Abstract
Since the original proposal by Fearon that the Complement System linked innate and adaptive immunity (1), there has been a rapid expansion of studies on this topic. With the advance of intravital imaging, a number of recent papers have revealed an additional novel pathway in which complement C3 and its receptors enhance humoral immunity through delivery of antigen to the B cell compartment. In this review, we will discuss this pathway and highlight several novel exceptions recently found with a model influenza vaccine such as: (a) MBL opsonization of influenza and uptake by macrophages; (b) and capture of virus by dendritic cells residing in the medullary compartment of peripheral lymph nodes.
INTRODUCTION
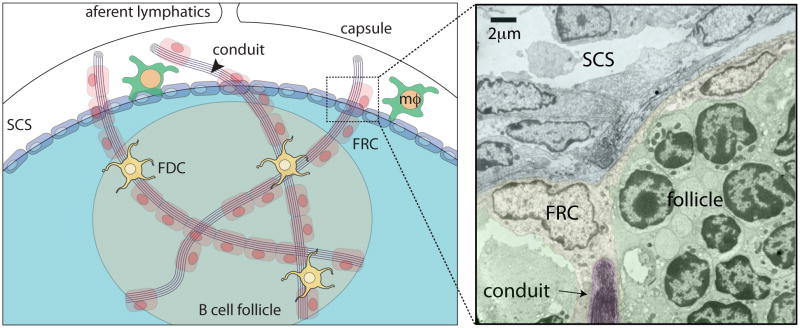
Peripheral lymph nodes (LN) along with the spleen make-up the secondary lymphoid organ tissue which provide a specialized environment for circulating B and T lymphocytes to interact and encounter cognate antigen(2). While T cells home to the paracortical region of LNs, B cells traffick to the follicles in search of antigen. This directed migration is dependent on chemokines produced by stromal cells in the respective compartments. Recent elegant intravital imaging of T and B cell trafficking within the peripheral LN reveal a directed migration along stromal “highways” (3, 4). Fibroblast reticular cells (FRC) not only secrete the collagen-rich fibers that form the network within the paracortical region but also secrete T cell chemokines CCL19 and CCL21. B cell migration within the follicles is dependent on both FDC dendritic processes as well as a less dense network of FRC fibers. Although the reticular network within LNs was characterized over 3 decades ago (5) , it is only more recent that it became apparent that they act as conduits for the delivery of cytokines, chemokines and small protein antigens to both the T (6–9)and B cell areas (10, 11). B cell conduits are structurally and immunochemically similar to those in the T cell area. They differ primarily by specificity of the chemokine secreted, i.e. follicular FRC secrete CXCL-13, whereas, paracortical FRC secrete CCL-19 & 21. Although the outer diameter of conduits is approximately 1–2 μm they are tightly packed with collagen fibers with a spacing of 5–8 nm that acts as molecular sieve (Figure 1). Thus, only proteins less than approximately 60 kDa enter into the conduits. Whether conduit structures are altered to accommodate larger antigens during infection is not clear.
Figure 1.
The conduit network, formed by collagen fibers, is secreted by the fibroblastic reticular cells (FRC) and drains small antigens from the subcapsular sinus (SCS) area of the lymph node to the B cell follicle. Follicular dendritic cells (FDC) present in the follicle are closely associated with the collagen fibers. MΦ; indicates subcapsular sinus macrophages. Arrows indicate conduit opening into the sinus in electron micrograph on right panel. EM is from S. Gonzalez, unpublished.
Trafficking of lymph-borne antigens into B cell follicles
Small protein antigens gain direct access into the B cell follicles via either gaps in the sub-capsular sinus floor (12) or through the FRC conduits (10, 11) (Figure 2a). The latter pathway provides a directed flow of small antigen to the FDC for either transient retention or in the presence of antibody and complement long term binding via specific receptors. While, cognate B cells can access antigen draining via the conduits (10), their principal role is more likely directing the antigen to FDC for stable retention. While these initial experiments involved model antigens such as lysozyme (10) or OVA (11), in the natural setting it seems likely that a major source of antigen is degraded products of pathogens that drain from tissues via the lymphatics as suggested by Jenkins and colleagues (13).
