected against Indo-induced small bowel injury, however, revaprazan pretreatment and Indo-PC resulted in significantly fewer erosions (>50% reduction) or bleeding (>80% reduction).
Conclusion
PPIs showed no small bowel protective effect in vitro or in vivo. Revaprazan showed a small bowel protective effect in vivo whereas Indo-PC was protective both in vitro and in vivo.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are extensively used for their analgesic, antipyretic and anti-inflammatory properties. However, NSAIDs can induce gastrointestinal (GI) injury which is an important issue in clinical practice. Proton pump inhibitors (PPI) are widely used to reduce NSAID-induced peptic ulcer and are currently recommended for the prevention of NSAID-induced peptic ulcer in high risk groups[1]. NSAID induced small intestinal injury has been reported in 60–70% of users [2–5]. Although NSAID-induced small intestinal injury is typically asymptomatic, it can result in clinical outcomes such as overt bleeding, stricture or small bowel perforation. The pathophysiologic mechanism for small intestinal injury induced by NSAIDs has not been fully elucidated, nor have reliably protective drugs been identified.
Recently, studies in rats have shown that some PPIs have a protective role against NSAID-induced small intestinal injury acting through anti-inflammatory and antioxidant mechanisms, and not by the suppression of acid secretion[6–8]. However, in other studies, PPIs have been reported to exacerbate NSAID-induced lower gut injury [9]. This paper sought to test and compare the effects of an acid pump antagonist (APA), revaprazan [10] and administration of the NSAID indomethacin linked to phosphatidylcholine (PC) (i.e., the PC-associated NSAIDs indomethacin, Indo-PC][11]. Protection against indomethacin (Indo)-induced small intestinal injury was evaluated using both in vitro and in vivo systems.
Methods
Materials
Omeprazole was purchased from LKT laboratories Inc. (St. Paul, MN), and lansoprazole and Indo from Sigma Aldrich (St. Louis, MO). Revaprazan was a gift from Yuhan Corp. (Seoul, South Korea). Indo-PC was prepared according to published protocols [11] and was a 1:2 preparation (by weight) of Indo and Phospholipon 90G (Lipoid Corp., Germany).
In vitro system
To assess whether there was direct protective effect of drugs in vitro, we conducted the following experiments: rat Intestinal epithelial cells (IEC-6) were maintained in Dulbecco’s modified eagle media (DMEM) containing 10% fetal bovine serum, 4 μg/mL insulin and penicillin/streptomycin under humidified atmosphere containing 5% CO2 at 37C. Cells were studied in 24-well tissue culture plates starting with 105 cells per well. Omeprazole, lansoprazole, and revaprazan were solubilized in dimethyl sulfoxide and diluted at least 100-fold in DMEM media for cell studies. We determined beforehand that DMSO had no activity to either injure or protect cells at the concentrations and times used. Indo was solubilized by forming the sodium salt, and Indo-PC was resuspended by vortex and sonication in phosphate buffered saline prior to dilution in DMEM. Initially, we determined the maximum concentration of omeprazole, lansoprazole and revaprazan which did not affect cell viability over 24 hours exposure. Next the concentration of Indo showing 50% cell viability compared to saline control following exposure for 24h was determined. Using these optimized concentrations, omeprazole, lansoprazole and revaprazan were incubated on IEC-6 cells for 1 hour prior to Indo addition and incubation was continued for 24 hours. An equivalent concentration of Indo-PC was used alone for 24 hours.
Cell viability was assessed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) uptake into live cells. The MTT assay was performed on the plated cells by incubation with 0.05% MTT in media for 2 hours using a previously reported protocol [12].
Indo-induced apoptosis was measured using Apo-ONE Caspase-3/7 Assay kits (Promega Corp., Madison WI), according to manufacturers instructions. Following cell incubations as described above, the assay reagent was added to cultures and fluorescence was measured after 1.5 hours.
