Abstract
Objective
To evaluate the efficacy of a single dose of intravenous zoledronate for the treatment of HIV-associated osteopenia and osteoporosis.
Design
A double-blinded, randomized, placebo-controlled, 12 month trial of 5 mg intravenous zoledronate dose to treat 30 HIV-infected men and women with osteopenia and osteoporosis.
Methods
Following zoledronate or placebo infusions, participants were followed for 12 months on daily calcium and vitamin D supplements. Lumbar spine and hip bone density was assessed at baseline, 6 and 12 months. Biomarkers of bone metabolism were measured at baseline, 2 weeks, 3, 6, 9, and 12 months. Student’s t-test and repeated measure analyses were used to evaluate bone density and bone marker changes over time.
Results
In the 30 HIV-infected men (27) and women (3) in the trial, median T-scores at entry were -1.7 for the lumbar spine and -1.4 for the hip. Median CD4 count was 461 cells/μL, 93% had HIV-RNA viral loads <400 copies/mL, and 97% were taking antiretroviral medications. Bone density measured either absolutely or as sex-adjusted T-scores significantly improved in zoledronate recipients as compared to minimal changes in those receiving placebo. Bone resorption markers significantly decreased over the study period in the zoledronate recipients as compared to placebo controls. No acute infusion reactions were detected, but one patient developed uveitis, a recognized complication of zoledronate, which responded to therapy.
Conclusions
In this small study, annual zoledronate appears to be a safe and effective therapy for HIV-associated bone loss.
Keywords: metabolic bone diseases, osteoporosis, zoledronic acid, HIV, bisphosphonates
INTRODUCTION
In the current era of highly active antiretroviral therapy (HAART), HIV-infected patients are living longer owing to effective medical treatment [1, 2]. Antiretroviral medications have also been linked to a number of toxic side effects, including bone loss (osteopenia and osteoporosis)[3]. HIV-associated osteopenia and osteoporosis is common with reported prevalence rates of 51 to 67% in various cohorts [4, 5] and can result in significant morbidity [6]. Simplified regimens for HIV-associated osteopenia and osteoporosis could help to ensure compliance to all medications, especially since they must be taken concurrently with antiretroviral therapy.
Bisphosphonates are pyrophosphate analogues that inhibit bone resorption by binding to hydroxyapatite crystals [7]. Bisphosphonates have been used in HIV-infected patients with osteopenia and osteoporosis to improve bone density[8-13]. However, gastrointestinal intolerance [14] of bisphosphonate therapy can exacerbate compliance issues that result from the polypharmacy required to treat HIV infection and associated diseases. Recent studies using intravenous zoledronate, a third-generation, long-acting bisphosphonate, in postmenopausal women with osteoporosis (T-score ≤-2.5) have demonstrated beneficial improvements in bone density and reduction in fracture risk [15, 16]. Boland et al. conducted a randomized placebo controlled trial of 4 mg zoledronate in 43 HIV-infected men with low bone density, defined as a T-score<-0.5, and demonstrated significant increases in bone density [8]. Zoledronate therefore potentially offers a convenient means of providing therapy for clinically significant osteopenia in HIV-infected patients with adequate compliance. We thus evaluated the effectiveness of intravenous zoledronate in improving bone density and reducing bone turnover in HIV-infected persons with WHO-defined osteopenia and osteoporosis.
METHODS
Participants
Ambulatory HIV-infected men (n=27) and women (n=3), who were recruited from the San Diego area, met criteria at screening of 1) bone mineral density at the lumbar spine or hip from -3.5 SD to -1.5 SD below the mean value for young adults, 2) no more than one asymptomatic vertebral fracture, 3), HIV viral load ≤ 5000 copies/mL, 4) CD4 count ≥100 cells/mm3, and 5) stable antiretroviral regimen (including no treatment) with no plans to change. Major exclusion criteria included 1) evidence of endocrinologic, hepatic or renal disease, disorders of parathyroid or thyroid glands, 2) prior treatment with bisphosphonates, and 3) current therapy with any other drug known to affect the skeleton. Women who were postmenopausal were required to take hormone replacement therapy during the study period. Persons who had a current history of osteomyelitis of the jaw, ongoing dental infection, and recent tooth extraction or major dental procedure within the past 3 weeks were ineligible owing to reports of osteonecrosis of the jaw with bisphosphonate therapy [17, 18]. Seven persons with low 25-hydroxyvitamin D levels (<20 ng/mL) were repleted with a one-time dose of 50,000 IU vitamin D prior to the baseline evaluation.