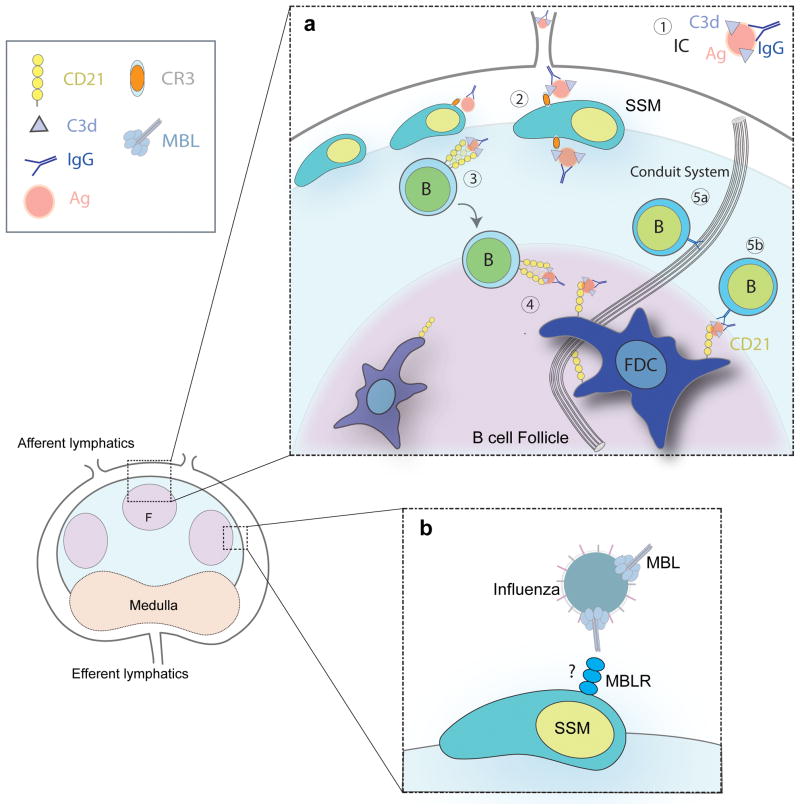
Figure 2.
(a) Pathways for the circulation of antigen (Ag) in the LN. (1) Complement C3 opsonizes antigen in presence of antibody. C3-coated Immune complexes (C3-IC) are formed by the deposition of complement proteins and IgG on the surface of the antigen. (2) The retention of C3-IC on the surface of the subcapsular sinus macrophages (SSM) is CR3 and FcRIIb dependent. (3) B cells transport C3-IC from the surface of the SSM to the FDC in a CD21 dependent manner. (4) C3-IC are transferred to FDC. (5) Cognate B cells acquire small Ag drained via FRC-conduits directly or from FDC surface. (b) Influenza virus uptake by SSM is MBL dependent.
Lymph-borne particulate antigens such as vesicular stomatitis virus (VSV) (14) and protein coated beads (15) are rapidly taken-up by macrophages that line the sub-capsular sinus (SSM) (16). Interestingly, the particulate antigens are shuttled to the underlying surface where they are made available to cognate B cells. Similarly, large protein antigens injected sub-cutaneously (s.q.) into passively immunized mice also appear to bind rapidly to SSM. However, in the later example, capture by SSM is complement dependent. Thus, formation of immune complexes (IC) activates complement resulting in formation of C3-coated immune complexes (C3-IC) that enhance uptake via CR3 (Mac-1) and FcRIIb on the SSM (17). Subsequently, C3-IC are relayed to the underlying B cell compartment where they are transferred to naïve B cells (18) (Figure 2a). FcRIIb is known to recycle to the surface following internalization and not go through a lysosomal compartment (19). So it is possible that C3-IC are partially protected by this cycling process. How C3-IC are actually transferred to B cells is not clear; however, uptake on the naïve B cells is CD21/35 -dependent and highly efficient. Strikingly, over 25% of naïve B cells within the LN follicles take-up C3-IC within 8 hrs of s.q. injection of antigen into immune mice (17) (10). In naïve mice where antibody is not preexisting, it is not clear how large protein antigens within the lymph are captured by sinus- lining macrophages. The lymph, in general, is thought to include a similar repertoire of recognition proteins that activate complement as found in blood such as, MBL, Ficolins and pentraxin. Alternatively, protein antigens might be directly taken-up by scavenger or lectin receptors expressed on the sinus-lining macrophages. MM which are more similar to marginal zone macrophages of the spleen express SIGN-R1 and mannose receptor in addition to CR3. However, SSM are more similar to the metalophilic macrophages of the marginal zone and express MOMA-1 (18).