In vivo system
Approval for all protocols was obtained from the institutional Animal Welfare Committee which follows the guidelines and is accredited by the US Public Health Service. Male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN, USA) were used in all studies. Animals were maintained on a 12 hours light/dark cycle with food and water provided ad libitum, except as noted. Sprague-Dawley rats (male, N = 72, average weight = 298 gram) were used to investigate the protective role of each drug against Indo in vivo. Omeprazole, lansoprazole and revaprazan were suspended in 0.5% carboxymethylcellulose for oral administration (1 mL/rat). Omeprazole (30 and 100 mg/kg), lansoprazole (30 and 100 mg/kg) and revaprazan (30 mg/kg) were administered orally 30 minutes prior to Indo. Indo was dissolved in 1.25% sodium bicarbonate to 2.5 mg/mL, and the pH adjusted to 7.4 before oral administration (10 mg/kg or 1 mL/250 g body wt). To confirm a therapeutic effect of each PPI, in separate experiments we measured the gastric fluid pH 1 hour after PPIs were administered to rats. At PPI doses of 100 mg/kg the gastric fluid pH was maintained at a pH>6.8, compared to control values of pH<4. Indo-PC (10 mg/kg) was administered orally to rats after dispersion by vortex and sonication in saline.
Rats were sacrificed 24 hours after Indo administration to determine small intestinal injury. The entire small intestine was resected along the antimesenteric border and was visually examined along its length. A segment of 15 cm that included the most severe lesions was selected form the distal half of the small intestine and erosions were counted independently by two persons (e.g., number of erosions/15 cm). We calculated numbers of erosion using the sum of longitudinal length.