Protocol and Intervention
The study protocol was approved by the UCSD IRB and registered at ClinicalTrials.gov (NCT00102908). Participants were randomly allocated to receive an administration of either zoledronate 5 mg, given as a 30-min intravenous infusion in 100 ml 0.9% NaCl, or placebo. Randomization was performed using computer-generated random numbers (Excel 2000; Microsoft Corp., Redmond, WA). Infusions were prepared by a pharmacist who had no contact with the patients and were labeled with only the subject’s study number. Premedication with 1 gram acetaminophen was offered to all participants prior to the infusion. All study staff and participants remained blinded to treatment allocation throughout the study. All participants received 1 gram calcium and 400 IU vitamin D supplements daily and were re-evaluated at 2 weeks and at 3 month intervals thereafter up to 12 months after the infusion.
Outcome Measures
At each study visit, evaluations included a fasting blood draw for bone marker measurements, HIV disease markers (including CD4 count and HIV viral load), weight, height measurements. Nutritional and physical activity assessments and DXA scans for bone density were performed at baseline, 6 and 12 months.
Nutritional Assessments
At the baseline, 6 and 12 month visits, participants underwent body fat and dietary assessment. Body fat percentage was determined by bioimpedance analysis (Bioelectrical Body Composition Analyzer, Quantum II, Clinton Twp, MI) using Cyprus 2.7 software [19]. Daily dietary calcium intake was assessed from 24-hour dietary recall data using Nutritionist Pro (Axxya Systems, version 3.1.0).
Physical Activity Assessment
Physical activity was assessed using the Modifiable Activity Questionnaire (MAQ) at the baseline, 6 and 12 month visits. The MAQ assesses past-year occupational and leisure activities. For the purposes of this study, physical activity was assessed over the past 6 months given the study visit frequency. Only physical activities that demand energy expenditure greater than that required by activities of daily living are assessed and each activity is weighted by its relative intensity. Estimates of physical activity performed during leisure and occupational activity were calculated as minutes per day. The MAQ has been shown to be reliable and valid in adults [20, 21].
Bone Metabolic Biomarker Assays
We measured serum intact osteocalcin by competitive immunoassay to quantify bone formation. The osteocalcin assay has a minimum detection limit of 0.45 ng/mL and a normal reference interval of 3.7 to 10.0 ng/mL. C-terminal telopeptides of Type I collagen and cross-linked N-telopeptides of type I collagen were measured in human serum as indicators of bone resorption. C-terminal telopeptides of Type I collagen were measured via an ELISA assay with a detection limit of 0.02 ng/mL and a usual range of 0.12-0.75 ng/mL among males, 0.11-0.74 ng/mL among premenopausal women, and 0.14-1.35 ng/mL among postmenopausal women. N-telopeptides of type I collagen were also measured via an ELISA assay with a measurement range of 3.2 to 40 nM BCE (bone collagen equivalents). 25-hydroxyvitamin D levels were performed by ARUP Laboratories; the lower limit of normal of this assay is 20 ng/mL.
Bone Density Assessments
Bone mineral density of the lumbar spine, and hip (total hip and femoral neck) were measured by dual X-ray absorptiometry (DXA) using the Hologic Discovery W (Hologic Inc., Waltham, Massachusetts, USA). The in vivo precision for the measurement of bone density using the DXA technique is 0.5 – 1.5% at the lumbar spine and the standard deviation of the lumbar spine bone density is 0.01 g/cm2. Osteopenia and osteoporosis were defined according to World Health Organization criteria [6] (osteopenia: T score <-1.0 SD and >-2.5 SD; osteoporosis: T score ≤-2.5 SD). Racioethnic-specific T-scores provided by the manufacturer were used to determine osteopenia and osteoporosis (Hologic, Inc.).
