Abstract
Status epilepticus (SE) produced by excitatory amino acids is a well established model in adult rodents. Limbic neuronal degeneration and synaptic reorganization observed after, for example, kainic acid-induced SE are considered relevant to human epilepsy. Kainic acid also produces severe seizures in infant rats, but neuronal injury and sprouting have not been demonstrated. The results of the present study show that corticotropin releasing hormone (CRH)-induced SE causes limbic neuronal death and reorganization in infant rats. In adults, CRH produced seizures at much higher doses, and no neuronal degeneration. As a modulator of the CNS stress response, CRH is activated in various ‘stressful’ circumstances. Its age-dependent ability to kill neurons represents a unique form of cell death potentially important in human medicine.
Keywords: Hippocampus, Cell death, Amygdala, Status epilepticus, Infant, Corticotropin releasing hormone, Excitotoxicity
Introduction
Status epilepticus induced by systemic administration of a rigid glutamate analog, kainic acid, results in cell loss in discrete brain regions.1 The hippocampal formation, amygdala and piriform cortex are significantly affected,1,2 followed by sprouting of granule cell mossy fibers and synaptic reorganization3 in the dentate gyrus. Current thinking is that sprouting is a consequence of loss of mossy fiber targets, hilar or CA3 neurons.4,5 Surviving animals develop spontaneous seizures, and this paradigm of excitotoxicity has been extensively studied as a model for human temporal lobe epilepsy.1–5 Systemic kainic acid (KA) induces severe limbic status epilepticus (SE) in infant rats. However, KA-induced SE does not result in excitotoxic neuronal injury prior to the end of the third postnatal week.6–8 Other systemic convulsants, as well as amygdala kindling, do not result in neuronal death or mossy fiber sprouting in the infant hippocampus.7 This has been interpreted as evidence for resistance of immature neurons to seizure-induced excitotoxicity,7 possibly due to immature mossy fiber innervation of hilar neurons.9
Corticotropin releasing hormone (CRH) is a peptide convulsant inducing limbic seizures in immature rats in picomolar doses10,11; its potency in adults is 200 fold lower.12 Synthesis and secretion of endogenous hypothalamic CRH are decreased by agents effective for certain age-specific human epilepsies of infants.13 These observations led to the hypothesis that CRH-induced SE may cause neuronai loss in immature limbic structures, potentially via mechanisms independent of glutamate receptors.
Materials and Methods
Infant and adult Sprague–Dawley derived rats were housed in AALAC approved facilities.10–11 Animal procedures were in accordance with guidelines of the institutional Animal Care Committee. Surgical methodology for multiple limbic bipolar electrode implantation, and the validation of the limbic origin of CRH-induced seizures have been described.11 For CRH infusion into the cerebral ventricles (i.c.v.), a stainless steel cannula was implanted under halothane anesthesia 24 h prior to experiments.10,11
KA and CRH administration were carried out between 08:00 and 10:00 h. KA was administered i.p., using age-specific maximal tolerated doses determined previously.8 CRH, 150–750 pmol per dose, was infused i.c.v. in 0.5–2 µl. SE was monitored, and a separate group of rats was implanted with limbic electrodes for electrophysiological verification.11 Rats subjected to KA on postnatal day 12 (n = 3), 32 or in adulthood (n = 4) survived for 3 weeks. CRH-injected rats (n = 7 for day 12; n = 4 for days 32 and adult) survived 3–8 weeks. Repetitive CRH infusions were carried out on postnatal days 10–13, with a 3 week survival (n = 5). For each age group, controls included naive rats, and animals subjected to surgery (cannula implantation) and injection of saline (n = 10). For the 12-day-old group, additional controls received a peptide CRH analog devoid of convulsant activity (n = 2).