Intestinal bleeding was assessed by the measuring hemoglobin (Hb) concentration of small intestinal fluid collected by flushing 8 mL of normal saline followed by 2 mL of air through the lumen of whole small intestine. Intestinal fluid Hb level was measured by a modification of the benzidine method as previously reported [13,14].
Experiments were analysed initially by one-way ANOVA, followed by post hoc testing with the Fisher LSD test. The level of significance was set at P < 0.05.
Results
In vitro system
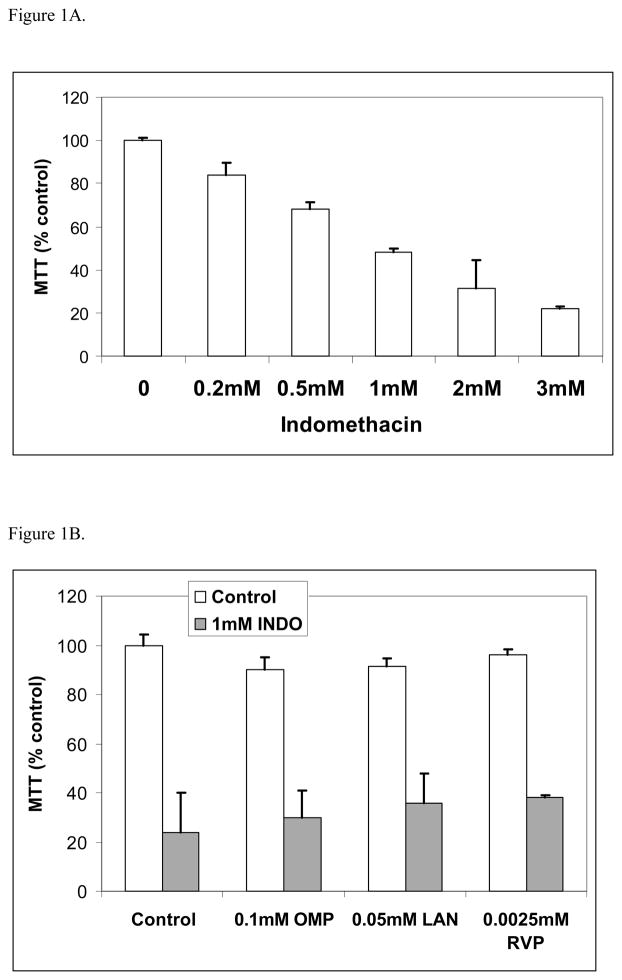
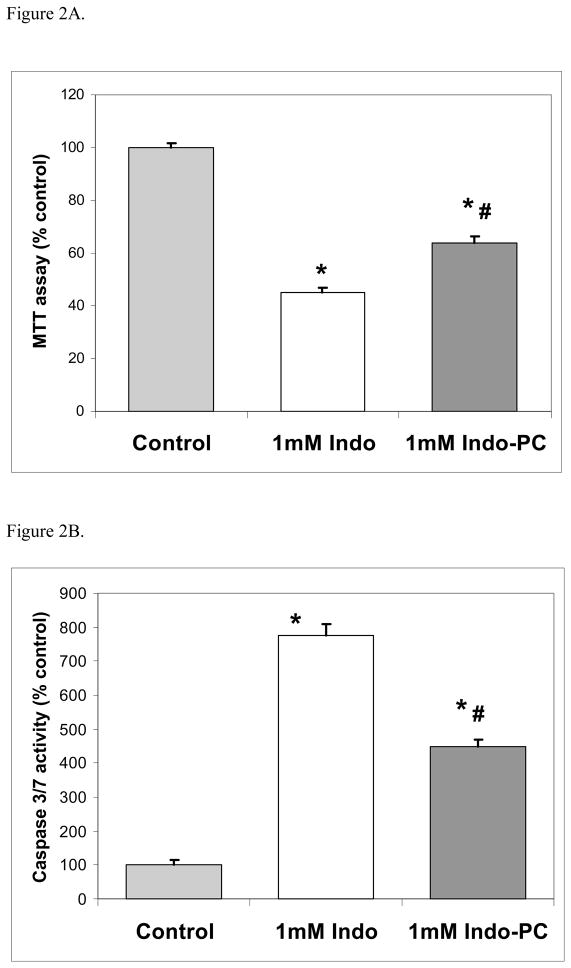
The cell viability was dose-dependently reduced by increasing concentrations of Indo (Figure 1A). The concentration of Indo that reduced cell viability 50% compared to the untreated control was 1 mM and it was used in further studies. The concentrations of drug that did not affect viability were 0.1 mM concentration of omeprazole, 0.05 mM of lansoprazole or 0.0025mM of revaprazan (data not shown). MTT assays showed that omeprazole, lansoprazole and revaprazan failed to protect against 1 mM Indo-induced intestinal cell injury (Figure 1B). In contrast, Indo-PC at 1 mM was significantly less damaging (approximately 35%) than Indo alone (Figure 2A). Using caspase-3/7 activity as a marker of apoptosis also showed that Indo-induced apoptosis was significantly less with Indo-PC compared to Indo alone (Figure 2B).
Figure 1.
In vitro studies - Indo. (A) MTT assay showed that indomethacin decreased cell viability in a dose-dependent manner. (B) MTT assay showed that omeprazole, lansoprazole and revaprazan did not reverse the Indomethacin-induced reduction in intestinal cell viability.
Figure 2.
In vitro studies – Indo-PC. (A) MTT assay showed Indo-PC at 1mM was less injurious than Indo alone. (B) Caspase-3/7 assay showed that Indo-PC induced less apoptosis than Indo alone. *=p<0.001 versus Control; #=p<0.001 versus Indo.