HIV-related Biomarkers
HIV RNA in plasma was measured by reverse transcriptase–polymerase chain reaction (RT–PCR; Amplicor, Roche Diagnostics, Indianapolis, IN, USA) using the standard assay with a nominal detection limit of 400 copies/ml. CD4 counts were measured using standard flow cytometry technique.
Statistical Analysis
Sample size was calculated as the number of patients needed to detect a difference between the zoledronic acid and the placebo groups of at least 4 percent in the degree of change in lumbar-spine bone mineral density from baseline to 12 months [16]. Given a central t distribution with a type I error of 0.05, a power of 80 percent, a two-sided alternative, and a standard deviation of 3 percent determined from other longitudinal studies of bone density among HIV-infected patients [22, 23], we calculated that 13 patients would be needed in each treatment group in order to allow detection of a difference of 4 percent. To allow for a possible 15 percent dropout rate, a total sample size of 30 was selected.
Differences between groups for continuous variables were assessed using Student’s t test for normally distributed variables, Wilcoxon rank sum for non-normally distributed variables, and the χ2 test for categorical groups. Select correlational analyses between continuous variables were performed with Pearson’s or Spearman’s correlation tests according to variable distributions. Multivariate modeling was used to examine the effects of treatment group on change in bone density, while controlling for baseline measures with significant differences between groups. A mixed model approach to repeated measures (analysis of covariance) was used to examine the time course of response in treatment and control arms for bone density and body composition measurements and bone turnover markers. Analyses were performed according to intention to treat, and all available data from subjects were used. Missing values were not imputed or replaced. For multivariate regression methods, we log-transformed some variables to improve the symmetry of their distributions. Statistical analyses were performed using JMP 5.0 (SAS Institute, Inc., Cary, NC).
RESULTS
Participants
Of 143 subjects screened for the study, 113 subjects did not meet study DXA criteria for osteopenia and 7 demonstrated low vitamin D levels at the screening visit. All seven subjects were enrolled following vitamin D repletion per study protocol.
Demographic and disease characteristics of the study population are presented in Table 1. At baseline, zoledronate and placebo groups were similar in age, sex, racioethnic background, weight, bone marker levels, bone density measurements and bone disease categorization. Baseline CD4 counts were significantly higher in recipients of zoledronate compared to placebo (663 v. 384 cells/μL, p=0.002), but plasma HIV RNA levels (viral loads) were similar. All except one participant were on stable antiretroviral therapy at the time of study, and exposure to tenofovir, smoking and alcohol history was similar between groups. Three women entered the study; two premenopausal and one, postmenopausal.