B cell transport of C3-IC is CD21/35 dependent
In mice, complement receptors CD21 and CD35 are encoded at the Cr2 locus (20, 21). CD21 represents a splice product of the CD35 mRNA; whereas, in humans they are encoded at separate loci (22, 23). Murine CD21 and CD35 (CD21/35) are co-expressed on B cells, FDC and a subset of T cells. CD35 binds activated C3b and C4b and like CD21 binds the cleavage products iC3b, C3d,g and C3d covalently attached to antigens(20, 24–26) . They play a critical role in humoral immunity as blocking with antibodies(27, 28),a soluble receptor (29) or deletion of the receptors (30) (31)leads to impaired humoral immunity to model protein antigens, bacteria(32), and viruses (33, 34) (Figure 3). On B cells, CD21/35 form a co-receptor with CD19 and CD81 and coligation with the BCR lowers the threshold of B cell activation(35–37).
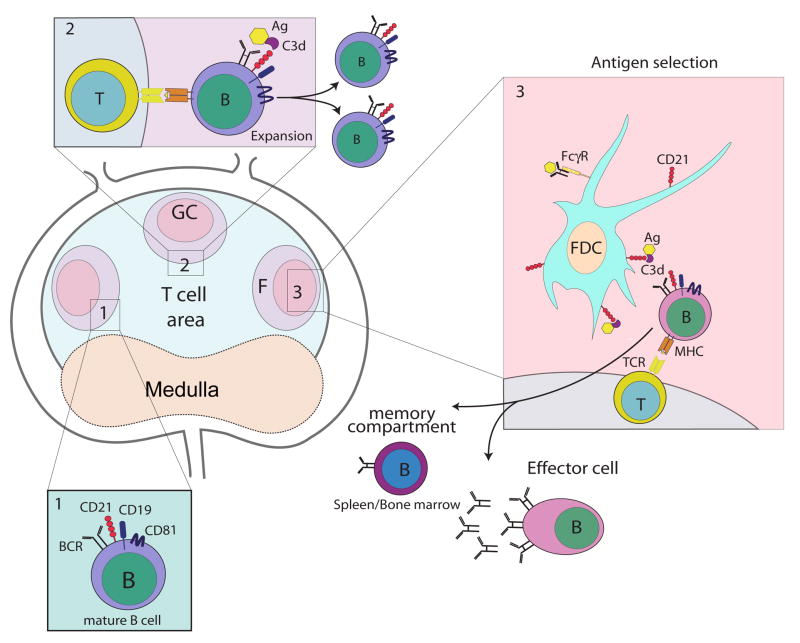
Figure 3.
Recognition of C3-tagged antigen via CD21 and CD35 enhances B cell differentiation at three major stages. Complement receptors CD21 and CD35 play an important role in at least three stages of B cell differentiation. Stage 1: co-ligation of C3d-antigen with BCR lowers threshold of B cell activation leading to migration of the activated B cell to the T cell:B cell boundary where cognate interaction occurs and B cells receive co-stimulation via CD40. Stage 2: activated B cells enter a germinal center where they begin further differentiation including rapid cell division, somatic cell hypermutation (SHM) and class switch recombination (CSR). Stage 3: following clonal selection (binding of antigen on FDC) the GC B cell differentiates into an effector cell (plasma cell) or memory B cell. Maintenance of B effector and memory cells is dependent on presence of antigen on FDC.