In vivo system
Gross small intestinal lesion scores
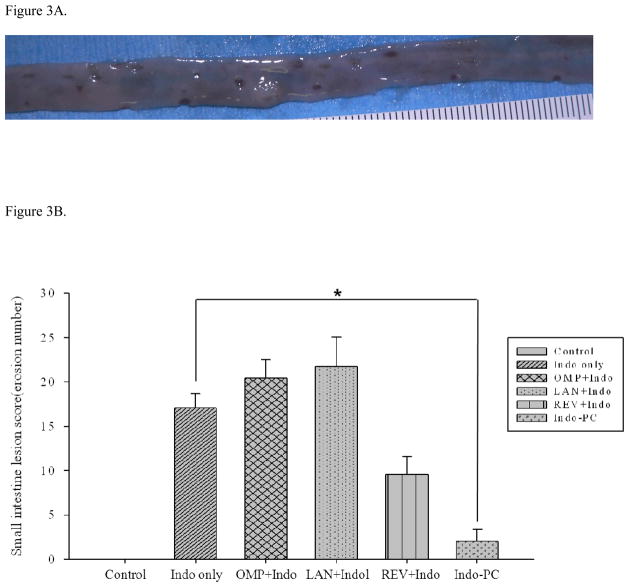
When administered to rats, Indo at 10 mg/kg caused small intestinal injury consisting of punctuate hemorrhagic spots or erosions on the small intestinal mucosa (Figure 3A). There was no concomitant stomach or large intestinal injury during the same time frame in this rat model. Because pretreatment with lansoprazole or omeprazole (30 mg/kg) did not reduce Indo-induced small intestinal injury (not shown), a higher dose of 100 mg/kg of each PPI was used. Even at this higher dose, neither PPI protected against Indo-induced intestinal injury (Figure 3B). In contrast, revaprazan pretreatment (30 mg/kg) apparently reduced the small intestinal lesion scores by approximately 50%, although it did not reach statistical significance (p=0.06). Indo-PC (10 mg/kg) was also associated with a markedly fewer small intestinal lesions (reduction of more than 80%; p<0.05) compared to the Indo alone group (Figure 3B).
Figure 3.
In vivo studies – lesions. (A) A 10 mg/kg dose of indomethacin produced punctate hemorrhagic spots and small punch-out lesions on the small intestine in rats. (B) Small intestinal lesion scores. Omeprazole and lansoprazole pretreatment did not alter the indomethacin-induced mucosal lesion score, while revaprazan pretreatment tended to reduce the lesion score (p=0.06). Indo-PC showed significantly fewer gross small intestine mucosal lesions than Indomethacin alone (p<0.05)*.
Hemoglobin assay of small intestinal fluid
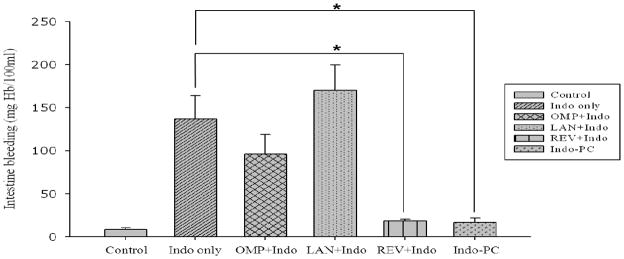
Vehicle control (carboxymethycellulose) produced very low Hb concentration similar to the saline control group (data not shown). In contrast, Indo treatment significantly increased the intestinal fluid Hb concentration, while lansoprazole and omeprazole pretreatment prior to administration of Indo induced the release of Hb in the intestinal fluid that was similar to the levels observed in response to the administration of Indo alone (Figure 4). Both the revaprazan pretreatment group and indo-PC group showed a significant reduction in intestinal fluid Hb concentration (i.e., over 80%) compared to Indo alone (p<0.05).
Figure 4.
In vivo studies – bleeding. Intestinal fluid hemoglobin assay. Revaprazan-pretreatment group and Indomethacin-phosphatidylcholine (Indo-PC)-group had statistically significant decreased hemoglobin levels of the collected intestinal fluid compared to indomethacin alone (p<0.05)*. In contrast, omeprazole and lansoprazole did not reduce intestinal bleeding induced by indomethacin.