TABLE 1.
Population Demographics and Baseline Measures
| Zoledronate (N=15) | Placebo (N=15) | p-value between groups | |
|---|---|---|---|
|
| |||
| Age (years) | 48 ± 13 | 49 ± 7 | 0.95 |
|
| |||
| Race (%) | 87% White | 93% | 0. 39Ф |
| 7% Black | 0% | ||
| 0% Asian | 7% | ||
| 7% Other | 0% | ||
|
| |||
| Ethnicity (%) | 27% Hispanic | 20% | 0.66 Ф |
| 73% Non-Hispanic | 80% | ||
|
| |||
| Male: Female | 14: 1 | 13: 2 | 0.54Ф |
|
| |||
| BMI (kg/m2) | 25.0 ± 3.0 | 23.5 ± 3.0 | 0.27 |
|
| |||
| % Body Fat | 18.1 ± 5.7 | 17.7 ± 6.4 | 0.87 |
|
| |||
| CD4 count (cells/μL) | 663 ± 306 | 384 ± 104 | 0.002 |
|
| |||
| HIV viral load (copies/mL)* | <400 (<400, <400) | <400 (<400, <400) | >0.99¥ |
|
| |||
| Current tenofovir exposure N (%) | 6 (40%) | 10 (67%) | 0.14Ф |
|
| |||
| Smoking history N (%) | Current 9 (60%) | 9 (60%) | 0.58 Ф |
| Past 1 (7%) | 0 (0%) | ||
| Never 5 (33%) | 6 (40%) | ||
|
| |||
| Alcohol exposure N (%) | >3-5 drinks/week 1 (7%) | 3 (20%) | 0.13 Ф |
| 1-2 drinks/week 1 (7%) | 5 (33%) | ||
| 0-2 drinks/mo 5 (33%) | 2 (14%) | ||
| None 8 (53%) | 5 (33%) | ||
|
| |||
| 25-vitamin D level (ng/mL)** | 37 ± 12 | 43 ± 15 | 0.25 |
|
| |||
| Dietary Calcium Intake (mg/d) | 1088 ± 692 | 1328 ± 596 | 0.33 |
|
| |||
| Minutes of Physical Activity/day* | 133 (30, 241) | 49 (25, 235) | 0.52¥ |
|
| |||
| Bone density at lumbar spine (g/cm2) | 0.94 ± 0.14 | 0.90 ± 0.06 | 0.37 |
|
| |||
| T-score at lumbar spine | -1.5 ± 1.3 | -1.8 ± 0.5 | 0.39 |
|
| |||
| Bone density at hip (g/cm2) | 0.85 ± 0.08 | 0.85 ± 0.08 | 0.97 |
|
| |||
| T-score at hip | -1.3 ± 0.6 | -1.2 ± 0.6 | 0.60 |
|
| |||
| Bone disease categorization | 80% Osteopenia | 93% Osteopenia | 0.28Ф |
| 20% Osteoporosis | 7% Osteoporosis | ||
|
| |||
| N-telopeptide (nM BCE) | 5.20 ± 2.29 | 5.63 ± 2.50 | 0.62 |
|
| |||
| C-telopeptide (ng/mL) | 1.12 ± 0.73 | 1.21 ± 0.74 | 0.73 |
|
| |||
| Osteocalcin (ng/mL) | 9.12 ± 6.06 | 8.38 ± 7.11 | 0.74 |
Answers expressed as mean ± standard deviation unless otherwise noted.
Expressed as median (interquartile range).
p-value by Student’s t-test unless otherwise indicated.
p-value by Wilcoxon rank sum test.
p-value by chi-square statistics.
after hypovitaminosis D corrected.
Bone density
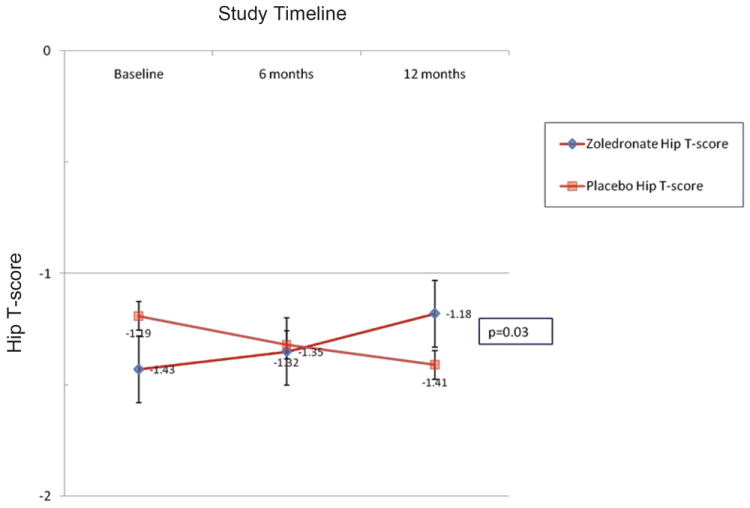
At 12 months patients receiving zoledronate increased their bone density at the lumbar spine by 3.7 ± 4.1% (mean ± S.D.) compared to patients receiving placebo (0.7 ± 3.1%, p=0.04). Likewise, bone density at the hip increased at 12 months by 3.2 ± 2.2% in zoledronate recipients compared to controls (-1.8 ± 9.3%) (p=0.016). T-scores also significantly increased at both the lumbar spine and hip over the 12 month study period in the zoledronate group compared to placebo (Figures 1A and 1B). Bone density improvements in the lumbar spine peaked at 6 months among zoledronate recipients, but continued to improve over 12 months at the hip. At study completion, prevalence of osteopenia (71% vs. 77%, zoledronate vs. control, respectively) and osteoporosis (21% vs. 15%, zoledronate vs. control) did not differ between groups.