A second major function of CD21/35 is retention of antigen on FDC. They represent the major antigen receptors on FDC(38–40). Antigen is also retained for long periods via FcRIIb; however, its expression is not constitutive but upregulated on activated FDC(41). FDC are thought to be the major source of antigen required for clonal selection of B cells during the germinal center (GC) reaction (42)and possibly for maintenance of memory (43–45). They not only retain antigen but secrete B cell growth factors and the B cell attractant CXCL-13 as mentioned above (46). Mice deficient in C3 or CD21/35 have fewer and smaller GC and B cells fail to survive(47, 48).
There is growing evidence that B cells acquire antigen and are most efficiently activated when it is attached to membrane(49–51). Therefore, retention of antigen and C3d on the FDC surface could enhance formation of the B cell synapse. Support for a role for FDC as an important source of antigen comes from the recent studies on Cyster et al as they demonstrate direct capture of antigen from FDC surface by cognate B cells using multi-photon intravital microscopy (MP-IVM) (52). Importantly they also identify a role for CD21/35 in efficient uptake of medium affinity antigens. In their model system, MD4 B cells deficient in CD21/35 acquired less antigen from FDC. One explanation of their results is that the co-receptor acts to enhance BCR signaling in the synapse between the BCR and FDC.
A third novel role for CD21/35 is transport of lymph-borne C3-IC into the B cell follicles and transfer to FDC. As discussed above, naïve B cells acquire C3-IC from SSM lining the sub-capsular sinus via their CD21/35 receptors. Whether uptake induces a direct signal via CD21/35 or involves the co-receptor CD19 and CD81 is not clear. In a recent study using mice in which CD21/35 are uncoupled from CD19 (Cr2Δ/Δ) naïve B cells in the mutant line appeared to take-up C3-coated immune complexes normally and deliver them to FDC(43). CD21/35 receptors include a cytoplasmic domain so it is possible that uptake of C3-IC induces a signal by CD21 independent of CD19 (53) and triggers B cell migration to the FDC. An alternative possibility, is that B cells within the follicles migrate constitutively to FDC based on a chemokine gradient in a manner similar to that described for marginal zone (MZ) B cells (54); therefore direct signaling by C3-IC may not be not essential. Thus, as B cells enter the peripheral LNs via HEV, they cycle to the sub-capsular sinus area which is rich in MRC (marginal reticular cells) that secrete B cell chemokine (55)and subsequently to FDC which are also a source of chemokine(46).
Earlier studies identified a role for C3 and CD21/35 in capture of IC by MZ B cells and transport to FDC within B cell follicles (56–58). Using intravital imaging, Cyster and colleagues identified a similar pathway for capture of C3-IC in the spleen as reported for the LN (54). They found that MZ B cells continuously shuttled between the splenic marginal zone and the B cell follicles by a CXCR5-chemokine-sphingosine 1- phosphate receptor -dependent mechanism. Thus, naïve MZ B cells capture C3-coated antigens within the marginal zone sinus and transport them into the follicles where they are transferred to FDC. How C3-IC are handed-off to FDC is not clear; however the uptake on the FDC is dependent on CD21/35 and FcRIIb. Since the latter receptors (FcRIIb) are not constitutively expressed but upregulated following FDC activation, complement receptors are critical for the initial capture and retention of C3-IC (41).
Influenza virus is captured via a novel pathway
Influenza infection represents a major pathogen of humans; in general it is controlled by annual vaccination and induction of a protective humoral response (59) (60, 61). Complement C3 and CD21/35 are a important component in humoral immunity to influenza based on earlier studies (62) (63) (34). While much of the focus on the enhancing role of complement has been on the B cell co-receptor, the C3-CD21/35 pathway is also important for transport and retention of antigen on FDC as discussed above.
Recent studies have identified a novel pathway by which viral antigen is captured and possibly transported to the FDC. Gonzalez et al used an UV (ultra-violet light) inactivated form of influenza virus (strain PR8) as a model vaccine. Using a fluorescent-tagged form of UV-PR8 injected in the footpad (s.q.) and imaging by MP-IVM they identified rapid filling of the sinus and capture by macrophages in both the sub-capsular (SSM) and medullary (MM) regions. As discussed earlier, macrophages lining these regions represent distinct populations as distinguished by functional maturity and cell surface markers (18). For example, MM which express SIGN-R1, F4/80 and mannose receptor appear more mature than SSM based endocytosis and degradation of immune complexes.