Discussion
NSAIDs have been widely used for decades to relieve pain, inflammation and fever. However, the main factor that limits use of NSAIDs is the GI side effects of dyspepsia, bleeding and peptic ulceration. Although the adverse side effect of NSAIDs on the upper GI tract is well known, NSAID-induced small intestinal injury is less appreciated and although the small intestine may be a common site for NSAID toxicity, injury is generally asymptomatic and is difficult to detect using conventional endoscopic methods. However, newly developed diagnostic modalities such as capsule endoscopy and double balloon enteroscopy have identified NSAID enteropathy as an emerging problem. For example, several recent studies have reported that lower GI events accounted for one-third of all clinically relevant GI events and that NSAID-induced lower GI complication (perforation, bleeding, obstruction) is increasing while upper GI complication is decreasing. In fact, the ratio of upper/lower complications was 4.1 in 1996 but only 1.4 in 2005 [15] and it has been suggested that the incidence of lower GI complications is now similar to that observed in the upper GI tract [15].
Unlike upper GI toxicity, cyclooxygenase-mediated mechanisms of the small intestine may be less important. The pathophysiologic mechanism of NSAID enteropathy is not fully elucidated. Factors involved include: 1) NSAID-induced direct damage of enterocyte mitochondria during absorption by uncoupling of mitochondrial oxidative phosphorylation [16], 2) increased intestinal motility [17], 3) NO derived from induced iNOS [18–20], 4) increased small intestinal permeability [16], and 5) luminal aggressive factors including bile, hydrolytic and proteolytic enzymes, bacterial degradation products, bacterial invasion of the mucosa, and the enterohepatic circulation of NSAID [21–23].
Up to now, no medications have been shown to reliably and specifically prevent or heal NSAID-induced small intestinal injury. However NSAIDs may differ in their tendency to produce small bowel injury, For example, selective COX-2 inhibition with celecoxib after short-term (2 weeks) use had fewer mucosal breaks when compared to naproxen plus omeprazole but no improvement in intestinal permeability[24]. However, subjects with more than 3 months of selective COX-2 inhibition with a variety of coxibs developed injury similar to non-selective COX inhibition [3]. In a 6 month trial of celecoxib versus enteric-coated diclofenac plus omeprazole, there were fewer cases of clinically significant GI events with celecoxib compared to the nonselective NSAID however, they did not directly examine either mucosal injury or permeability[25]. Capsule endoscopy showed that misoprostol (prostaglandin E2) co-administration with the NSAID diclofenac was associated with significantly fewer small bowel mucosal breaks than NSAID administration [26]. Misoprostol also decreased low-dose aspirin-induced small bowel injuries [27]. Rebamipide, a so called mucosal protective agent, has also been reported to prevent diclofenac-induced small bowel injury as observed with capsule endoscopy after seven days of treatment in healthy patients [28]. There was also a tendency for protection in a two week trial but it did not achieve statistical significance [29]. In a similar trial design against a one week diclofenac challenge, the anti-ulcer drug geranylgeranylacetone also provided significant intestinal protection [30], but it has not been further evaluated for more long term efficacy.
A number of animal studies where protection against NSAID-induced intestinal injury was examined have reported that both recombinant human lactoferrin [31] and the probiotic Lactobacillus casei strain Shirota [32] exerted intestinal protection against indomethacin and other NSAIDs.
PPIs are widely available and there have been reports [6–8] that lansoprazole prevented indo-induced small intestinal injury in a rat model by upregulation of heme oxygenase-1 producing anti-inflammatory and anti-oxidative responses. Here we conducted experiments to elucidate whether PPIs in comparison to an acid pump antagonist could protect against NSAID-induced intestinal injury. We were unable to confirm a protective effect of traditional PPIs against Indo-induced intestinal injury in an acute rodent model system. PPIs also did not prevent Indo-induced cell injury in vitro. The explanation for this lack of protection is not clear, as we attempted to replicate the published study conditions. It is reported that chronic use of PPI can shift bacterial flora in the intestine and that this PPI-induced dysbiosis can aggravate NSAID enteropathy [9]. Whether this dysbiosis can occur 24 h after only one PPI dose as in our studies appears unlikely, but remains to be investigated further. We did however attempt to eliminate some possible explanations for the lack of a PPI protective effect. For example, gastric pH was measured to confirm that the PPI doses tested produced an appropriate anti-secretory effect. Also, controls for the vehicle (carboxymethycellulose) showed that this solution itself was not toxic to the small intestine. Thus, contrary to previous reports, we found that PPIs had no protective effect against NSAID-induced intestinal injuries.