FIGURE 1.
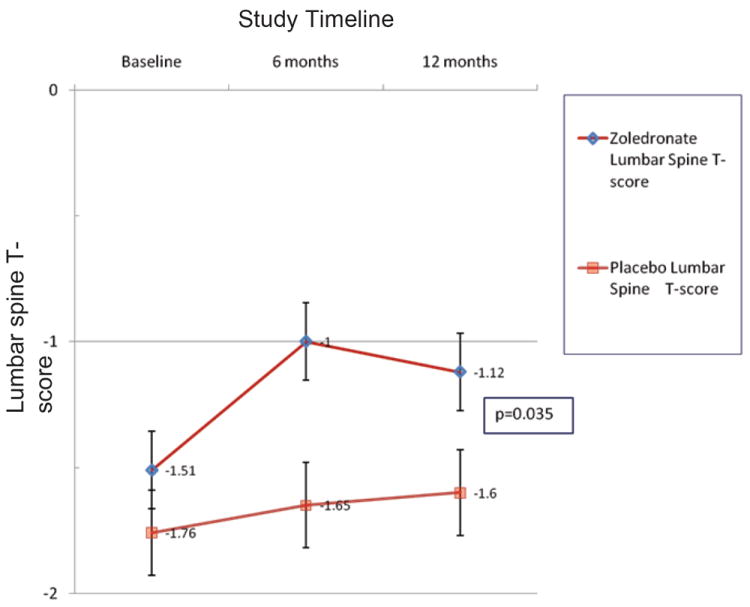
A. Lumbar spine T-scores according to treatment.
B. Hip T-scores according to treatment.
NOTE: Graphed data points represent mean ± SEM.
In multivariate modeling of changes in bone density, entering baseline CD4 counts and treatment group as outcome predictors, only treatment group remained predictive of changes in bone density at the hip (p=0.03) and at the spine (p=0.02).
Bone turnover
Both biomarkers of bone resorption were significantly reduced in the zoledronate group as compared to the placebo group over the 12 months of evaluation (N-telopeptides: F=3.04, p=0.03; C-telopeptides: F=2.70, p=0.048; Figure 2). Significant reduction was seen in both bone resorption markers by 2 weeks after the zoledronate infusion. In contrast, osteocalcin did not differ significantly by treatment group (F=0.38, p=0.54) or change significantly by treatment group over the study period (F=0.54, p=0.74).
FIGURE 2.
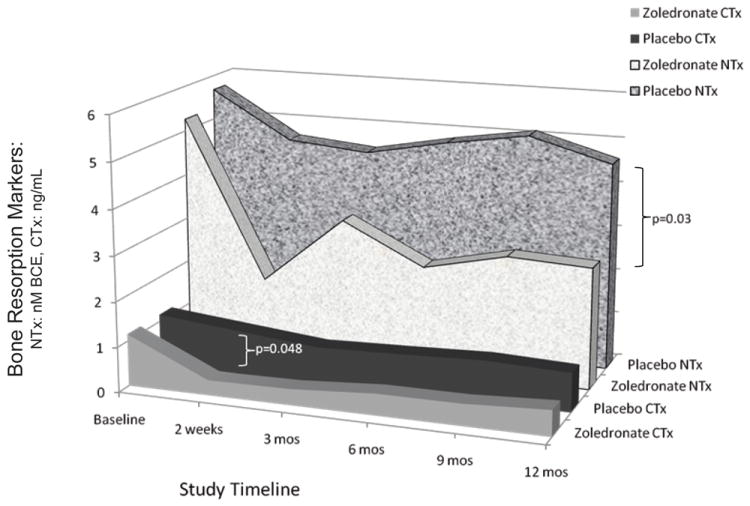
Bone resorption markers according to treatment.