The study by Gonzalez reported that labeled-UV-PR8 virus was rapidly bound by both the SSM and MM similar to that reported for VSV (14). In contrast to the earlier studies, UV-PR8 was not retained on the surface but was internalized. Binding and phagocytosis was dependent on MBL as SSM in MBL−/− mice failed to bind the virus. Opsonization by MBL is in agreement with earlier studies by Ezekowitz and colleagues who reported that guinea pig mannan-binding lectin bound and neutralized influenza (64). Interestingly, blocking of UV-PR8 uptake by either SSM alone or both SSM and MM did not impair humoral immunity. This was somewhat surprising as earlier reports suggested that capture of particulate antigens by SSM was important for activation of cognate B cells. However, capture of UV-PR8 by sinus-lining macrophages was important in limiting spread of the virus to downstream LNs and the spleen. Thus, the role of macrophages lining the sinus of pLNs is important in efficient capture and spread of virus but not important for humoral immunity to inactive influenza.
How MBL- opsonized UV-PR8 is phagocytosed by SSM is not clear. Since a known receptor for murine MBL is not clear (65) it is possible that uptake is via a complement ligand since the lectin pathway activates both C2 and C4 or C3 directly via a by-pass pathway as described in human serum (Figure 2b)(66).
Medullary dendritic cells bind influenza
In addition to capture of virus by sinus lining macrophages, Gonzalez et al reported that a resident population of dendritic cells (DC) also bound virus in the medullary region. Using flow cytometric assays, they identified CD11c+ CD11bhi, SIGN-R1+ population that bound a significantly high amount of virus. Interestingly about 50% of the PR8 binding DC expressed a receptor for the cysteine rich (CR) domain of the mannose receptor (MR) based on binding with an Ig Fc fusion protein with the CR domain, i.e. CR-Fc (67). The receptor for the CR domain of MR is associated with dendritic-like cells that accumulate within the B cell follicles following immunization and possibly represent cells transporting antigen (68).
Previous studies by Park and colleagues identified murine SIGN-R1 as the major receptor in uptake of S. pneumoniae by marginal zone macrophages (69). SIGN-R1 (specific intracellular adhesion molecule-grabbing nonintegrin R1) is structurally similar to human DC-SIGN and is a C-type lectin that binds glycans rich in mannose such as dextran and capsular polysaccharides of pneumococcus (70, 71). It is the major receptor for S. pneumoniae as pretreatment of mice with a monoclonal (clone 22D1) that down-regulates macrophage expression of SIGN-R1 led to an impaired humoral response and increased mortality (69). Notably, binding of bacteria or purified polysaccharide by SIGN-R1 induces receptor aggregation and activation of C1q and C3 deposition (69). Mice deficient in C3 have impaired immunity to S. pneumoniae and this is associated with impaired uptake of bacteria on FDC. Thus, one interpretation of the findings is that opsonization of bacteria with C3 via SIGN-R1 is critical for uptake on FDC and access by follicular B cells.
SIGN-R1 is required for local humoral immunity to influenza
Using a similar approach as Park et al (69), Gonzalez et al showed treatment of mice with a monoclonal antibody to SIGN-R1 that down regulates receptor expression significantly reduced uptake of UV-PR8 by resident DC(72).
Moreover, blocking of UV-viral uptake via SIGN-R1 in MBL−/− mice not only substantially reduced the capture of UV-PR8 in the draining LN; but the local B cell response was reduced by three-fold. However, in these experiments the systemic response wasn’t affected suggesting that blocking of viral capture locally led to efficient spreading of the virus and response in downstream LNs and spleen. Further support for the importance of the resident DC in viral capture and humoral immunity was determined by elimination of DC systemically in CD11c-DTR bone marrow chimeric mice (73). In this model, CD11c hi cells express the monkey diptheria toxin receptor (DTR) and are sensitive to ablation on treatment with diptheria toxin. Thus, the CD11c DC-ablated chimeric mice had a significantly reduced CD4 T cell-dependent (IgG) and - independent (IgM) day 10 humoral response to UV-PR8. Since the primary IgM response to inactive influenza under the conditions used in the study was largely independent of CD4 T cells (74) (M.C.C, unpublished), the results support a requirement for DC in transport of antigen to the B cell compartment. It is noted that under different conditions in which mice are infected with live influenza virus that the primary response is partly T cell-dependent (75).