Histological techniques
Degenerating neurons were visualized by silver staining.14 Under deep pentobarbital anesthesia, rats were perfused with saline, followed by a buffered 4% paraformaldehyde solution, 24 or 48 h after the fourth daily CRH infusion (i.e. on day 14 or 15). For visualization of mossy fiber sprouting, rats were perfused with a freshly made buffered 4.9% sodium sulfide solution (pH 7.4). This was followed by a perfusate consisting of 3% paraformaldehyde and 1% glutaraldehyde solution.9 Brains were left in situ overnight at 4°C, then dissected onto fresh perfusate, blocked and processed for Neo-Timm stain as described.9
Results
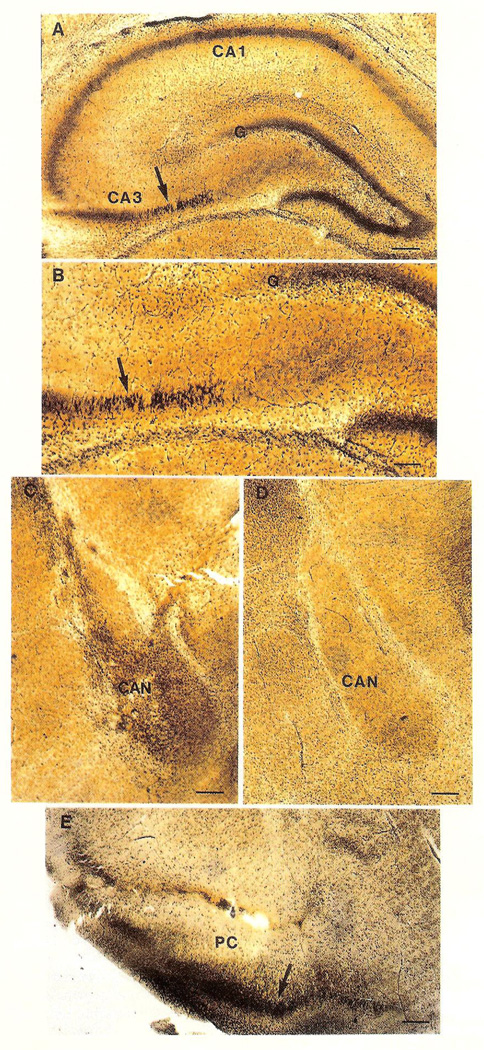
KA resulted in severe prolonged SE at all ages; CRH-SE was prolonged in infant but not in juvenile or adult rats. Silver-stained degenerating neurons were not found in hippocampi of control infant rats or in those with short CRH-induced SE. In those with prolonged seizures induced by single or multiple CRH infusions, the ipsilateral (and occasionally contralateral) CA3b region of the pyramidal cell layer contained numerous degenerating neurons (Fig. 1A, B). Silver-stained neurons were observed in ipsilateral (Fig. 1C) but not contralateral (Fig. 1D) central amygdaloid nucleus. Piriform cortex contained many labelled neurons as well (Fig. 1E).
FIG. 1.
Silver-stained coronal sections through limbic structures of rats subjected to i.c.v. CRH infusions on postnatal days 10–13. (A, B) Hippocampus. Low magnification (A), shows the pyramidal cell layers of CA1 and CA3 (arrow), as well as the granule cell layer (G) of the dentate gyrus. High magnification (B), reveals numerous degenerating neurons in CA3b (arrow). (C) Prominent silver staining in ipsilateral central amygdaloid nucleus (CAN). (D) No staining in the contralateral CAN (figure is photographically reversed for comparison purposes). (E) Dense silver deposits are visible in neurons of the superficial layers (arrow) of the piriform cortex (PC). Scale bar = 100 µm (B) or 200 µm (A, C, D, E).
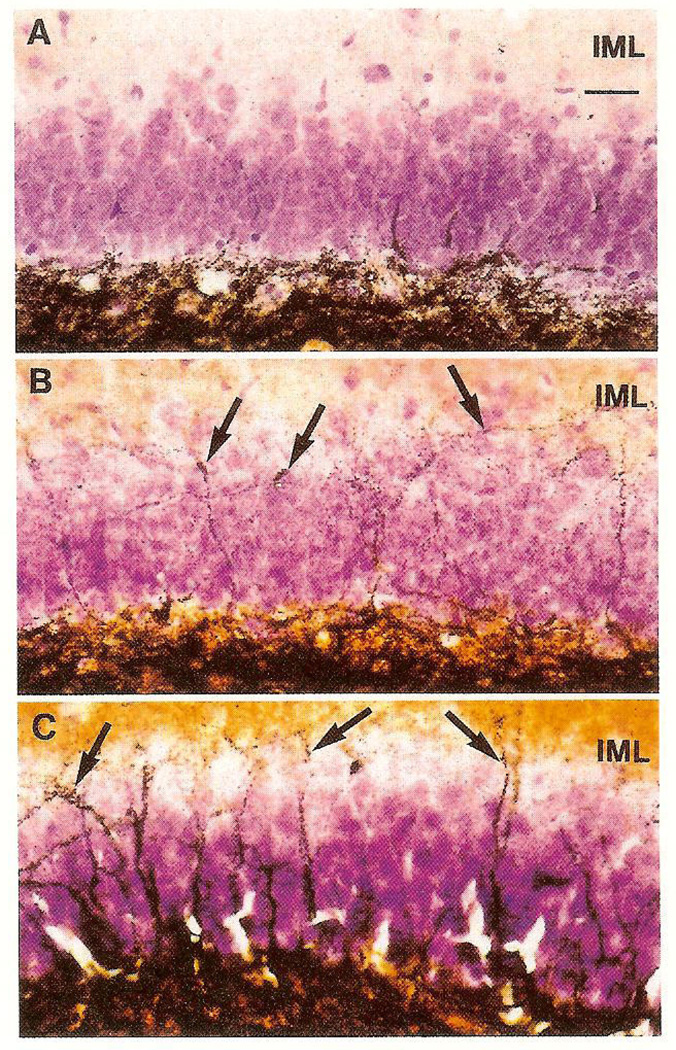
Induction, of mossy fiber sprouting was not evident in control or KA-treated infant rats (Fig. 2A, Table 1). CRH induced moderate sprouting when given once (Fig. 2B). Heavier, bilateral Timm staining of mossy fibers in the inner molecular layer of the fascia dentata resulted from CRH administration on four consecutive days (Fig. 2C). As opposed to the effects of KA, CRH did not induce mossy fiber sprouting when administered to 32-day-old or adult rats (Table 1).