Nutritional Evaluations
Dietary calcium intake significantly increased over time in both groups with study prescribed calcium supplementation (F=14.89, p<0.0001), but did not differ between groups (F=2.31, p=0.14) or by group over time (F=0.39, p=0.68). Body mass index and body fat percentage did not differ between groups (BMI: F=1.87, p=0.18; % body fat: F=0.01, p=0.94) or over time (BMI: F=0.35, p=0.88; % body fat: F=0.31, p=0.74). Physical activity also did not differ by any group or time comparisons (data not shown).
HIV-related Biomarkers
CD4 counts were significantly higher in the zoledronate group compared to placebo (F=11.59, p=0.002), but CD4 counts did not vary with time (F=0.06, p=0.94) or by group over time (F=0.43, p=0.66). In contrast, no differences in HIV viremia were found in any between-group and/or by time comparisons (data not shown).
Correlations between changes in bone density and other measures
Changes in calcium intake or BMI did not correlate with changes in bone density at either the lumbar spine or hip [change in calcium intake: vs. lumbar spine bone density change r=0.04, p=0.84, vs. hip bone density change ρ=0.14, p=0.49; and change in BMI: vs. lumbar spine bone density change r=-0.18, p=0.38, vs. hip bone density change ρ=0.26, p=0.19]. Similarly, baseline CD4 count and bone density changes at the lumbar spine (r=0.09, p=0.63) and hip (ρ=-0.07, p=0.75) were not correlated.
Safety
Fifteen persons in each treatment group began the study, and twenty-eight participants completed 12 months of observation. One placebo recipient was lost at 12 months and one zoledronate recipient was lost at 6 months. No acute infusion reactions occurred, but one recipient developed uveitis, a known complication of zoledronate infusions, within 72 hours of the drug infusion. The patient was treated successfully with topical steroids and has no residual deficit. Serum calcium levels dropped slightly over the study period (F=2.28, p=0.09) in zoledronate recipients as compared to placebo, but none required medical intervention.
DISCUSSION
Osteopenia and altered bone metabolism are a clinical concern among providers of HAART to HIV-infected patients. Anti-resorptive therapy has been demonstrated to be an effective therapy in HIV-infected patients with bone loss [9] but dosing can be limited by associated side effects and compliance issues. This study provides evidence of the efficacy of intravenous annual zoledronate for HIV-associated osteopenia.
Treatment of HIV-related bone disease is controversial because the relationship between low bone density and risk for bone fracture is unclear, and maintenance of and increases in bone density over time independent of usual treatments for bone-related diseases have been reported [22, 23]. The mechanism of increased bone density without specific treatment in the HIV-infected population remains unclear, but may result from improvements in HIV disease or nutritional status with antiretroviral therapy [12, 23]. Nevertheless, these findings suggest that therapies for bone loss may be unnecessary for some HIV-infected patients with osteopenia. Although treatment criteria for HIV-associated osteopenia have not been fully established, studies to date (including ours) have primarily used WHO categorizations for osteopenia and osteoporosis to determine need for therapy. National Osteoporosis Foundation recommendations support a cut-off T-score at -1.5 with the presence of 1 major or 2 minor risk factors as treatment criteria [24].
Bisphosphonate therapy has been evaluated previously as therapy for HIV-associated bone disease. Alendronate, in particular, has increased bone density at both lumbar spine and hip in HIV-infected patients who meet WHO-defined criteria for osteopenia and osteoporosis in most studies [10-13]. In the study by Boland et al., annual zoledronate reduced bone resorption and increased bone density at the lumbar spine and hip among HIV-infected men with subclinical osteopenia (T-score<-0.5 at the lumbar spine or hip) [8]. Our study confirms these findings in a group of patients with more advanced bone disease using T-score criteria that are more consistent with national bone health organizations’ recommendations for the treatment of osteopenia and osteoporosis [24]. We also demonstrate notable improvements in T-scores, the clinical determinant score of fracture risk, at both the lumbar spine and hip among zoledronate recipients. Although a marked imbalance occurred in average CD4 counts between our treatment groups, this probably did not affect our findings because bone density responses were independent of CD4 counts.