Revaprazan (YH-1885), 5,6-dimethyl-2-(4-fluorophenylamino)-4-(1-methyl-1, 2, 3, 4-tetrahydroisoquinoline-2-yl) pyrimidine hydrochloride, is an acid pump antagonist that is currently approved by the Korean Food and Drug Administration as a new drug for the treatment of gastric diseases including peptic ulcer and gastritis [10,33]. Revaprazan competes with K+ on the proton pump and represents a reversible model of action against H+/K+-ATPase, which is distinct from that of PPIs known for irreversible inhibition. The other advantage of an acid pump antagonist has a fast onset of action as it does not need to be converted to an active form, and it has a high selective affinity for H+/K+ ATPase. Revaprazan has been shown to prevent NSAID-induced gastropathy through the preservation of heat shock protein 27 in a rat model [34]. However, in present study, we failed in finding any significant change of heat shock potein (Heat shock protein 24, 70) in revaprazan pretreatment group. Our results showed revaprazan did not directly protect against indo-induced intestinal cell injury in the IEC-6 cell line. However, it was effective at reducing intestinal bleeding in the in vivo rat experiment. The protective effect of revaprazan may be indirect and unrelated to acid inhibition, as the PPI drugs did not show similar protection. The underlying pathophysiologic mechanism of the protective effect of revaprazan remains to be elucidated.
Bile acids augment indomethacin-induced GI toxicity in vivo[35–37. This toxicity of Indo with bile acids has also been shown in vitro cell systems [12]. The cytotoxic action of NSAIDs in combination with natural bile and/or synthetic bile acids can be reversed in a dose-dependent fashion by the addition of phosphatidylcholine [38]. Therefore, it follows that indomethacin that is pre-associated with PC (Indo-PC) may have less GI toxicity than the NSAID alone. Previous animal studies indeed showed that Indo-PC has little GI toxicity and has similar anti-inflammatory, analgesic and bioavailability properties as compared to Indo[11]. The results of the current study showed that Indo PC was non-injurious in vivo. The mechanism for less injury by Indo-PC may be related to a proposed membrane-perturbing effect of Indo [12]which could be prevented by PC. In vitro studies showed that Indo-PC resulted in higher viability of IEC-6 cells compared to Indo when measured using the MTT assay, supporting a reduction in the direct injurious action of Indo. It has been reported by others [39]that a 16 hour incubation of GI cells with 0.5–1 mM Indo results in apoptosis. We assessed the apoptotic activity of both Indo and Indo-PC and found that the Indo-PC produced less apoptotic activity leading to reduced programmed cell death. We speculate that a less membrane injurious effect of Indo-PC may contribute to a reduced toxicity over time, and that reduced apoptosis may be either a direct effect or a reflection of this lower toxicity.
In summary, we report that PPIs failed to protect the small intestine for indomethacin-induced injury. In contrast, the acid pump antagonist revaprazan was protective. We also showed that, in contrast to the parent NSAID, PC-associated indomethacin also was less injurious to the small intestine. Both revaprazan and Indo-PC should be further evaluated for protective activity against NSAID-induced intestinal injury.
Footnotes
Disclosure
The study was supported in part by NIH grant RC1DK086304, P30DK056338 and Yuhan Corp. Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Dr. Lichtenberger and Dr. Graham are supported in part by Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. Dr. Lichtenberger is a stockholder and consultant to PLx Pharma Inc. which is developing Indomethacin-PC for therapeutic uses.