FIG. 2.
Light micrographs of Timm-stained mossy fiber sprouting in dentate gyrus of infant rats. (A) Kainic acid injection on postnatal day 12. No Timm staining in the inner molecular layer (IML) is visible. (B) Single CRH infusion on postnatal day 12. Modest sprouting (arrows), is visible as Timm-stained boutons in the IML. (C) Multiple CRH injections on postnatal days 10–13. Heavy mossy fiber sprouting (arrows) is visible in the IML. Magnification is the same for A,B,C: bar (in A) = 25 µm.
Table 1.
Mossy fiber sprouting induction by corticotropin releasing hormone (CRH) and kainic acid (KA) induced status epilepticus: age dependence
| Age (days) | CRH | KA | Control |
|---|---|---|---|
| 10–13 | +++ | 0 | 0 |
| 32 | + | +++ | 0 |
| Adult | 0 | ++++ | 0 |
CRH was infused daily on days 10–13. KA was injected on day 12. Neo-Timm staining in the dentate gyms was graded as absent (0), light (+), moderate (++), heavy (+++) or very profuse (++++).
Discussion
CRH-induced neuronal death and mossy fiber sprouting in infant brain may derive from direct toxicity or from excess neuronal activation. Direct application of CRH to hippocampal organ cultures (0.15 µM), to cultured cortical neurons (0.1 µM) or to hippocampal slices (0.6 µM) has not resulted in neuronal death.15 Conversely, the distribution of CRH-SE induced cell death is congruent with the localization of CRH-induced epileptiform discharges.11 The presence of bilateral sprouting and of lesions ‘distant’ from the site of CRH administration, and the striking similarities between the distribution of neuronal loss sub-Control sequent to i.c.v. or systemic KA in adult brain also support an excitotoxic mechanism.
The neuroanatomical distribution of KA-induced neuronal loss in the adult rodent has been studied extensively.1,2,6 Broadly, vulnerable structures are interconnected via the ‘limbic’ or olfactory circuitry. Within the hippocampal formation, CA3 pyramidal cells are among the most susceptible to both KA- and CRH-induced SE. Piriform cortex neurons, affected by CRH, are universally vulnerable to KA.1,2,6 Studies of glucose consumption and immediate early gene induction16 point to the involvement of piriform cortex in the propagation of KA-induccd SE. Within the amygdaloid complex, KA causes severe neuronal degeneration particularly in the basolateral group and posterior cortical nucleus, as well as the central nucleus.1,2 With CRH, the latter was primarily affected, possibly due to the preferential presence of CRH inter-neurons and receptors in this nucleus.17–18
KA excitotoxic lesions are glutamate-receptor mediated, and involve increased intracellular calcium.19 Patterns of selective vulnerability and resistance to KA-SE-induced neuronal death in developing limbic neurons may stem from developmental regulation of glutamate-receptor mediated calcium permeability.20 Recently, CRH has been found to increase intracellular calcium in a number of different cell types.21 This receptor-mediated channel, coupled to pertussis-toxin sensitive G protein(s), seems independent of glutamate receptors.21
CRH was originally isolated from the mammalian hypothalamus as a neuromediator of neuroendocrine responses to stress.22 Age specificity of the convulsant effects of synthetic CRH has been demonstrated,10,11 possibly secondary to the developmental profile of the endogenous peptide and its receptors.13,18 Hippocampal CRH receptor number peaks at the end of the first postnatal week.18 This may predispose CA3 pyramidal cells to CRH-induced death, with consequent mossy fiber sprouting in the dentate gyrus. The implications of CRH-induced neuronal loss and sprouting to the human condition are not fully resolved. Sprouting has recently been reported in limbic tissue from young children with recurrent seizures,23 but a causal relationship remains unclear.24 CRH provides a mechanism for such injury and anatomical reorganization. The unique efficacy of ACTH and prednisone, which down-regulate CRH, in infantile spasms, the malignant, age-specific form of infant epilepsy, may provide important clues.13 In addition, acute stress of many kinds increases CRH gene expression.25
Conclusion
CRH-induced neuronal death may provide a neurobiological mechanism for the longterm sequelae of stress26 and for neurological disability which may follow severe seizures. The precise mechanisms of this form of neuronal degeneration remain unresolved. Nevertheless, these observations suggest CRH-mediated neurotoxicity as a novel, important mechanism of cell death unique to immature neurons and probably independent of glutamate receptors.