Evaluations of other etiologies known to affect bone metabolism were evaluated in this study cohort. Prior studies in the HIV-infected population have suggested that HIV disease status may affect progression of bone disease [23]. In our study, treatment and placebo groups did differ by CD4 count at baseline; however, baseline CD4 count did not correlate with bone density outcomes. CD4 count and viral load also did not vary according to treatment group over the course of the study. In regards to nutritional status, all study participants had replete vitamin D stores at the time of study initiation. Although dietary calcium intake did increase over the course of the study, this increase did not correlate with the observed bone density changes. Also, body mass index and body composition measures did not differ between groups at all study measurement points. Similarly, physical activity measures did not differ between groups or over time.
There are currently a variety of bisphosphonate therapies for persons who meet clinical criteria for treatment of low bone density. Oral bisphosphonate therapies can now be dosed as infrequently as monthly, but only weekly regimens have been tested to date in the HIV-infected population [9-12]. However, gastrointestinal side effects still contribute to reduced compliance with oral bisphosphonate therapies [14] and may be an even more significant issue in the HIV-infected population where current antiretroviral regimens also commonly cause gastrointestinal distress. Adherence to medical therapy is an important clinical determinant of successful reduction of HIV viremia [25] and is related to the complexity (i.e. dosing frequency, food-dosing restrictions, and pill burden) of therapeutic regimens [26]. Therefore, physicians must take into account an already demanding antiretroviral medication schedule before prescribing additional remedies.
In our study, zoledronic acid was generally well tolerated, and the rate of study subject retention was high. In contrast with other studies of zoledronic acid [8, 15, 16], none of our subjects experienced a reaction to the zoledronate infusion, possibly because acetaminophen premedication was used in this protocol Uveitis, which occurred in one zoledronate recipient, has been previously described [27-29] and resolved completely with medical management.
Our study has several limitations. First, our study cohort was relatively small and did not include an adequate number of women to assess their responses separately. Second, although we attempted to exclude secondary causes of osteopenia and osteoporosis in the study design, we did allow persons with low vitamin D levels after repletion and post-menopausal women on hormone replacement therapy into the study. Repeat data analysis excluding data from these persons (N=8), continue to demonstrate significant bone density improvement at the lumbar spine (p=0.002) and hip (p=0.03) in subjects receiving zoledronate as compared to placebo. Thirdly, only one dose of zoledronate was given with 12 months of monitoring and thus long-term safety of zoledronate in the HIV-infected population could not be assessed. However, follow-up studies in HIV-infected patients after two doses of zoledronate suggest that toxicity does not increase with a second dose [8]. Larger and longer studies are required to evaluate women, the persistence of benefits in bone density after treatment, and potential long-term clinical benefits such as reduction in fracture rates.
In conclusion, we have confirmed that annual dosing of zoledronate treats osteopenia and osteoporosis in HIV-infected men. As in other populations, this therapy probably acts by reducing bone resorption as shown by biomarkers. Zoledronate was well-tolerated by our study cohort except for one episode of uveitis. Its combination of good tolerability, low medication burden, and effectiveness make zoledronate an excellent candidate to treat a common complication of chronic therapy for a life-threatening disease.
Acknowledgments
JH conceived of the study, participated in its design, coordination, and data analysis, and helped draft the manuscript. LM and SF coordinated study recruitment, carried out the study protocol, and supervised data integrity and analysis. JAM participated in the study design and helped draft the manuscript. All authors approved the final manuscript.
Funding/research support sources: Grant NIH R21 AI58756 and Novartis Pharmaceuticals, Inc.