References
- 1.Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39:433–464. doi: 10.1016/j.gtc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 3.Maiden L, Thjodleifsson B, Seigal A, Bjarnason, Scott D, Birgisson S, Bjarnason S. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44:64–71. doi: 10.1007/s00535-008-2248-8. [DOI] [PubMed] [Google Scholar]
- 5.Caunedo-Alvarez A, Gomez-Rodriguez BJ, Romero-Vazquez J, Arguelles-Arias F, Romero-Castro R, Garcia-Montes JM, Pellicer-Bautista FJ, Herrerias-Gutierrez JM. Macroscopic small bowel mucosal injury caused by chronic nonsteroidal anti-inflammatory drugs (NSAID) use as assessed by capsule endoscopy. Rev Esp Enferm Dig. 2010;102:80–85. doi: 10.4321/s1130-01082010000200002. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda MN, Yoshida H, Ichikawa T, Takagi T, Okuda Y, Naito T, Okanoue T, Yoshikawa T. Lansoprazole, a proton pump inhibitor, reduces the severity of indomethacin-induced rat enteritis. Int J Mol Med. 2006;17:89–93. [PubMed] [Google Scholar]
- 7.Pozzoli C, Menozzi A, Grandi D, Solenghi E, Ossiprandi MC, Zullian C, Bertini B, Cavestro GM, Coruzzi G. Protective effects of proton pump inhibitors against indomethacin-induced lesions in the rat small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:283–291. doi: 10.1007/s00210-006-0121-y. [DOI] [PubMed] [Google Scholar]
- 8.Yoda Y, Amagase K, Kato S, Tokioka S, Murano M, Kakimoto K, Nishio H, Umegaki E, Takeuchi K, Higuchi K. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin -induced small intestinal ulceration in rats through induction of heme oxygenase-1. J Physiol Pharmacol. 2010;61:287–294. [PubMed] [Google Scholar]
- 9.Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. Proton Pump Inhibitors Exacerbate NSAID-Induced Small Intestinal Injury by Inducing Dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 10.Kim HK, Park SH, Cheung DY, Cho YS, Kim JI, Kim SS, Chae HS, Kim JK, Chung IS. Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects. J Gastroenterol Hepatol. 2010;25:1618–1625. doi: 10.1111/j.1440-1746.2010.06408.x. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenberger LM, Romero JJ, Dial EJ. Gastrointestinal safety and therapeutic efficacy of parenterally administered phosphatidylcholine-associated indomethacin in rodent model systems. Br J Pharmacol. 2009;157:252–257. doi: 10.1111/j.1476-5381.2009.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Dial EJ, Doyen R, Lichtenberger LM. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G722–731. doi: 10.1152/ajpgi.00387.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenberger LM, Wang ZM, Romero JJ, Ulloa C, Perez JC, Giraud MN, Barreto JC. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- 14.Crosby WH, Furth FW. A modification of the benzidine method for measurement of hemoglobin in plasma and urine. Blood. 1956;11:380–383. [PubMed] [Google Scholar]
- 15.Lanas A, Sopeña F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am. 2009;38:333–352. doi: 10.1016/j.gtc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30–41. doi: 10.1159/000064419. [DOI] [PubMed] [Google Scholar]
- 18.Whittle BJ, Laszlo F, Evans SM, Moncada S. Induction of nitric oxide synthase and microvascular injury in the rat jejunum provoked by indomethacin. Br J Pharmacol. 1995;116:2286–2290. doi: 10.1111/j.1476-5381.1995.tb15066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konaka A, Nishijima M, Tanaka A, Kunikata T, Kato S, Takeuchi K. Nitric oxide, superoxide radicals and mast cells in pathogenesis of indomethacin-induced small intestinal lesions in rats. J Physiol Pharmacol. 1999;50:25–38. [PubMed] [Google Scholar]
- 20.Tanaka A, Kunikata T, Mizoguchi H, Kato S, Takeuchi K. Dual action of nitric oxide in pathogenesis of indomethacin-induced small intestinal ulceration in rats. J Physiol Pharmacol. 1999;50:405–417. [PubMed] [Google Scholar]