General Summary.
A major controversy in human epilepsy is whether severe seizures in infants or young children cause brain damage. Animal models to date have failed to show neuronal death related to such seizures. The natural peptide CRH is particularly powerful in causing seizures in infant compared with mature animals. CRH is present in brain and mediates the response to stress. We wondered whether giving CRH in doses causing severe seizures would induce loss of neurons and reorganization of connections of the surviving ones. Infusing CRH into the brain of infant rats caused cell loss in areas involved in the seizures but far from the site of infusion. Surviving cells reorganized in pursuit of new targets (‘sprouted’). In comparison, a conventional seizure-provoking drug caused severe seizures—but no cell death. Therefore, CRH may kill immature neurons via a unique mechanism, independent of the well-described glutamate-receptor mediated death.
ACKNOWLEDGEMENTS
We thank Drs M. Baudry and S. R. Snodgrass for review of the manuscript, and D. P, Sarco, Y. Jhurani and L. Schultz for technical assistance. Supported by NIH NS 15669 and NS 28912.
References
- 1.Ben-Ari Y, Tremblay E, Riche D, et al. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 2.Lothman EW, Collins RC. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 3.Cronin J, Dudek FE. Brain Res. 1988;474:181–184. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- 4.Nadler JW, Perry BW, Gentry C, et al. J Comp Neurol. 1981;196:549–569. doi: 10.1002/cne.901960404. [DOI] [PubMed] [Google Scholar]
- 5.Cavazos JE, Sutula TP. Brain Res. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- 6.Nitecka L, Tremblay E, Charton G, et al. Neuroscience. 1984;13:1073–1084. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- 7.Sperber EF, Stanton PK, Haas K, et al. Molecular Neurobiology of Epilepsy. Amsterdam: Elsevier; 1992. Developmental differences in the neurobiology of epileptic brain damage; pp. 67–81. [PubMed] [Google Scholar]
- 8.Chang D, Baram TZ. Dev Brain Res. 1994;77:133–136. doi: 10.1016/0165-3806(94)90220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribak CE, Navetta MS. Dev Brain Res. 1994;79:47–62. doi: 10.1016/0165-3806(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 10.Baram TZ, Schultz LS. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baram TZ, Hirsch E, Snead OC, et al. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlers CL, Henriksen SJ, Wang M, et al. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 13.Baram TZ. Ann Neurol. 1993;33:231–237. doi: 10.1002/ana.410330302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadler JW, Evenson DA. Methods Enzymol. 1983;103:393–400. doi: 10.1016/s0076-6879(83)03027-x. [DOI] [PubMed] [Google Scholar]
- 15.Baudry M, Weiss JH, Dudek FE, et al. In preparation. [Google Scholar]
- 16.Schreiber SS, Najm I, Tocco G, et al. NeuroReport. 1993;5:269–272. doi: 10.1097/00001756-199312000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Uryu K, Okumura T, Shibasaki T, et al. Brain Res. 1992;577:175–179. doi: 10.1016/0006-8993(92)90554-m. [DOI] [PubMed] [Google Scholar]
- 18.Grigoriadis DE, Heroux JA, De Souza EB. CRF, CIBA Found. Symp. Vol. 172. Chichester: Wiley; 1993. pp. 85–107. [DOI] [PubMed] [Google Scholar]
- 19.Choi DW. Science. 1992;258:241–244. doi: 10.1126/science.1357748. [DOI] [PubMed] [Google Scholar]
- 20.Monyer H, Seeberg PH, Wisden W. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- 21.Kiang JG. Eur J Pharmacol. 1994;267:135–142. doi: 10.1016/0922-4106(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 22.Vale W, Spiess J, Rivier C. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 23.Mathern GW, Leite JP, Pretorius JK. Dev Brain Res. 1994;78:70–80. doi: 10.1016/0165-3806(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 24.Moshe SL. Neurology. 1993;43(suppl. 5):3–7. [PubMed] [Google Scholar]
- 25.Lightmen SL, Harbuz MS. CRF, CIBA Found. Symp. Vol. 172. Chichester: Wiley; 1993. pp. 173–198. [DOI] [PubMed] [Google Scholar]
- 26.Plotsky PM, Meaney MJ. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]