Abstract
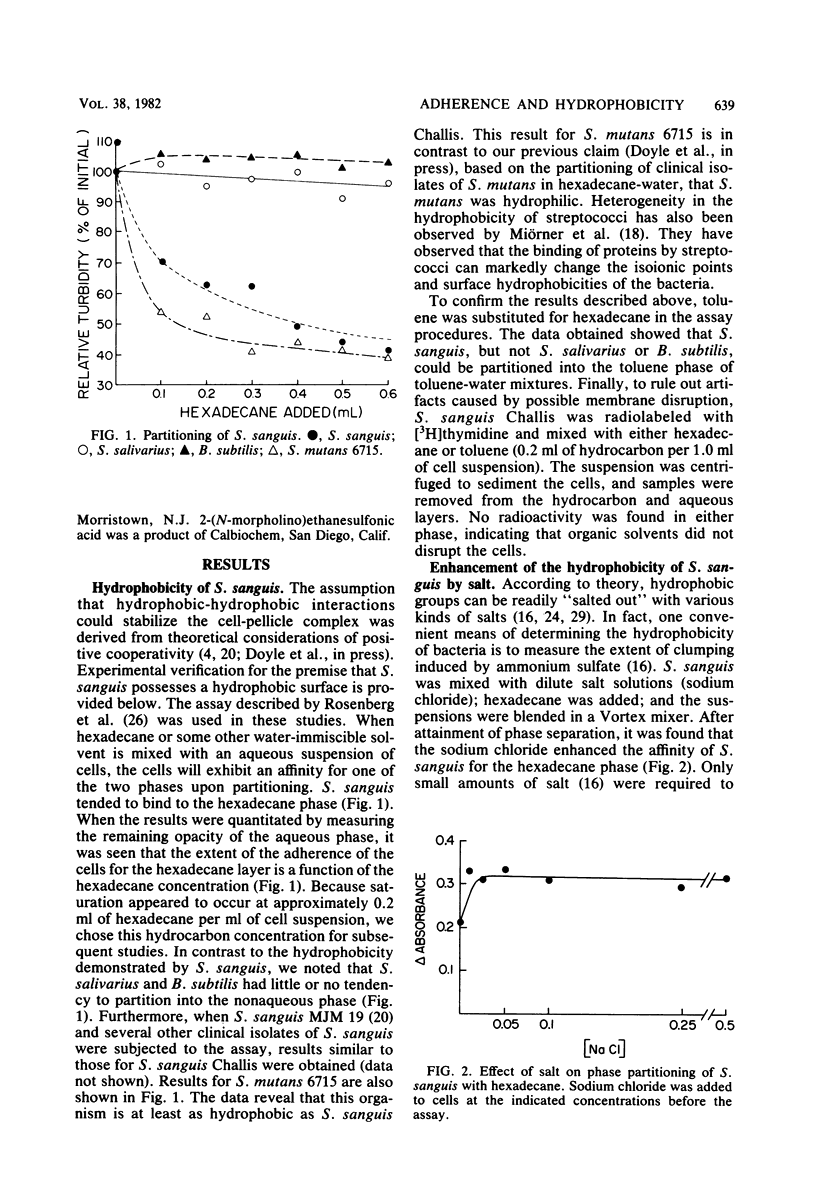
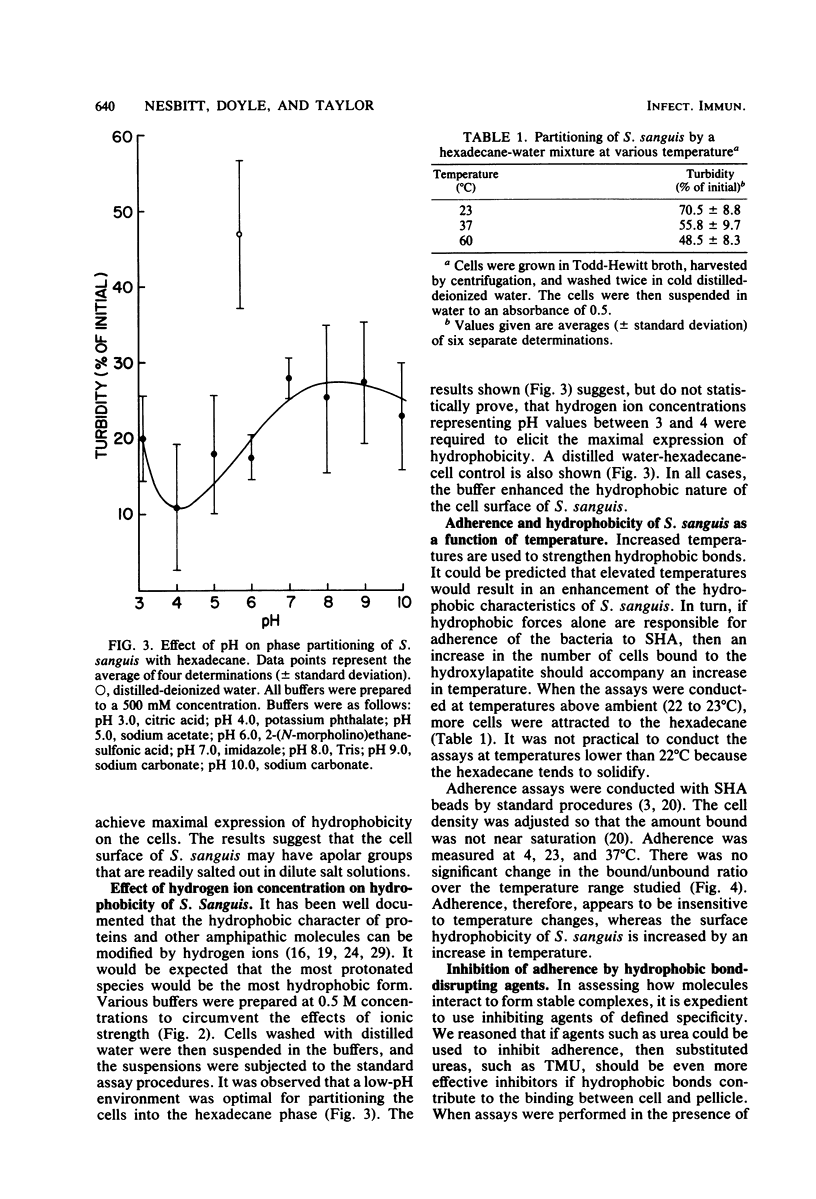
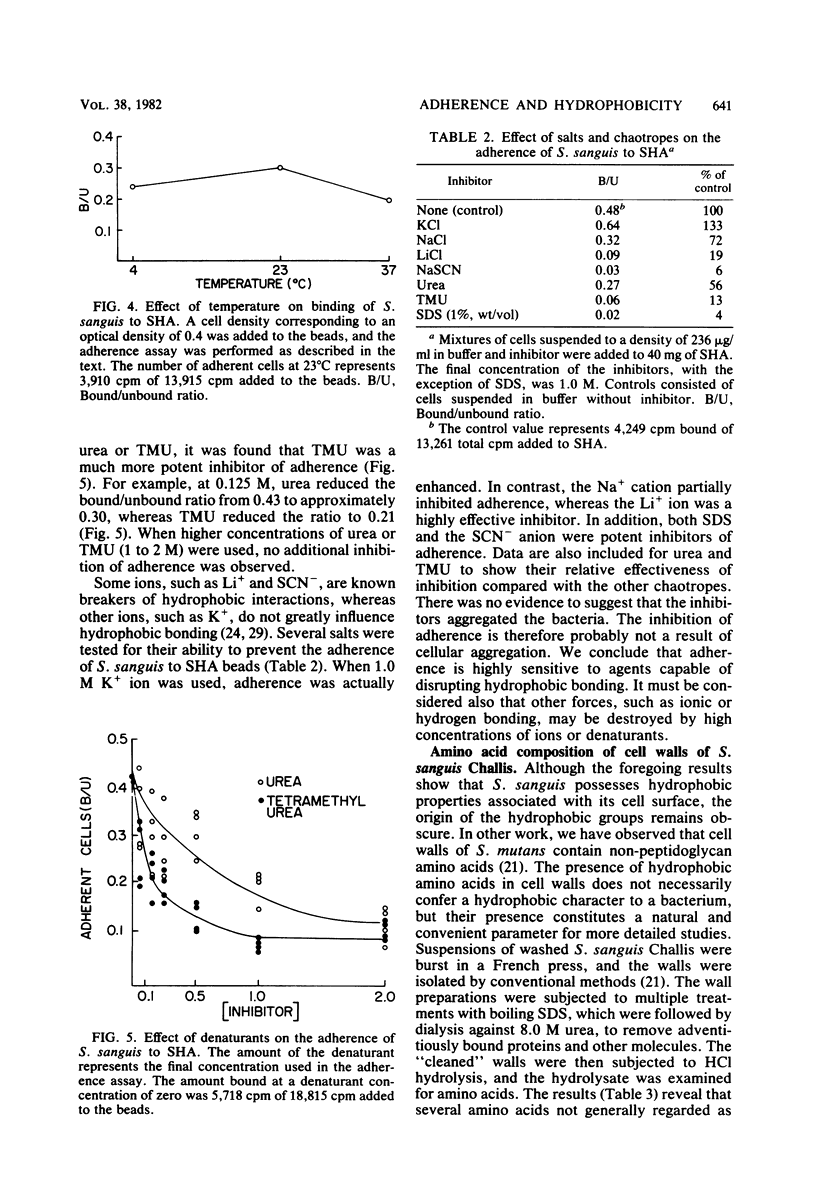
Streptococcus sanguis demonstrated a high affinity for hydrocarbon solvents. When aqueous suspensions of the organism were mixed with either hexadecane or toluene, the cells tended to bind to the nonaqueous solvent. Increases in temperature resulted in a greater affinity of cells for hexadecane. Interaction between the cells and hexadecane was also enhanced by dilute aqueous sodium chloride and by low pH (pH less than 5). The results suggest that the cell surface of S. sanguis has hydrophobic properties. Isolated cell walls also tended to partition into the nonaqueous solvent. Amino acid analyses of the walls revealed the presence of several amino acids which possess hydrophobic side chains. It is likely that the hydrophobic amino acids associated with the cell wall contribute to the hydrophobicity of intact S. sanguis. When the adherence of S. sanguis to saliva-coated hydroxylapatite was measured, it was found that hydrophobic bond-disrupting agents, such as the Li+ cation, the SCN- anion, and sodium dodecyl sulfate, were capable of inhibiting the cell-hydroxylapatite union. In addition, it was observed that both urea and tetramethylurea were inhibitors of the adherence, although the latter reagent was the superior inhibitor. The results suggest that the adherence of S. sanguis to saliva-coated smooth surfaces is at least partially dependent on the formation of hydrophobic bonds between the cell and adsorbed salivary proteins. Hydrophobic bonding may contribute to cooperative interactions involving S. sanguis and saliva-coated hydroxylapatite (Nesbitt et al., Infect. Immun. 