Additional support for a role for resident DC in transport of UV-PR8 into the B cell compartment came from MP-IVM analysis of CD11c-EYFP mice injected with labeled influenza. Tracking of CD11c hi cells both before and after injection of labeled virus revealed that only those cells that bound virus moved in a non-random manner and that their net displacement and velocity was significantly increased relative to neighboring DC that did not bind virus. Moreover, calculation of the net vector of directionality, i.e. net overall direction of migration, indicates that DC that bind UV-PR8 migrate towards the B cell follicles (72).
It is tempting to speculate that capture of influenza by SIGN-R1 on DC surface leads to activation of C1q and deposition of C3 on the inactive virus as reported with S. pneumonia. Thus, it will be important in future studies to learn if LN resident DC deliver C3d-coupled inactive virus to the FDC much like that identified for naïve B cells (Figure 4).
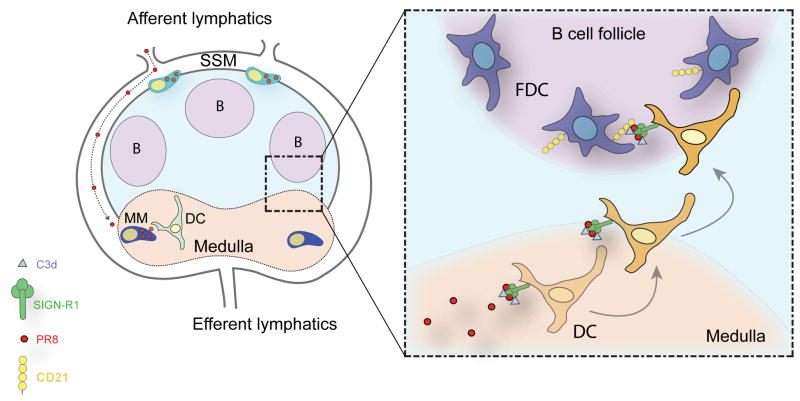
Figure 4.
Lymph-borne UV-inactive influenza (strainPR8) fills the subcapsular (SCS) of the LN, where it is partially taken up by SSM. Binding of virus to SSM is MBL-dependent. Virions are channeled to the LN medulla where medullary macrophages (MM) and resident dendritic cells (DC) capture them in a SIGN-R1-dependent manner. Model speculates that C1q is activated by SIGN-R1 leading to C3 activation and deposition on PR8. Subsequently, resident DC transport C3-coated virus complex to the follicles where it is transferred to FDC.
Conclusions
In this review, we discuss a novel role for complement in transport of soluble antigens, immune complexes and viral antigens to the B cell compartment. The finding that complement C3 and its receptors, i.e. CD21/35 and CR3 (CD11b/18), participate in capture and transport of lymph borne antigens to the FDC provides another important mechanism by which complement enhances adaptive immunity. Moreover, the identification of a network of conduits that directly funnel small antigens to the FDC further elucidates how antigens are made accessible to naïve B cells. We also highlight a new pathway in which capture of inactive-influenza via the SIGN-R1 receptor signals the resident DC to migrate towards the B cell compartment. It will be important in future experiments to determine if C3 is activated and deposited on the virus following binding by SIGN-R1 and to track resident DC with bound PR8 into the B cell follicles and determine whether they hand-off viral antigen to directly to FDC (Figure 4).
Acknowledgments
The authors wish to thank Ms. Alex Gillmore for assistance with assembly of the manuscript and figures and members of the lab for discussions on antigen trafficking.
We also acknowledge support from US National Institutes of Health: 5 RO1 AI039246, 1 PO1 AI078897; 5 RO1 AI067706 to MCC ; RO1 DK074500 to S.J.T.; Glaxo-Smith Kline (post-doctoral fellowship award to MK); Marie Curie International Outgoing Fellowship for Career Development (220044 to S.F.G.) and NIH Transfusion Biology and Medicine TG (5T32HL066987-09) to L.A.P.).