References
- 1.Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984-1997. Jama. 2001;285:1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. Aids. 2000;14:F63–67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. Aids. 2008;22:395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- 5.Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A. Osteopenia in HIV-infected patients: is it the disease or is it the treatment? Aids. 2001;15:807–808. doi: 10.1097/00002030-200104130-00022. [DOI] [PubMed] [Google Scholar]
- 6.Guaraldi G, Ventura P, Albuzza M, Orlando G, Bedini A, Amorico G, Esposito R. Pathological fractures in AIDS patients with osteopenia and osteoporosis induced by antiretroviral therapy. Aids. 2001;15:137–138. doi: 10.1097/00002030-200101050-00025. [DOI] [PubMed] [Google Scholar]
- 7.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolland MJ, Grey AB, Horne AM, Briggs SE, Thomas MG, Ellis-Pegler RB, et al. Annual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: a randomized controlled trial. J Clin Endocrinol Metab. 2007;92:1283–1288. doi: 10.1210/jc.2006-2216. [DOI] [PubMed] [Google Scholar]
- 9.Clay PG, Voss LE, Williams C, Daume EC. Valid treatment options for osteoporosis and osteopenia in HIV-infected persons. Ann Pharmacother. 2008;42:670–679. doi: 10.1345/aph.1K465. [DOI] [PubMed] [Google Scholar]
- 10.Guaraldi G, Orlando G, Madeddu G, Vescini F, Ventura P, Campostrini S, et al. Alendronate reduces bone resorption in HIV-associated osteopenia/osteoporosis. HIV Clin Trials. 2004;5:269–277. doi: 10.1310/MD8V-5DLG-EN3T-BRHX. [DOI] [PubMed] [Google Scholar]
- 11.McComsey GA, Kendall MA, Tebas P, Swindells S, Hogg E, Alston-Smith B, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. Aids. 2007;21:2473–2482. doi: 10.1097/QAD.0b013e3282ef961d. [DOI] [PubMed] [Google Scholar]
- 12.Mondy K, Powderly WG, Claxton SA, Yarasheski KH, Royal M, Stoneman JS, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr. 2005;38:426–431. doi: 10.1097/01.qai.0000145352.04440.1e. [DOI] [PubMed] [Google Scholar]
- 13.Negredo E, Martinez-Lopez E, Paredes R, Rosales J, Perez-Alvarez N, Holgado S, et al. Reversal of HIV-1-associated osteoporosis with once-weekly alendronate. Aids. 2005;19:343–345. [PubMed] [Google Scholar]
- 14.Lewiecki EM, Babbitt AM, Piziak VK, Ozturk ZE, Bone HG. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther. 2008;30:605–621. doi: 10.1016/j.clinthera.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 16.Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonate-induced avascular osteonecrosis of the jaws: a clinical report of 11 cases. Int J Oral Maxillofac Surg. 2006;35:588–593. doi: 10.1016/j.ijom.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 19.Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996;64:489S–497S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 20.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 21.Schulz LO, Harper IT, Smith CJ, Kriska AM, Ravussin E. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;2:541–548. doi: 10.1002/j.1550-8528.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolland MJ, Grey AB, Horne AM, Briggs SE, Thomas MG, Ellis-Pegler RB, et al. Bone mineral density remains stable in HAART-treated HIV-infected men over 2 years. Clin Endocrinol (Oxf) 2007;67:270–275. doi: 10.1111/j.1365-2265.2007.02875.x. [DOI] [PubMed] [Google Scholar]
- 23.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 24.National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2005. [Google Scholar]
- 25.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. Aids. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 26.Stone VE. Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin Infect Dis. 2001;33:865–872. doi: 10.1086/322698. [DOI] [PubMed] [Google Scholar]
- 27.Kilickap S, Ozdamar Y, Altundag MK, Dizdar O. A case report: zoledronic acid-induced anterior uveitis. Med Oncol. 2008;25:238–240. doi: 10.1007/s12032-007-9006-2. [DOI] [PubMed] [Google Scholar]
- 28.El Saghir NS, Otrock ZK, Bleik JH. Unilateral anterior uveitis complicating zoledronic acid therapy in breast cancer. BMC Cancer. 2005;5:156. doi: 10.1186/1471-2407-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stack R, Tarr K. Drug-induced optic neuritis and uveitis secondary to bisphosphonates. N Z Med J. 2006;119:U1888. [PubMed] [Google Scholar]