35:157-165, 1982).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978;48:270–299. doi: 10.1016/s0076-6879(78)48015-2. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Matthews T. H., Streips U. N. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol. 1980 Jul;143(1):471–480. doi: 10.1128/jb.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., McDannel M. L., Streips U. N., Birdsell D. C., Young F. E. Polyelectrolyte nature of bacterial teichoic acids. J Bacteriol. 1974 May;118(2):606–615. doi: 10.1128/jb.118.2.606-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum D., Pandolfelli E. R., Herskovits T. T. Denaturation of human and Glycera dibranchiata hemoglobins by the urea and amide classes of denaturants. Biochemistry. 1974 Mar 12;13(6):1278–1284. doi: 10.1021/bi00703a034. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits T. T., Jaillet H., Gadegbeku B. On the structural stability and solvent denaturation of proteins. II. Denaturation by the ureas. J Biol Chem. 1970 Sep 10;245(17):4544–4550. [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. Extracellular proteases modify cell wall turnover in Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1199–1208. doi: 10.1128/jb.141.3.1199-1208.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Newbrun E. Effect of PH and some reagents on the sucrose-independent non-specific sorption of the oral bacterium Streptococcus mutans to glass. Arch Oral Biol. 1981;26(9):735–743. doi: 10.1016/0003-9969(81)90191-6. [DOI] [PubMed] [Google Scholar]