- 21.Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977;14:333–341. doi: 10.1016/0090-6980(77)90178-2. [DOI] [PubMed] [Google Scholar]
- 22.Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109–117. doi: 10.1016/s0016-5085(97)70225-7. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Takeuchi K, Arakawa T. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–187. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 25.Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376:173–179. doi: 10.1016/S0140-6736(10)60673-3. [DOI] [PubMed] [Google Scholar]
- 26.Fujimori S, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339–1346. doi: 10.1016/j.gie.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Sugimori S, Kameda N, Machida H, Okazaki H, Tanigawa T, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–1282. doi: 10.1016/j.cgh.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Niwa Y, Nakamura M, Ohmiya N, Maeda O, Ando T, Itoh A, Hirooka Y, Goto H. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, crossover study. J Gastroenterol. 2008;43:270–276. doi: 10.1007/s00535-007-2155-4. [DOI] [PubMed] [Google Scholar]
- 29.Fujimori S, Takahashi Y, Gudis K, Seo T, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: a double-blind, randomized, controlled trial evaluated by capsule endoscopy. J Gastroenterol. 2011;46:57–64. doi: 10.1007/s00535-010-0332-3. [DOI] [PubMed] [Google Scholar]
- 30.Niwa Y, Nakamura M, Miyahara R, Ohmiya N, Watanabe O, Ando T, Kawashima H, Itoh A, Hirooka Y, Goto H. Geranylgeranylacetone protects against diclofenac-induced gastric and small intestinal mucosal injuries in healthy subjects: a prospective randomized placebo-controlled double-blind cross-over study. Digestion. 2009;80:260–266. doi: 10.1159/000236032. [DOI] [PubMed] [Google Scholar]
- 31.Dial EJ, Dohrman AJ, Romero JJ, Lichtenberger LM. Recombinant human lactoferrin prevents NSAID-induced intestinal bleeding in rodents. J Pharm Pharmacol. 2005;57:93–99. doi: 10.1211/0022357055191. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Higuchi K, Takeuchi K, Arakawa T. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol. 2009;297:G506–513. doi: 10.1152/ajpgi.90553.2008. [DOI] [PubMed] [Google Scholar]
- 33.Yu KS, Bae KS, Shon JH, Cho JY, Yi SY, Chung JY, Lim HS, Jang IJ, Shin SJ, Song KS, Moon BS. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol. 2004;44:73–82. doi: 10.1177/0091270003261321. [DOI] [PubMed] [Google Scholar]
- 34.Ock CY, Lim YJ, Kim YJ, Chung JW, Kwon KA, Kim JH, Hahm KB. Acid pump antagonist-provoked HSP27 dephosphorylation and accentuation rescues stomach from indomethacin-induced damages. J Dig Dis. 2011;12:71–81. doi: 10.1111/j.1751-2980.2011.00482.x. [DOI] [PubMed] [Google Scholar]
- 35.Duggan DE, Hooke KF, Noll RM, Kwan KC. Enterohepatic circulation of indomethacin and its role in intestinal irritation. Biochem Pharmacol. 1975;24:1749–1754. doi: 10.1016/0006-2952(75)90450-5. [DOI] [PubMed] [Google Scholar]
- 36.Yamada T, Deitch E, Specian RD, Perry MA, Sartor RB, Grisham MB. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993;17:641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]
- 37.Jacob M, Foster R, Sigthorsson G, Simpson R, Bjarnason I. Role of bile in pathogenesis of indomethacin-induced enteropathy. Arch Toxicol. 2007;81:291–298. doi: 10.1007/s00204-006-0149-2. [DOI] [PubMed] [Google Scholar]
- 38.Dial EJ, Darling RL, Lichtenberger LM. Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J Gastroenterol Hepatol. 2008;23:e384–389. doi: 10.1111/j.1440-1746.2007.05266.x. [DOI] [PubMed] [Google Scholar]
- 39.Tomisato W, Tsutsumi S, Rokutan K, Tsuchiya T, Mizushima T. NSAIDs induce both necrosis and apoptosis in guinea pig gastric mucosal cells in primary culture. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1092–1100. doi: 10.1152/ajpgi.2001.281.4.G1092. [DOI] [PubMed] [Google Scholar]