Literature Cited
- 1.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 2.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 3.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25:867–869. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Anderson AO, Anderson ND. Studies on the structure and permeability of the microvasculature in normal rat lymph nodes. Am J Pathol. 1975;80:387–418. [PMC free article] [PubMed] [Google Scholar]
- 6.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 7.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Anderson AO, Shaw S. Conduit for privileged communications in the lymph node. Immunity. 2005;22:3–5. doi: 10.1016/j.immuni.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajenoff M, Germain RN. B cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood. 2009;114:4989–4997. doi: 10.1182/blood-2009-06-229567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pape KA, Catron DM, Itano AA, Jenkins MK. The Humoral Immune Response Is Initiated in Lymph Nodes by B Cells that Acquire Soluble Antigen Directly in the Follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Catron DM, Pape KA, Fife BT, van Rooijen N, Jenkins MK. A protease-dependent mechanism for initiating T-dependent B cell responses to large particulate antigens. J Immunol. 2010;184:3609–3617. doi: 10.4049/jimmunol.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 15.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Pomares L, Gordon S. Antigen presentation the macrophage way. Cell. 2007;131:641–643. doi: 10.1016/j.cell.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 18.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Molina H, Kinoshita T, Inoue K, Carel JC, Holers VM. A molecular and immunochemical characterization of mouse CR2. J Immunol. 1990;145:2974–2983. [PubMed] [Google Scholar]
- 21.Molina H, Wong W, Kinoshita T, Brenner C, Foley S, Holers VM. Distinct receptor and regulatory properties of recombinant mouse complement receptor 1 (CR1) and Crry, the two genetic homologues of human CR1. J Exp Med. 1992;175:121–129. doi: 10.1084/jem.175.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll MC, Alicot EM, Katzman PJ, Klickstein LB, Smith JA, Fearon DT. Organization of the genes encoding complement receptors type 1 and 2, decay-accelerating factor, and C4-binding protein in the RCA locus on human chromosome 1. J Exp Med. 1988;167:1271. doi: 10.1084/jem.167.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 24.Kalli KR, Fearon DT. Binding of C3b and C4b by the CR1- like site in murine CR1. J Immunol. 1994;152:2899–2903. [PubMed] [Google Scholar]
- 25.Isenman DE. The role of the thioester bond in C3 and C4 in the determination of the conformational and functional states of the molecule. Ann N Y Acad Sci. 1983;421:277–290. doi: 10.1111/j.1749-6632.1983.tb18115.x. [DOI] [PubMed] [Google Scholar]
- 26.Law SK, Dodds AW, Porter RR. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. Embo J. 1984;3:1819–1823. doi: 10.1002/j.1460-2075.1984.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustavsson S, Kinoshita T, Heyman B. Antibodies to murine complement receptor 1 and 2 can inhibit the antibody response in vivo without inhibiting T helper cell induction. J Immunol. 1995;154:6524–6528. [PubMed] [Google Scholar]
- 28.Gustavsson S, Kinoshita T, Heyman B. Antibodies to CR1/CR2 inhibit the antibody response without affecting T-helper cells. Mol Immunol. 1993;30:13. [Google Scholar]
- 29.Hebell T, Ahearn JM, Fearon DT. Supression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991;254:102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- 30.Ahearn J, Fischer M, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard R, Rothstein T, Carroll M. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 31.Molina H, V, Holers M, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas KM, Hasegawa M, Steeber DA, Poe JC, Zabel MD, Bock CB, Karp DR, Briles DE, Weis JH, Tedder TF. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17:713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- 33.DaCosta X, Brockman M, Alicot E, Ma M, Fischer M, Zhou X, Knipe D, Carroll M. Humoral response to herpes simplex virus is complement dependent. Proc Natl Acad Sci USA. 1999;96:12708. doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez Gonzalez S, Jayasekera JP, Carroll MC. Complement and natural antibody are required in the long-term memory response to influenza virus. Vaccine. 2008;26(Suppl 8):I86–93. doi: 10.1016/j.vaccine.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 35.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TT. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841. [PubMed] [Google Scholar]