- Miörner H., Albertsson P. A., Kronvall G. Isoelectric points and surface hydrophobicity of Gram-positive cocci as determined by cross-partition and hydrophobic affinity partition in aqueous two-phase systems. Infect Immun. 1982 Apr;36(1):227–234. doi: 10.1128/iai.36.1.227-234.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Staat R. H., Rosan B., Taylor K. G., Doyle R. J. Association of protein with the cell wall of Streptococcus mutans. Infect Immun. 1980 Apr;28(1):118–126. doi: 10.1128/iai.28.1.118-126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D. The in-vitro attachment of an oral Streptococcus sp. to the acquired tooth enamel pellicle. Arch Oral Biol. 1978;23(3):167–173. doi: 10.1016/0003-9969(78)90212-1. [DOI] [PubMed] [Google Scholar]
- Pavlic A., Lapanje S. Interactions of human serum albumin with some alkylureas. Biochim Biophys Acta. 1981 Jun 29;669(1):60–64. doi: 10.1016/0005-2795(81)90223-3. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. R., JENCKS W. P. THE EFFECT OF CONCENTRATED SALT SOLUTIONS ON THE ACTIVITY COEFFICIENT OF ACETYLTETRAGLYCINE ETHYL ESTER. J Am Chem Soc. 1965 Jun 5;87:2470–2479. doi: 10.1021/ja01089a029. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Jinks D. C., Merrick J. M. Adherence of Streptococcus mutans and Streptococcus sanguis to salivary components bound to glass. Infect Immun. 1981 May;32(2):583–591. doi: 10.1128/iai.32.2.583-591.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Peticolas V., Schack L., Karlson L. Model studies on the effects of neutral salts on the conformational stability of biological macromolecules. I. Ion binding to polyacrylamide and polystyrene columns. Biochemistry. 1973 Mar 27;12(7):1256–1264. doi: 10.1021/bi00731a003. [DOI] [PubMed] [Google Scholar]