- 36.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 37.Carter RH, Fearon DT. CD19:Lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 38.Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004;41:141–146. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Fang Y, Xu C, Fu Y, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 40.Brockman MA, Verschoor A, Zhu J, Carroll MC, Knipe DM. Optimal long-term humoral responses to replication-defective herpes simplex virus require CD21/CD35 complement receptor expression on stromal cells. J Virol. 2006;80:7111–7117. doi: 10.1128/JVI.01421-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Shikh ME, El Sayed R, Szakal AK, Tew JG. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur J Immunol. 2006;36:2715–2724. doi: 10.1002/eji.200636122. [DOI] [PubMed] [Google Scholar]
- 42.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 43.Barrington RA, Schneider TJ, Pitcher LA, Mempel TR, Ma M, Barteneva NS, Carroll MC. Uncoupling CD21 and CD19 of the B- cell coreceptor. Proc Natl Acad Sci U S A. 2009;106:14490–14495. doi: 10.1073/pnas.0903477106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. J Exp Med. 2002;196:1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verschoor A, Brockman MA, Knipe DM, Carroll MC. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J Immunol. 2001;167:2446–2451. doi: 10.4049/jimmunol.167.5.2446. [DOI] [PubMed] [Google Scholar]
- 46.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer M, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard R, Rothstein T, Kremmer E, Rosen F, Carroll M. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- 48.Fischer MB, Goerg S, Shen L, Prodeus AP, Goodnow CC, Kelsoe G, Carroll MC. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 49.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 50.Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–599. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 51.Randall KL, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, Whittle B, Tze LE, Enders A, Crockford TL, Bouriez-Jones T, Alston D, Cyster JG, Lenardo MJ, Mackay F, Deenick EK, Tangye SG, Chan TD, Camidge T, Brink R, Vinuesa CG, Batista FD, Cornall RJ, Goodnow CC. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouillie S, Barel M, Frade R. Signaling through the EBV/C3d receptor (CR2, CD21) in human B lymphocytes: activation of phosphatidylinositol 3-kinase via a CD19-independent pathway. J Immunol. 1999;162:136–143. [PubMed] [Google Scholar]
- 54.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 57.Youd ME, Ferguson AR, Corley RB. Synergistic roles of IgM and complement in antigen trapping and follicular localization. Eur J Immunol. 2002;32:2328–2337. doi: 10.1002/1521-4141(200208)32:8<2328::AID-IMMU2328>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 58.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 59.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 60.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skehel J. An overview of influenza haemagglutinin and neuraminidase. Biologicals. 2009;37:177–178. doi: 10.1016/j.biologicals.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Ross T, Xu Y, Bright R, Robinson H. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1:127. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe I, Ross TM, Tamura S, Ichinohe T, Ito S, Takahashi H, Sawa H, Chiba J, Kurata T, Sata T, Hasegawa H. Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin. Vaccine. 2003;21:4532–4538. doi: 10.1016/s0264-410x(03)00510-3. [DOI] [PubMed] [Google Scholar]
- 64.Anders EM, Hartley CA, Reading PC, Ezekowitz RA. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75(Pt 3):615–622. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 65.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose- binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 66.Selander B, Martensson U, Weintraub A, Holmstrom E, Matsushita M, Thiel S, Jensenius JC, Truedsson L, Sjoholm AG. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116:1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S, Bonnefoy JY, Gordon S. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med. 1996;184:1927–1937. doi: 10.1084/jem.184.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berney C, Herren S, Power CA, Gordon S, Martinez-Pomares L, Kosco-Vilbois MH. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med. 1999;190:851–860. doi: 10.1084/jem.190.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 70.Geijtenbeek TB, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GC, Kraal G, van Oosterhout AJ, van Kooyk Y. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 71.Kang YS, Yamazaki S, Iyoda T, Pack M, Bruening SA, Kim JY, Takahara K, Inaba K, Steinman RM, Park CG. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim Y-A, Cloninger M, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010 Mar 21; doi: 10.1038/ni.1856. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sha Z, Compans RW. Induction of CD4(+) T-cell- independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]